淡水生態(tài)系統(tǒng)中幾種大DNA病毒研究概述
張奇亞
(1. 中國(guó)科學(xué)院水生生物研究所淡水生態(tài)與生物技術(shù)國(guó)家重點(diǎn)實(shí)驗(yàn)室, 武漢 430072;2. 中國(guó)科學(xué)院種子設(shè)計(jì)創(chuàng)新研究院, 北京 100101)
為滿足全球日益增長(zhǎng)的人口對(duì)優(yōu)質(zhì)蛋白質(zhì)的需求, 水產(chǎn)養(yǎng)殖業(yè)正快速發(fā)展[1,2], 且中國(guó)水產(chǎn)養(yǎng)殖的成功經(jīng)驗(yàn)已提供給全球共享[3]。但仍面臨動(dòng)物種類多、養(yǎng)殖密度大、在多變或劣質(zhì)水環(huán)境中易受流行病侵染的困擾[4]。尤其是病毒引起的水產(chǎn)動(dòng)物疾病, 發(fā)病快、死亡率高、傳播廣、危害大, 尚無特效藥物與解決方案, 被認(rèn)為是水產(chǎn)養(yǎng)殖業(yè)發(fā)展的限制因素之一[5—7]。而深入認(rèn)識(shí)病毒病原本質(zhì)特征, 則成為有效檢測(cè)、預(yù)防控制水產(chǎn)動(dòng)物病毒病的關(guān)鍵[6,8—11]。
具有雙鏈DNA基因組、其分子量接近或大于100 kb的病毒通常被稱為大DNA病毒(或核質(zhì)大DNA病毒, NCLDVs)[12,13]。包括感染動(dòng)物的痘病毒科(Poxviridae)、虹彩病毒科(Iridoviridae)、魚蛙皰疹病毒科(Alloherpesviridae)、線頭病毒科(Nimaviridae)、非洲豬瘟病毒科(Asfarviridae)、桿狀病毒科(Baculoviridae)和囊泡病毒科(Ascoviridae)成員,感染真核藻的藻類DNA病毒科(Phycodnaviridae)成員, 感染原核藻或藍(lán)藻菌的肌尾病毒科(Myoviridae)成員, 感染原生生物的擬菌病毒科(Mimiviridae)、馬賽病毒科(Marseilleviridae)、潘多拉病毒科(Pandoraviridae)、闊口罐病毒科(Pithoviridae)成員等[14]。水生大DNA病毒變異率雖比RNA病毒要低, 但它們的宿主范圍及分布環(huán)境極廣, 存在于各種水體和沉積物中[15—17]。已開展針對(duì)大DNA病毒的遺傳進(jìn)化及其宿主適應(yīng)性的研究[18—21], 其中有感染水產(chǎn)動(dòng)物重要的病毒病原, 如: 虹彩病毒(Iridoviruses)、皰疹病毒(Herpesviruses)、線頭病毒(Nimaviruses)[22,23]和感染藍(lán)藻菌的肌尾噬藻體(Myovirus)[24]。近期查明水環(huán)境中大DNA病毒和巨病毒(Giant viruses)的部分基因來源于宿主[25], 且能增加病毒對(duì)宿主的適應(yīng)性[26]。相關(guān)研究不僅拓寬病毒知識(shí)的邊界, 也將促進(jìn)水產(chǎn)動(dòng)物病毒病遠(yuǎn)程診斷、生物調(diào)控等智慧漁業(yè)水平的提升。
中國(guó)科學(xué)院水生生物研究所(簡(jiǎn)稱中科院水生所)對(duì)魚病防控及魚池控藻的研究始于20世紀(jì)50年代, 由倪達(dá)書負(fù)責(zé)成立魚病工作站, 并在蘇、浙、粵等省展開魚病調(diào)查[27]。同時(shí), 饒欽止[28]研究和報(bào)道了消滅魚池微囊藻湖靛(水華)的有效方法。20世紀(jì)70年代后期, 陳燕燊等[29]則對(duì)草魚出血病病毒病原開展研究, 以集體署名方式在《水生生物學(xué)集刊》上發(fā)表相關(guān)結(jié)果。1996年, 《水生生物學(xué)報(bào)》報(bào)道了我國(guó)分離鑒定的第一株水生脊椎動(dòng)物大DNA病毒——沼澤綠牛蛙蛙病毒(Rana gryliovirus,RGV)的相關(guān)研究[30,31](圖 1), 并由此開始兩棲類以及魚類虹彩病毒的研究, 內(nèi)容涉及病原的分離鑒定和基因組測(cè)序, 如: 魚淋巴囊腫病毒中國(guó)株 (Lymphocystis disease virus-China, LCDV-C, AY380826)[32]、牛蛙蛙病毒RGV (JQ654586)[33]和大鯢蛙病毒 (Andrias davidianusranvirus, ADRV, KC865735)[34]; 確定病毒在宿主體內(nèi)或細(xì)胞中的分布與定位[35], 比較不同毒株主要結(jié)構(gòu)蛋白基因的異同[36]; 鑒定一批功能基因[37—41], 并闡明它們?cè)诓《緩?fù)制中的作用, 新建雙熒光標(biāo)記可控基因表達(dá)重組病毒技術(shù)[42]; 揭示幾種大DNA病毒與水產(chǎn)動(dòng)物宿主相互作用的分子機(jī)制[43]。病原體》(Ranaviruses: lethal pathogens of ectothermic vertebrates)的撰寫[46]、獲湖北省自然科學(xué)獎(jiǎng)的“重要水產(chǎn)動(dòng)物病毒病原的鑒定及致病機(jī)理研究” (2004Z-034-2-010-007)和“水產(chǎn)動(dòng)物不同病毒基因組解析及病毒與宿主相互作的用分子機(jī)制”(2017Z-023-2-010-008)等工作, 記錄和見證了“水生病毒學(xué)”新枝萌發(fā)的過程。中科院水生所報(bào)道的蛙病毒RGV[31,33]、大鯢蛙病毒ADRV[34]及淋巴囊腫病毒LCDV-C[32], 中山大學(xué)報(bào)道的鱖魚傳染性脾腎壞死病毒ISKNV(AF371960)[47]和虎紋蛙病毒TFV(AF389451)、國(guó)家海洋局報(bào)道的大黃魚虹彩病毒LYCIV(AY779031)[48]、中國(guó)水產(chǎn)科學(xué)研究院黃海水產(chǎn)研究所報(bào)道的大菱鲆紅體病虹彩病毒TRBIV(GQ273492)[49]及新加坡石斑魚虹彩病毒SGIV(AY521625)[50]等作為虹彩病毒參考毒株, 被2020年更新發(fā)布的《國(guó)際病毒分類委員會(huì)報(bào)告》(The International Committee on Taxonomy of Viruses, ICTV Report)收錄[51,52], 為大DNA病毒種群共性特征提供借鑒[53]。
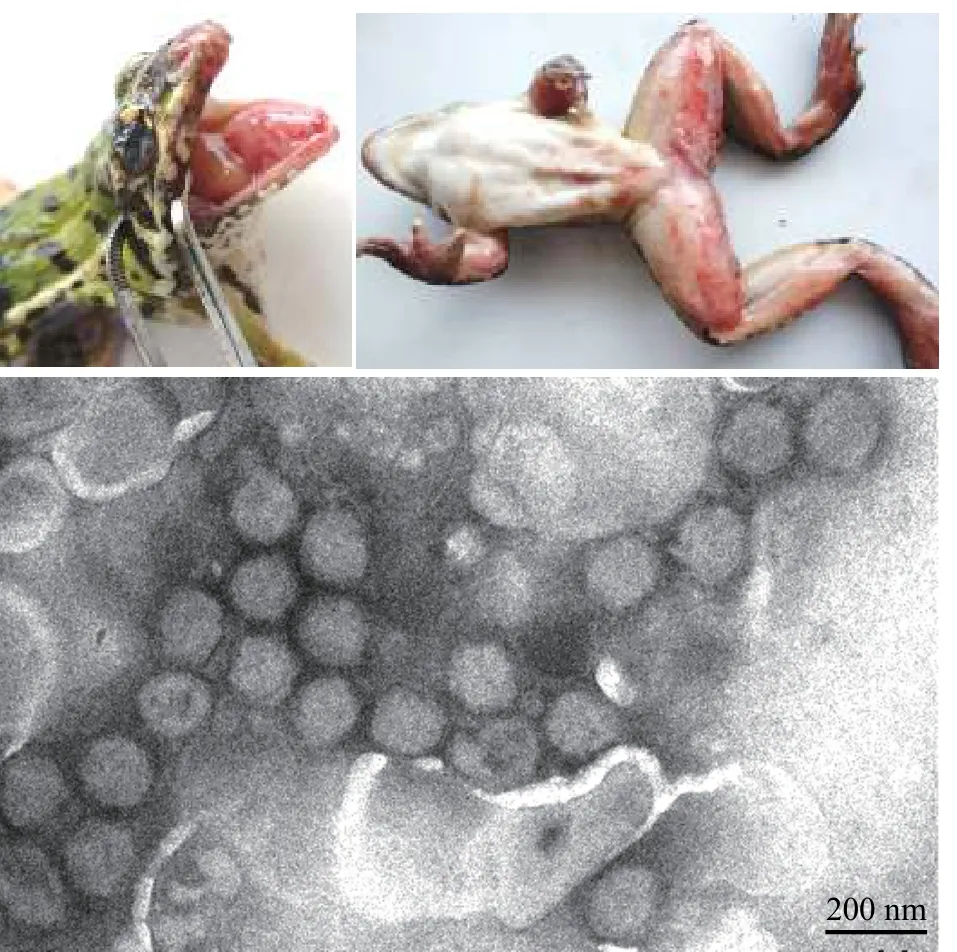
圖1 蛙病毒RGV引起的蛙致死性出血綜合癥及其負(fù)染電鏡圖(黃曉紅 圖)Fig. 1 Rana grylio virus (RGV) caused frog disease with lethal hemorrhagic syndrome and negative staining electron micrograph of the ranavirus particles. Bar=200 nm
盡管人類病毒性疫病會(huì)引起公共衛(wèi)生挑戰(zhàn), 甚至引發(fā)全球政治經(jīng)濟(jì)格局變化[54,55], 但病毒的感染與毒性有特定宿主范圍[56,57]。現(xiàn)有研究表明, 水生病毒僅感染低等脊椎動(dòng)物與其他水生生物, 尚無魚類病毒會(huì)感染人類的直接證據(jù)。水產(chǎn)品是人類安全和高質(zhì)量蛋白質(zhì)的重要來源。世界動(dòng)物衛(wèi)生組織 (OIE)指出: 水產(chǎn)養(yǎng)殖的好處是無窮的[58]。水產(chǎn)健康養(yǎng)殖能更好地促進(jìn)水產(chǎn)動(dòng)物、水環(huán)境與人類的整體健康[59]。水生病毒學(xué)當(dāng)前的重點(diǎn)任務(wù)就是要在闡釋病毒本質(zhì)及其與宿主和水環(huán)境相互作用的基礎(chǔ)上, 降低水生動(dòng)物發(fā)生病毒病的風(fēng)險(xiǎn), 并借助噬藻體等藍(lán)藻菌病毒優(yōu)化水生態(tài)系統(tǒng), 促進(jìn)生態(tài)健康的水產(chǎn)養(yǎng)殖業(yè)可持續(xù)增長(zhǎng)[9]。
1 研究動(dòng)態(tài)
歷經(jīng)半個(gè)世紀(jì), “水生病毒學(xué)”學(xué)科已發(fā)展成水生生物學(xué)的特色學(xué)科之一。《水生病毒學(xué)》與中英文雙語專著《水生病毒和及水生病毒病圖鑒》[44,45]、由國(guó)際知名蛙病毒專家、美國(guó)Chinchar和Gray教授為主編的英文專著《蛙病毒:變溫脊椎動(dòng)物的致命淡水大DNA病毒在形態(tài)、基因結(jié)構(gòu)、進(jìn)化及生態(tài)作用等方面都具有多樣性[21,60,61]。本節(jié)主要就兩棲類蛙病毒、鯽皰疹病毒、克氏原螯蝦(小龍蝦)線頭病毒及藍(lán)藻菌噬藻體這幾種淡水大DNA病毒的研究動(dòng)態(tài)做一簡(jiǎn)介。
1.1 沼澤綠牛蛙蛙病毒(Rana grylio virus, RGV)和大鯢蛙病毒(Andrias davidianus ranavirus, ADRV)
蛙病毒是能感染世界各地養(yǎng)殖和野生水生動(dòng)物、有囊膜、基因組大小105—150 kb、直徑為100—200 nm的球形大DNA病毒[62], 已測(cè)全基因組序列的蛙病毒毒株超過22株[52]。在同一物種中分離到不同蛙毒株的事件也時(shí)有發(fā)生, 如從牛蛙中分離鑒定了RGV-9506、RGV-9807、RGV-9808等毒株[36]; 而從發(fā)病大鯢中分離鑒定CGSIV-HN1104(KF512820)[63]、ADRV’(KF033124)[64]及未分類毒株(KC243313)等。蛙病毒還能跨種感染不同水生動(dòng)物[65,66]。因此, 專家呼吁要重視并避免這類病毒病傳播[67]。
大鯢細(xì)胞系的建立與蛙病毒研究已建立的魚類細(xì)胞系現(xiàn)超過300個(gè)[68—70], 與之相比, 兩棲動(dòng)物細(xì)胞系則要少得多, 并限于無尾(如蛙類)動(dòng)物細(xì)胞系[71—73]。直至2015年, 由中科院水生所建立大鯢胸腺細(xì)胞系(Chinese giant salamander thymus cell line, GSTC)、大鯢脾細(xì)胞系(Chinese giant salamander spleen cell line, GSSC)及大鯢腎細(xì)胞系(Chinese giant salamander kidney cell line, GSKC)之后, 方見有尾動(dòng)物(如大鯢)細(xì)胞系用于科研的報(bào)道[74]。其中, GSTC也是源于兩棲動(dòng)物胸腺組織的第一株細(xì)胞系。分別測(cè)試大鯢胸腺細(xì)胞系GSTC、爪蟾腎細(xì)胞系A(chǔ)6及鯉上皮瘤細(xì)胞系EPC這三種來源不同物種的細(xì)胞系對(duì)大鯢蛙病毒ADRV的敏感性。引起GSTC細(xì)胞病變所需時(shí)間最短、病變程度最嚴(yán)重、病毒滴度最高,TCID50達(dá)108/mL, 顯示GSTC細(xì)胞對(duì)ADRV很敏感。再用帶綠色熒光蛋白標(biāo)記的重組牛蛙蛙病毒(rRGV)感染, 不僅很快形成空斑, 且與rRGV產(chǎn)生的綠色熒光信號(hào)相吻合[75]。可見, GSTC不僅可用于測(cè)試蛙病毒感染, 也可用做蛙病毒基因擴(kuò)增、表達(dá)及與宿主相互作用研究的工具[76]。
重組蛙病毒構(gòu)建及其應(yīng)用推導(dǎo)蛙病毒基因組可編碼95—162個(gè)基因, 其中僅三分之一可利用序列同源性推定功能, 而對(duì)蛙病毒與宿主相互作用基因則知之甚少[77]。病毒重組技術(shù)是研究基因功能的重要技術(shù)之一, 還可用于篩選免疫活性分子、基因表達(dá)調(diào)控及疫苗研發(fā)[78]、模擬病毒入侵宿主過程和提供病毒與宿主之間相互作用的研究模型[79]等。基于對(duì)蛙病毒RGV尿嘧啶脫氧核糖核苷三磷酸酶基因RGV-dUTP(RGV-67R)[80]、淋巴囊腫病毒-中國(guó)株胸苷酸合成酶基因LCDV-CTS(LCDV-C11L)的鑒定[81], 以蛙病毒RGV的胸苷激酶基因TK(RGV-92R)和囊膜蛋白基因(RGV-53R)作為外源基因靶點(diǎn),構(gòu)建熒光報(bào)告基因或缺失特定基因位點(diǎn)重組病毒[82],獲得既保留親本毒株生物學(xué)特性, 又?jǐn)y帶外源基因的幾種重組蛙病毒[42,83,84]; 還構(gòu)建可調(diào)節(jié)特定基因表達(dá)、含乳糖操縱子和雙熒光標(biāo)記的條件致死型重組蛙病毒[85]。能否成功構(gòu)建重組病毒, 選擇適合的外源基因插入位點(diǎn)很重要[86]。
蛙病毒功能蛋白的鑒定有囊膜病毒可借助囊膜受體識(shí)別分子與細(xì)胞受體結(jié)合, 介導(dǎo)病毒內(nèi)吞進(jìn)入宿主細(xì)胞[87]。為查明蛙病毒囊膜蛋白在入侵時(shí)有何作用, 選擇與蛙病毒屬成員有高度同源性的膜蛋白R(shí)GV-43R進(jìn)行分析。結(jié)果顯示, 該蛋白跨膜結(jié)構(gòu)域決定其在胞質(zhì)中的定位; 而缺失基因43R的重組蛙病毒?43R-RGV與野生型RGV相比,前者DNA復(fù)制及超微形態(tài)不受影響, 但使細(xì)胞病變程度及病毒滴度卻顯著降低, 表明該蛋白是蛙病毒入侵的關(guān)鍵蛋白[88]。
病毒核心蛋白(Core proteins)指不同種屬毒株之間高度同源、涉及結(jié)構(gòu)及與復(fù)制的病毒蛋白。虹彩病毒科成員有26個(gè)核心蛋白[89], 其中, 蛙病毒蛋白R(shí)GV-63R被推導(dǎo)為DNA聚合酶, 具有3′-5′外切酶結(jié)構(gòu)域和DNA聚合酶B家族催化結(jié)構(gòu)域。研究揭示該蛋白不僅與病毒加工廠共定位, 還能與作為增殖細(xì)胞核抗原(Proliferating cell nuclear antigen,PCNA)的RGV-91R蛋白相互作用, 當(dāng)這兩個(gè)蛋白單獨(dú)或共同過表達(dá)時(shí), 均能促進(jìn)蛙病毒RGV在不同來源細(xì)胞系中復(fù)制[90]。對(duì)大鯢蛙病毒ADRV-96L蛋白進(jìn)行分析, 顯示這是一個(gè)具有ATPase活性、促進(jìn)宿主細(xì)胞增殖和生長(zhǎng)、有助于產(chǎn)生更多子代病毒[91]的重要蛋白。測(cè)試兩種蛙病毒的同源核心蛋白R(shí)GV-27R和ADRV-85L, 結(jié)果顯示它們不僅能在兩棲動(dòng)物細(xì)胞中高效表達(dá), 且都具有相同的抗原特性[92]。
蛙病毒與宿主的相互作用靶向破壞病毒囊膜就能抑制經(jīng)囊膜蛋白吸附細(xì)胞表面受體而入侵的病毒[93]。盡管糖蛋白或糖脂可作為哺乳動(dòng)物病毒受體, 但不同的表面分子結(jié)構(gòu)會(huì)導(dǎo)致病毒對(duì)物種或組織的取向不同[94]。研究揭示蛙病毒RGV和ADRV能以不同細(xì)胞表面的硫酸乙酰肝素作為受體, 經(jīng)此跨種入侵[95]。蛙病毒成熟和囊膜形成也可發(fā)生在不同細(xì)胞的囊泡中[31,96,97]。這些研究為蛙病毒跨種感染提供了新注釋。已知病毒能用miRNA操縱宿主細(xì)胞和病毒基因的表達(dá)[98], 借助miRNAs干擾與病毒重組等進(jìn)行分析, 結(jié)果顯示敲除病毒膜蛋白基因RGV-2L和RGV-53R能顯著抑制病毒裝配[42,99]。這表明蛙病毒囊膜蛋白不僅是其吸附入侵的要素, 且能顯著影響病毒裝配與成熟。分別對(duì)大鯢正常血清和感染蛙病毒血清及正常黏液和感染蛙病毒黏液的蛋白圖譜進(jìn)行測(cè)試比較, 顯示不同程度發(fā)生變化[100], 預(yù)示蛙病毒感染可引起宿主機(jī)體的生理生化反應(yīng)。蛙病毒RGV和ADRV的基因有99%同源性, 將其分別感染養(yǎng)殖大鯢,構(gòu)建15個(gè)轉(zhuǎn)錄組文庫, 并進(jìn)行測(cè)序, 結(jié)果從8.2億個(gè)有效讀數(shù)中獲得12.8萬個(gè)注釋基因; 在蛙病毒感染自然宿主或跨種感染過程中具有不同的基因表達(dá)模式, 所引起宿主應(yīng)答也各有不同。在跨種感染時(shí),蛙病毒進(jìn)入宿主后, 迅速表達(dá)自身基因、快速復(fù)制,但宿主應(yīng)答較弱, 以此提升其適應(yīng)性進(jìn)化及在種間傳播的時(shí)效性[101]。
以蛙病毒囊膜蛋白基因ADRV-2L和ADRV-58L分別構(gòu)建重組質(zhì)粒pcDNA-2L和pcDNA-58L, 并就其對(duì)大鯢的免疫保護(hù)作用進(jìn)行評(píng)價(jià)。對(duì)經(jīng)pcDNA-2L免疫過的大鯢進(jìn)行蛙病毒ADRV攻毒,其I型干擾素(IFN-1)、抗病毒蛋白(Mx)、主要組織相容性復(fù)合物(MHC-IA)和免疫球蛋白M (IgM)表達(dá)水平都能明顯上調(diào), 存活率為 66.7%, 顯著高于用pcDNA-58L免疫的大鯢存活率(3.3%)[78], 經(jīng)比較可篩出候選疫苗。蛙病毒會(huì)采取拮抗或免疫逃逸策略[102,103], 或有效變異, 增強(qiáng)對(duì)新物種宿主的適應(yīng)性, 以突破物種屏障感染新物種[104—106]。
1.2 鯽皰疹病毒(Crucian carp herpesvirus, CaHV)
皰疹病毒目(Herpesvirales)成員是有囊膜、基因組大小為125—290kb的大DNA病毒,在二十面體核衣殼外有層蛋白質(zhì)基質(zhì)被膜, 再被囊膜包裹[107]。魚蛙皰疹病毒科(Alloherpesviridae)以魚類和兩棲類為宿主[108],分為蛙皰疹病毒屬(Batrachovirus)、鯉皰疹病毒屬(Cyprinivirus)、鮰皰疹病毒屬(Ictalurivirus)和鮭皰疹病毒屬(Salmonivirus)[6,109,110]。2016年從急性鰓出血癥的鯽中分離鯽皰疹病毒CaHV[111](圖 2), 基因組為275 kb的線性雙鏈DNA(KU199244)[112], 推測(cè)可編碼150個(gè)基因, 是基因組架構(gòu)及引發(fā)病癥與已知鯉皰疹病毒都不同的新毒株[23]。
CaHV的G蛋白偶聯(lián)受體G蛋白偶聯(lián)受體(G Protein-Coupled Receptors, GPCR)是一類有七個(gè)跨膜結(jié)構(gòu)域的膜蛋白受體, 可作為信號(hào)通路或參與信號(hào)轉(zhuǎn)導(dǎo), 并可利用激活細(xì)胞內(nèi)信號(hào)通路為病毒的復(fù)制提供保障[113]。鯽皰疹病毒G蛋白偶聯(lián)受體CaHV-25L(或稱CaHV-GPCR), 其C端含賴氨酸殘基、蛋白激酶C磷酸化位點(diǎn)及豆蔻酰化位點(diǎn)。經(jīng)截短、缺失或替換等方式, 構(gòu)建了CaHV-GPCR的C端系列突變子, 并在魚類細(xì)胞中表達(dá)。結(jié)果證實(shí)其C端不同氨基酸對(duì)蛋白亞細(xì)胞定位與分布狀態(tài)有不同影響[114]。
鯽皰疹病毒膜蛋白及其靶向分子有研究表明皰疹病毒膜蛋白呈動(dòng)態(tài)分布且有不同作用[115]。CaHV-138L是有兩個(gè)跨膜結(jié)構(gòu)域的鯽皰疹病毒膜蛋白, 分析顯示全長(zhǎng)CaHV-138L呈點(diǎn)狀分布于質(zhì)膜或核膜周圍, 且與線粒體共定位。當(dāng)截短其單一或雙跨膜結(jié)構(gòu)域時(shí), 就會(huì)改變其亞細(xì)胞定位, 使之在胞質(zhì)和胞核中呈斑塊狀分布。經(jīng)酵母雙雜交和免疫共沉淀篩查到能與該病毒蛋白相互作用的宿主線粒體蛋白FoF1-ATP酶, 并證實(shí)CaHV-138L能靶向線粒體蛋白FoF1-ATP酶[116]。這預(yù)示該蛋白可通過介導(dǎo)線粒體ATP合成, 為病毒復(fù)制提供能量。另外, 對(duì)CaHV與CyHV-2高度同源、且含RNase E/G家族典型結(jié)構(gòu)域的蛋白CaHV-31R進(jìn)行分析, 結(jié)果表明它能與內(nèi)質(zhì)網(wǎng)和高爾基體等有單層膜結(jié)構(gòu)的細(xì)胞器共定位, 可能涉及病毒胞內(nèi)運(yùn)輸與釋放[117]。
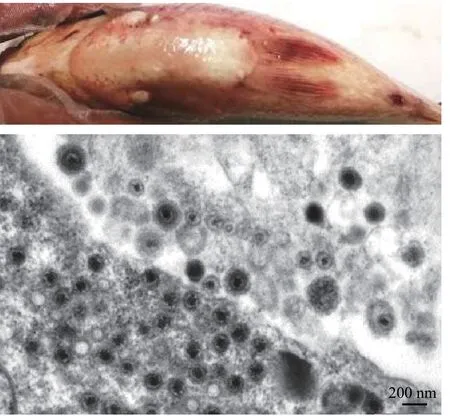
圖2 鯽皰疹病毒CaHV引起的高致死系統(tǒng)性出血癥及感染鯽頭腎超薄切片的電鏡圖 (方進(jìn) 圖)Fig. 2 Crucian carp herpesvirus (CaHV) caused disease with highlylethal systemic hemorrhagic symptoms and electron electronmicrograph of the infected Carassius auratus head kidney ultrathin section. Bar=200 nm
鯽皰疹病毒病防控對(duì)鯽皰疹病毒CaHV攻毒和感染的不同品系異育銀鯽轉(zhuǎn)錄組進(jìn)行分析, 測(cè)試其對(duì)病毒的抗性。結(jié)果, 三個(gè)雌核發(fā)育異育銀鯽品系對(duì)鯽皰疹病毒分別顯示出高(H)、中(F)和低(A+)抗性。又從不同品系中鑒定顯著差異表達(dá)的基因、免疫相關(guān)途徑及干擾素系統(tǒng)基因等[118]。在H、F和A+品系中, 依次有26條、7條和15條途徑與感染或免疫相關(guān)基因。鑒定出與病毒載量呈正相關(guān)或負(fù)相關(guān)的表達(dá)模塊[119]。這不僅顯示H品系的免疫力更強(qiáng), 且為分子標(biāo)記輔助選擇育種及鯽抗病毒分子育種實(shí)踐提供了新思路。還從被CaHV感染的異育銀鯽中, 鑒定出28個(gè)含不同免疫球蛋白結(jié)構(gòu)域的蛋白會(huì)上調(diào)表達(dá); 而且鯽蛋白DICPs能通過激活脂質(zhì)A驅(qū)動(dòng)熒光素酶, 與肉瘤病毒Src基因同源結(jié)構(gòu)域1蛋白酪氨酸磷酸酶(src-homology 1 protein tyrosine phosphatase, SHP-1)及SHP-2相互作用, 從而抑制干擾素及干擾素刺激基因(Interferon-stimulated genes, ISGs)表達(dá)[120]。所鑒定的干擾素系統(tǒng)基因有RIG-Is、LGP2s、IRF1-B、IRF3s、IRF7s、IRF9-B、Mxs及干擾素刺激因子Viperins等。進(jìn)一步研究顯示, CaHV侵染會(huì)啟動(dòng)干擾素調(diào)節(jié)因子RIG-I、遺傳學(xué)和生理學(xué)實(shí)驗(yàn)室蛋白2 (Laboratory of genetics and physiology 2, LGP2)表達(dá), 并激活線粒體抗病毒信號(hào)通路, 誘導(dǎo)表達(dá)干擾素調(diào)節(jié)因子[121]。從中等抗性的F品系中, 鑒定兩個(gè)大小不同的3′UTRs干擾素基因, 證實(shí)3′UTR參與干擾素基因的轉(zhuǎn)錄和翻譯, 是調(diào)節(jié)抗病毒免疫的潛在因素[122]。
通過替代藥物來控制水產(chǎn)病害越來越受到關(guān)注, 如有益微生物(益生菌probiotics)就被認(rèn)為是抗生素的有效且生態(tài)友好替代品[123,124]。經(jīng)對(duì)未喂餌益生菌而直接用CaHV攻毒的鯽, 與已喂餌益生菌后再攻毒的鯽, 分別測(cè)試其成活率和免疫相關(guān)基因。結(jié)果表明: 喂餌益生菌使鯽抗病毒的應(yīng)答水平及群體存活率顯著提高[125], 該研究為魚類抗病毒病添加了候選方案。
1.3 克氏螯蝦線頭病毒(Procambarus clarkia nimavirus, PCV)
克氏螯蝦(Procambarus clarkia)也稱小龍蝦。中國(guó)已成為世界養(yǎng)殖小龍蝦的最大生產(chǎn)國(guó)[126], 其需求和產(chǎn)量仍在增長(zhǎng), 但小龍蝦病毒病種類及其危害也隨之增加[127—131]。線頭病毒是有雙層囊膜、基因組大小 280—309 kb、一端帶尾、囊膜大小約430 nm×120 nm 的大DNA病毒。曾有小龍蝦受線頭病毒科(Nimaviridae)成員白班綜合癥病毒(White spot syndrome virus, WSSV)感染, 并出現(xiàn)白斑癥狀的報(bào)道[132,133]。或?qū)⑿↓埼r作為WSSV的實(shí)驗(yàn)動(dòng)物[134], 感染后也能觀察到白斑癥狀。但從自然感染小龍蝦中分離鑒定線頭病毒的文獻(xiàn)仍很少見。下面簡(jiǎn)介相關(guān)研究。
PCV的核酸檢測(cè)與超微形態(tài)某養(yǎng)殖場(chǎng)蝦群突然大量死亡卻無體表病癥, 采集幸存小龍蝦樣本, 并對(duì)這些無典型白斑癥的蝦解剖觀察。腸道無食物, 但因出血(或充血)而呈淡藍(lán)色, 肝胰腺呈淡黃或白色, 部分蝦鰓發(fā)黑。以幸存小龍蝦核酸作為模板, 設(shè)計(jì)小龍蝦線頭病毒PCV特有基因PCV-87R及五種對(duì)蝦病毒(WSSV、IHHNV、TSV、YHV及MrNV)保守基因的引物[135—137], 進(jìn)行PCR或RTPCR檢測(cè)。結(jié)果檢出PCV-87R與wssv-vp28為陽性,其他均呈陰性。這預(yù)示PCV是一株新線頭病毒, 與已知白斑病毒成員之間存在關(guān)鍵基因的遺傳與變異。
對(duì)自然感染無病癥小龍蝦組織制備的超薄切進(jìn)行電鏡觀察, 可見病毒顆粒存在于不同組織和細(xì)胞中。如在鰓和腸細(xì)胞中, 有大量病毒分布在胞質(zhì)和核質(zhì)中, 或規(guī)則排列在核膜周圍, 并伴隨廣泛的組織病變。完整PCV顆粒大小約300 nm×110 nm、兩端鈍圓呈短桿狀。負(fù)染電鏡圖顯示: PCV核衣殼呈有節(jié)桿狀[109](圖 3)。

圖3 小龍蝦線頭病毒PCV無癥狀感染的小龍蝦及病毒核衣殼負(fù)染電鏡圖 (李濤 圖)Fig. 3 Procambarus clarkia nimavirus (PCV) infected red swamp crayfish were asymptomatic and the negative staining electron micrograph of the viral nucleocapsid. Bar=200 nm
PCV基因組架構(gòu)及其多變區(qū)PCV基因組DNA大小為287 kb (MH663976), 推定可編碼180個(gè)基因。有觀點(diǎn)認(rèn)為可將對(duì)蝦白斑綜合征病毒基因大片段缺失作為時(shí)空進(jìn)化的標(biāo)記[138]。將PCV基因組與已知對(duì)蝦白斑綜合征病毒基因組進(jìn)行比較, 顯示PCV在易重組、有進(jìn)化意義的核酸片段相應(yīng)位置[139]也有缺失。此外, PCV另有一個(gè)差異顯著的核酸片段及普遍存在的核酸插入、缺失、替換及基因突變。
1.4 淡水藍(lán)藻菌噬藻體及水生大DNA病毒新成員
病毒歷史遠(yuǎn)比人類史悠遠(yuǎn), 而且數(shù)量極大, 地球被稱為 “病毒星球” (A Planet of Viruses)[140]。地球上的病毒已超過宇宙中繁星的數(shù)量, 若將預(yù)測(cè)地球上的1031個(gè)病毒首尾相連, 其長(zhǎng)度將達(dá)1億光年[141]。肌尾噬藻體(Cyanophage)是感染原核藍(lán)藻菌的病毒, 屬有尾目(Caudovirales)中肌尾噬藻體科(Myoviridae)的成員, 病毒顆粒由頭部和尾部?jī)刹糠纸M成, 呈蜊蚪形, 其DNA基因組大小約120 kb[142]。噬藻體可感染藍(lán)藻甚至操控水華藍(lán)藻的種群密度, 并將宿主機(jī)體和細(xì)胞轉(zhuǎn)化為有機(jī)物, 從而驅(qū)動(dòng)地球物理化學(xué)循環(huán); 在介導(dǎo)微生物之間的基因水平轉(zhuǎn)移、維持水生微生物群落多樣性等方面也發(fā)揮重要作用[143—147]。水體中有許多感染真核藻的大DNA病毒, 如藻DNA病毒(Phycodnavirus), 屬于藻類DNA病毒科(Phycodnaviridae), 通常為二十面體, 其DNA基因組的分子量為160—380 kb。還有感染原生動(dòng)物變形蟲的巨病毒(Giant virus), 其基因組大小甚至可達(dá)500 kb, 被認(rèn)為是規(guī)模迅速擴(kuò)張的水生大DNA病毒家族新成員[148]。相關(guān)新認(rèn)知拓展了水生大DNA病毒的范疇, 挑戰(zhàn)了對(duì)病毒的傳統(tǒng)認(rèn)識(shí)[149], 日漸模糊了病毒與細(xì)胞間的界限[150]。
噬藻體感染藍(lán)藻菌不僅改變宿主種群密度, 也促進(jìn)噬藻體的適應(yīng)性及與宿主的共同進(jìn)化[151]。噬藻體修飾宿主細(xì)胞膜能增強(qiáng)光保護(hù)及病毒編碼光合蛋白的表達(dá), 產(chǎn)生新的代謝通路或網(wǎng)絡(luò)[61]。水體中病毒對(duì)宿主的致死率很高, 同時(shí), 包括藍(lán)藻菌在內(nèi)的水生細(xì)菌對(duì)病毒的抵抗力也在增強(qiáng)[152]。噬藻體感染通常取決宿主的防御效果[153], 并由此可獲得高突變率及重組率[154]。由于從基因組數(shù)據(jù)所獲微生物的功能信息及其可信度都有限。因此, 純培養(yǎng)仍是微生物利用的前提與基礎(chǔ)[155]。運(yùn)用無菌技術(shù), 經(jīng)優(yōu)化條件、反復(fù)篩選、純化鑒定、培養(yǎng)和保藏等過程, 才可獲得很少量噬藻體純培養(yǎng)物[156]。自然界中不可培養(yǎng)微生物(Viable but non-culturable, VBNC)仍占絕大多數(shù)[157], 導(dǎo)致對(duì)微生物活體總數(shù)的低估[158], 也突顯基礎(chǔ)微生物研究的難點(diǎn)及蘊(yùn)藏開發(fā)微生物資源的潛能[159]。
銅綠微囊藻肌尾噬藻體-滇池株(Microcystis aeruginosa myovirus in Lake Dianchi, MaMV-DC)的分離及超微形態(tài)銅綠微囊藻肌尾噬藻體-滇池株MaMV-DC是利用微囊藻、魚腥藻、聚球藻等21株藍(lán)藻菌, 對(duì)昆明滇池采集的水樣進(jìn)行篩查, 并經(jīng)噬藻體單斑分離、擴(kuò)大培養(yǎng)及純化所獲得的噬藻體。其宿主范圍窄, 僅感染銅綠微囊藻株Microcystic aeruginosaFACHB-524, 噬藻斑為圓形透亮空斑; 噬藻體呈蝌蚪形, 二十面體頭部直徑約70 nm,收縮尾長(zhǎng)約160 nm (圖 4)。MaMV-DC的裂解量約為80個(gè)感染活性單位[160]。此外, 還有些噬藻體有形態(tài)獨(dú)特的噬斑, 如噬藻體A-4L可形成同心圓噬斑, 而同心圓形成與光照節(jié)律有關(guān)[161]。
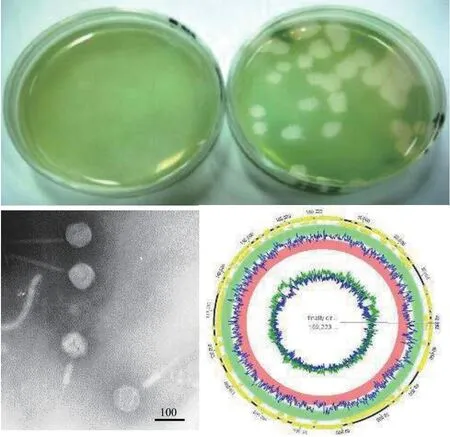
圖4 銅綠微囊藻肌尾噬藻體-滇池株MaMV-DC的噬斑、負(fù)染電鏡圖及基因組圖譜 (歐銅 圖)Fig. 4 The plaque, negative staining electron micrograph and genome map of Microcystis aeruginosa myovirus in Lake Dianchi(MaMV-DC). Bar=100 nm
MaMV-DC基因組的結(jié)構(gòu)測(cè)序分析顯示MaMV-DC基因組(KF356199)為末端循環(huán)冗余、雙鏈線性DNA大小169 kb, 推測(cè)可編碼170個(gè)基因, 其中含一個(gè)轉(zhuǎn)運(yùn)RNA (tRNA)基因。MaMV-DC與日本報(bào)道的銅綠微囊藻噬藻體Ma-LMM01(AB231700)基因組序列相似性為86%, 有150個(gè)同源基因[162]; 而與宿主銅綠微囊藻有29個(gè)同源基因。當(dāng)用主要衣殼蛋白構(gòu)建進(jìn)化樹時(shí), MaMV-DC與Ma-LMM01聚在一簇, 但兩者所攜帶的宿主或其他物種核酸片段卻差異顯著[163]。另外, 短尾噬藻體A-4L, 基因組DNA大小約為42 kb, 推測(cè)可編碼38個(gè)基因[164], 不屬大DNA病毒類群, 但能感染模式生物魚腥藻Anabaenasp. PCC 7120, 可作為載體, 用于研究噬藻體基因功能。
噬藻體功能基因噬藻體的生態(tài)功能是要憑借與宿主相互作用而體現(xiàn)[165]。對(duì)肌尾噬藻蛋白A-1(L)-ORF36功能進(jìn)行了鑒定, 顯示這是一個(gè)能與細(xì)胞表面脂多糖(LPS) O抗原結(jié)合的蛋白。還證實(shí)噬藻體正是利用該蛋白與細(xì)胞表面脂多糖特異性吸附而入侵藍(lán)藻菌的[166]。從絲狀藍(lán)藻噬藻體(Planktothrix agardhiivirus isolated from Lake Donghu,PaV-LD, NC_016564) 中鑒定藻膽體降解蛋白基因(NblA)、穿孔素基因和內(nèi)肽酶基因等[167,168]。銅綠微囊藻噬藻體基因MaMV-DC-5L也是編碼藻膽體降解蛋白基因, 能在模式集胞藻Synechocystissp.PCC 6803中表達(dá), 顯著消減宿主藻藍(lán)蛋白吸收峰,促進(jìn)噬藻體釋放[163]。已鑒定的噬藻體基因還有不同光合作用蛋白基因[169]和能量與代謝相關(guān)基因[170]。
噬藻體與宿主的相互作用微生物基因組所含規(guī)律間隔短回文重復(fù)序列及相關(guān)系統(tǒng)(Clustered regularly interspaced short palindromic repeats-associated endonuclease, CRISPR-Cas), 因?yàn)樗芘ccrRNA(CRISPR-derived RNA)堿基配對(duì)、識(shí)別并使入侵的噬菌體及其他病原體被Cas蛋白切割降解, 而被證明是細(xì)菌等原核生物的防御系統(tǒng)[171,172]。鑒定了功能多樣化、且有不同切割效率的V型CRISPRCas系統(tǒng)及由RNA引導(dǎo)的效應(yīng)蛋白Cas12[173]。還鑒定了絲狀藍(lán)藻菌長(zhǎng)尾噬菌體vB_AphaS-CL131編碼的V-U2 CRISPR-Cas系統(tǒng)[174], 顯示在藍(lán)藻菌中普遍存在V-U2的效應(yīng)蛋白[175]。而噬菌體也進(jìn)化出不同抗-CRISPR的蛋白(Acr), 能抑制或逃逸宿主CRISPR-Cas的防御作用[176]。將銅綠微囊藻肌尾噬藻體-滇池株MaMV-DC置于低溫冰箱(-80℃)中保存超過3年, 不僅保留對(duì)Microcystis aeruginosaFACHB-524的感染性, 也能感染微囊藻Microcystis flosaquaeTF09、Microcystis aeruginosaTA09和Microcystis wesenbergiiDW09, 但敏感性不同。再對(duì)不同微囊藻株的防御系統(tǒng)CRISPR-Cas進(jìn)行分析比較,顯示其分子結(jié)構(gòu)與含量都有變化。這證明CRISPR-Cas會(huì)影響微囊藻對(duì)噬藻體的敏感性, 或能決定噬藻體的宿主范圍[177]。CRISPR-Cas已作為基因編輯工具廣泛應(yīng)用[178], 并取得相應(yīng)成果, 將噬菌體酶靶向嵌入細(xì)菌特定基因位點(diǎn), 則能破壞細(xì)菌生物膜;而利用噬菌體將抗生素敏感基因?qū)肽退幘? 則可使耐藥菌致敏[179], 由此能獲得抗病毒新對(duì)策[180]。
2 機(jī)遇與挑戰(zhàn)
我國(guó)漁業(yè)已進(jìn)入設(shè)施化、智能化與生態(tài)化的歷史變革時(shí)期[181], 對(duì)水產(chǎn)健康生態(tài)養(yǎng)殖有更高要求, 也給水生病毒學(xué)學(xué)科發(fā)展帶來難得契機(jī)。水生大DNA病毒的性質(zhì)特征、遺傳進(jìn)化及與宿主及水生態(tài)關(guān)聯(lián)的研究基礎(chǔ)相對(duì)薄弱, 對(duì)水產(chǎn)動(dòng)物病毒病預(yù)測(cè)預(yù)警、智能健康水產(chǎn)養(yǎng)殖體系構(gòu)建、大DNA病毒群落多樣性及其水生態(tài)閾值評(píng)估的認(rèn)知也參差不齊, 正面臨前所未有的挑戰(zhàn)[182]。
2.1 先進(jìn)技術(shù)是提升水生大DNA病毒研究水平的載體
構(gòu)建和運(yùn)用大數(shù)據(jù)網(wǎng)絡(luò), 宏觀與微觀結(jié)合開展研究是水生大DNA病毒學(xué)新的生長(zhǎng)點(diǎn)。多國(guó)學(xué)者合作, 已從全球不同水體采集樣品重建大DNA病毒基因組信息, 圍繞其地理分布、基因多樣性、代謝特征等開展研究。闡明水體大DNA病毒全球分布的模式, 使其系統(tǒng)發(fā)育多樣性及功能多樣性數(shù)據(jù)各提升11倍和10倍; 發(fā)現(xiàn)病毒基因組編碼與光合作用及底物運(yùn)輸過程相關(guān)蛋白; 揭示大DNA病毒普遍具備使宿主重編程的功能[148]。單病毒顆粒示蹤、基因組解析、轉(zhuǎn)錄組分析也運(yùn)用于水生大DNA病毒研究中[101,183]; 剖析鑒定與疾病相關(guān)的DNA序列[184],可極大提高對(duì)疾病發(fā)生機(jī)制及病毒與水環(huán)境相關(guān)性的認(rèn)識(shí), 并推動(dòng)水產(chǎn)動(dòng)物病毒病由隨機(jī)檢測(cè)向系統(tǒng)防控戰(zhàn)略布局轉(zhuǎn)變。也由此可見, 掌握和運(yùn)用關(guān)鍵核心技術(shù)將為拓展水生病毒學(xué)學(xué)科注入鮮活動(dòng)力, 而新技術(shù)突破與經(jīng)典技術(shù)融合集成則會(huì)加速科研成果向?qū)嶋H應(yīng)用轉(zhuǎn)化。
2.2 整合、集成水生病毒學(xué)知識(shí)體系
淡水水生大DNA病毒研究是水生病毒學(xué)學(xué)科的重要內(nèi)容及原有基礎(chǔ)。隨著對(duì)水產(chǎn)品需求的不斷增長(zhǎng)及水產(chǎn)養(yǎng)殖規(guī)模持續(xù)擴(kuò)大, 水產(chǎn)動(dòng)物疾病也如影隨形。因此, 應(yīng)使水生病毒學(xué)知識(shí)從零星分散向交匯集成轉(zhuǎn)化, 尤其要強(qiáng)化水生病毒分類、溯源與遺傳進(jìn)化研究。
分類完全開放、不斷更新發(fā)布的《病毒分類報(bào)告》(http://ictv.global/report/), 是由國(guó)際病毒分類與命名的權(quán)威機(jī)構(gòu)——國(guó)際病毒分類委員會(huì)(The International Committee on Taxonomy of Viruses, ICTV), 按病毒性質(zhì)、親緣關(guān)系進(jìn)行歸類編排,為學(xué)者與公眾隨時(shí)了解新病毒的共性與特征而提供的綱要。隨著對(duì)更多水生大DNA病毒新成員及其與宿主相互作用、協(xié)同進(jìn)化軌跡的認(rèn)知[185], 將會(huì)使大DNA病毒的知識(shí)更加豐富和立體。
溯源尋覓病毒傳播源頭有益摸清病毒初始發(fā)生過程與傳播途徑, 預(yù)測(cè)病毒病流行趨勢(shì)與潛在風(fēng)險(xiǎn), 為疫病防控提供科學(xué)依據(jù)。但病毒只能在活細(xì)胞中復(fù)制, 沒留下化石, 其起源很難追蹤, 須從宿主或其他物種演化史中找答案。部分大DNA病毒有共同的祖先和起源[186], 或源自不同水生生物群落[187,188]; 有些大DNA病毒除了編碼復(fù)制和結(jié)構(gòu)形成所必需的蛋白質(zhì)核心基因外, 還會(huì)伴隨基因丟失或募集其他物種基因而形成新病毒[189]。但許多病毒的起源尚不清楚, 尤其是一些新發(fā)病毒病原。因此, 應(yīng)強(qiáng)化對(duì)水生大DNA病毒的溯源研究。
進(jìn)化轉(zhuǎn)換、插入、缺失、顛換、重組、重配及自然選擇等基因突變方式是病毒進(jìn)化與多樣性的源泉。病毒復(fù)制頻率高, 發(fā)生變異也較其他生物要快。病毒除了具備遺傳可變性, 還能通過跨宿主感染, 高效擴(kuò)大病毒的多樣性。有觀點(diǎn)認(rèn)為,病毒在地球生命的進(jìn)化中扮演重要的角色, 可導(dǎo)致重大進(jìn)化飛躍[190], 甚至還存在與宿主互惠共生病毒(Mutualistic viruses)。
2.3 魚水共護(hù), 促生態(tài)健康漁業(yè)發(fā)展
水生大DNA病毒病的流行方式與病毒、水生生物及水環(huán)境存在耦合關(guān)系[191]。病毒導(dǎo)致水產(chǎn)動(dòng)物病毒病流行, 或是噬藻體不作為任由水華暴發(fā)都會(huì)影響水生態(tài)健康及人與自然和諧共處[192]。以魚護(hù)水、以水養(yǎng)魚, 只有魚水共養(yǎng)護(hù)的健康生態(tài)漁業(yè)才可持續(xù)發(fā)展和有更旺盛的生命力。因此, 可利用分子生物學(xué)檢測(cè)方法和模型構(gòu)建預(yù)警網(wǎng)絡(luò)等途徑, 對(duì)機(jī)體及水生動(dòng)物健康狀況進(jìn)行評(píng)估[193]。但試圖通過投放藥物, 來防控水產(chǎn)動(dòng)物疾病的可操作性仍然很低[194,195]。總之, 面向綠色水產(chǎn)的全球重大需求, 要實(shí)現(xiàn)產(chǎn)品優(yōu)質(zhì)、環(huán)境優(yōu)美、生態(tài)優(yōu)穩(wěn)、養(yǎng)魚護(hù)水的漁業(yè)健康、可持續(xù)發(fā)展愿景(https://baijiahao.baidu.com/s?id=1654687744824397971&wfr=spider&for=pc), 就要拓展水生病毒學(xué)新方法、新技術(shù)與新知識(shí)的儲(chǔ)備; 探索水生大DNA病毒的感染、復(fù)制、發(fā)病機(jī)制、細(xì)胞/宿主取向, 及病毒、宿主與水環(huán)境相互作用等基礎(chǔ)理論問題; 深入研究水生動(dòng)物病毒病因、流行方式、預(yù)警閾值及防控管理等實(shí)踐中的新問題, 尋求噬藻體調(diào)控水環(huán)境核心技術(shù)的突破。研究者不斷探尋防御病毒新策略,為水生動(dòng)物提供更先進(jìn)的衛(wèi)生服務(wù), 以保障水產(chǎn)動(dòng)物健康, 創(chuàng)建魚水共護(hù)新機(jī)制。
參考文獻(xiàn):,
[1]Cressey D. Aquaculture: Future fish [J].Nature, 2009,458(7237): 398-400.
[2]Godfray H C, Beddington J R, Crute I R,et al. Food security: the challenge of feeding 9 billion people [J].Science, 2010, 327(5967): 812-818.
[3]Gui J F, Tang Q S, Li Z J,et al. Aquaculture in China:Success Stories and Modern Trends [M]. Wiley Blackwell, 2018: 1-711.
[4]Fisheries and Fisheries Administration Bureau of Ministry of Agriculture and Rural Affairs. National Fisheries Technology Extension Station, China Fisheries Association. Report on the health Status of Aquatic Animals in China [M]. Beijing: China Agriculture Press,2019: 1-106. [農(nóng)業(yè)農(nóng)村部漁業(yè)漁政管理局, 全國(guó)水產(chǎn)技術(shù)推廣總站, 中國(guó)水產(chǎn)學(xué)會(huì), 2019 中國(guó)水生動(dòng)物衛(wèi)生狀況報(bào)告 [M]. 北京: 中國(guó)農(nóng)業(yè)出版社, 2019: 1-106.]
[5]Stentiford G D, Neil D M, Peeler E J,et al. Disease will limit future food supply from the global crustacean fishery and aquaculture sectors [J].Journal of Invertebrate Pathology, 2012, 110(2): 141-157.
[6]Zhang Q Y, Gui J F. Virus genomes and virus-host interactions in aquaculture animals [J].Science China Life Sciences, 2015, 58(2): 156-169.
[7]Abdelrahman H, ElHady M, Alcivar-Warren A,et al.Aquaculture genomics, genetics and breeding in the United States: current status, challenges, and priorities for future research [J].BMC Genomics, 2017, 18(1):191.
[8]Gui J F, Zhu Z Y. Molecular basis and genetic improve-ment of economically important traits in aquaculture animals [J].Chinese Science Bulletin, 2012, 57(15): 1751-1760. [桂建芳, 朱作言. 水產(chǎn)動(dòng)物重要經(jīng)濟(jì)性狀的分子基礎(chǔ)及其遺傳改良 [J]. 科學(xué)通報(bào), 2012, 57(15): 1751-1760.]
[9]Gui J F. Fish biology and biotechnology is the source for sustainable aquaculture [J].Science China Life Sciences,2014, 44(12): 1195-1197. [桂建芳. 魚類生物學(xué)和生物技術(shù)是水產(chǎn)養(yǎng)殖可持續(xù)發(fā)展的源泉 [J]. 中國(guó)科學(xué): 生命科學(xué), 2014, 44(12): 1195-1197.]
[10]Naylor R L, Goldburg R J, Primavera J H,et al. Effect of aquaculture on world fish supplies [J].Nature, 2000,405(6790): 1017-1024.
[11]Smith M D, Roheim C A, Crowder L B,et al. Sustainability and global seafood [J].Science, 2010, 327(5967):784-786.
[12]Raoult D, Forterre P. Redefining viruses: lessons from Mimivirus [J].Nature Reviews Microbiology, 2008,6(4): 315-319.
[13]Jancovich J K, Qin Q, Zhang Q Y,et al. Ranavirus Teplication: Molecular, Cellular, and Immunological Events[M]//Gray M J, Chinchar V G (Eds.), Ranaviruses Lethal Pathogens of Ectothermic Vertebrates. New York:Springer, 2015: 105-139.
[14]WikiMili, Nucleocytoplasmic large DNA viruses.https://wikimili.com/en/Nucleocytoplasmic_large_DNA_viruses, 2020.
[15]Colson P, La Scola B, Levasseur A,et al. Mimivirus:leading the way in the discovery of giant viruses of amoebae [J].Nature Reviews Microbiology, 2017, 15(4):243-254.
[16]Koonin E V, Yutin N. Evolution of the large nucleocytoplasmic DNA viruses of eukaryotes and convergent origins of viral gigantism [J].Advances in Virus Research,2019(103): 167-202.
[17]Adriaenssens E M, Krupovic M, Knezevic P,et al. Taxonomy of prokaryotic viruses: 2016 update from the ICTV bacterial and archaeal viruses subcommittee [J].Archives of Virology, 2017, 162(4): 1153-1157.
[18]Monier A, Claverie J M, Ogata H. Taxonomic distribution of large DNA viruses in the sea [J].Genome Biology, 2008, 9(7): R106.
[19]Short S M, Short C M. Diversity of algal viruses in various North American freshwater environments [J].Aquatic Microbial Ecology, 2008, 51(1): 13-21.
[20]Elde N C, Child S J, Eickbush M T,et al. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses [J].Cell, 2012, 150(4): 831-884.
[21]Zhang Q Y, Gui J F. Diversity, evolutionary contribution and ecological roles of aquatic viruses [J].Science China Life Sciences, 2018, 61(12): 1486-1502.
[22]Chinchar V G, Hick P, Ince I A,et al. ICTV report consortium. ICTV virus taxonomy profile: iridoviridae [J].Journal of General Virology, 2017, 98(5): 890-891.
[23]Gui L, Zhang Q Y. Disease Prevention and Control[M]//Gui J F, Tang Q S, Li Z J,et al. Aquaculture in China: Success Stories and Modern Trends. Chichester:Wiley-Blackwell, 2018: 577-598.
[24]Zhang Q Y. Advances in studies on biodiversity of cyanophages [J].Microbiology China, 2014, 41(3): 545-559.[張奇亞. 噬藻體生物多樣性的研究動(dòng)態(tài) [J]. 微生物學(xué)通報(bào), 2014, 41(3): 545-559.]
[25]Schulz F, Yutin N, Ivanova N N,et al. Giant viruses with an expanded complement of translation system components [J].Science, 2017, 356(6333): 82-85.
[26]Abrah?o J, Silva L, Silva L S,et al. Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere [J].Nature Communications, 2018, 9(749): 1-12.
[27]China Pictorial, April Issue 1955. Prevention and Treatment of Fish Diseases [A]. [人民畫報(bào)1955年4月號(hào). 防治魚病] http://www.ihb.cas.cn/sq90/History/sq_lssj/202006/t20200609_5603618.html.
[28]Rao Q Z. An effective ways to prevent microsystis blooms in fishpond [J].Scientific Bulletin, 1952(Z1): 1-3. [饒欽止, 介紹一個(gè)消滅“湖靛”的有效方法 [J]. 科學(xué)通報(bào), 1952(Z1): 1-3.]
[29]Section of Virus Study, Third Laboratory, Institute of Hydrobiology, Academia Sinica. Studies on the causative agent of hemorrhage of the grass carp (Ctenopharyn-godon idellus) [J].Acta Hydrobiologica Sinica,1978, 2(3): 321-330. [中國(guó)科學(xué)院水生生物研究所第三室病毒組. 草魚出血病病原的研究 [J]. 水生生物學(xué)集刊, 1978, 2(3): 321-330.]
[30]Zhang Q Y, Li Z Q, Jiang Y L,et al. Preliminary studies on virus isolation and cell infection from diseased frogRnan grylio[J].Acta Hydrobiology Sinica, 1996, 20(4):390-392. [張奇亞, 李正秋, 江育林, 等. 沼澤綠牛蛙病毒的分離及其細(xì)胞感染的研究 [J]. 水生生物學(xué)報(bào),1996, 20(4): 390-392.]
[31]Zhang Q Y, Li Z Q, Gui J F. Studies on morphogenesis and cellular interactions ofRana gryliovirus in an infected fish cell line [J].Aquaculture, 1999, 175(3-4): 185-197.
[32]Zhang Q Y, Xiao F, Xie J,et al. Complete genome sequence of lymphocystis disease virus (LCDV-C) isolated from China [J].Journal of Virology, 2004, 78(13):6982-6994.
[33]Lei X Y, Ou T, Zhu R L,et al. Sequencing and analysis of the complete genome ofRana gryliovirus (RGV) [J].Archives of Virology, 2012(157): 1559-1564.
[34]Chen Z Y, Gui J F, Gao X C,et al. Genome architecture changes and major gene variations ofAndrias davidianusranavirus (ADRV) [J].Veterinary Research, 2013(44):101.
[35]Xie J, Li Z Q, Zhang Q Y,et al. Detection ofRana gryliovirus (RGV) in host frog tissues by using immunohistochemisrey assay [J].Acta Hydrobiologica Sinica,2002, 26(5): 438-443. [謝簡(jiǎn), 李正秋, 張奇亞, 等. 免疫組化法檢測(cè)美國(guó)青蛙組織中的蛙虹彩病毒 [J]. 水生生物學(xué)報(bào), 2002, 26(5): 438-443.]
[36]Zhang Q Y, Zhao Z, Xiao F,et al. Molecular characterization of threeRana gryliovirus (RGV) isolates andParalichthys olivaceuslymphocystis disease virus(LCDV-C) in iridoviruses [J].Aquaculture, 2006,251(1): 1-10.
[37]Sun W, Huang Y H, Zhao Z,et al. Characterization of theRana gryliovirus 3β-hydroxysteroid dehydrogenase and its novel role in suppressing virus-induced cytopathic effect [J].Biochemical and Biophysical Research Communications, 2006, 351(1): 44-50.
[38]Zhao Z, Shi Y, Ke F,et al. Constitutive expression of thymidylate synthase from LCDV-C induces foci formation and anchorage-independent growth in fish cells [J].Virology, 2008, 372(1): 118-126.
[39]Zhao Z, Ke F, Huang Y H,et al. Identification and characterization of a novel envelope protein inRana gryliovirus [J].Journal General Virology, 2008(89): 1866-1872.
[40]Zhao Z, Ke F, Shi Y,et al.Rana gryliovirus thymidine kinase gene: an early gene of iridovirus encoding for the cytoplasmic protein [J].Virus Genes, 2009(38): 345-352.
[41]Ke F, Zhao Z, Zhang QY. Cloning, expression and subcellular distribution of aRana gryliovirus late gene encoding ERV1 homologue [J].Molecular Biology Reports, 2009(36): 1651-1659.
[42]He L B, Ke F, Wang J,et al.Rana gryliovirus (RGV)envelope protein 2L: subcellular localization and essential roles in virus infectivity revealed by conditional lethal mutant [J].Journal of General Virology, 2014(95): 679-690.
[43]Gui L, Chinchar V G, Zhang Q Y. Molecular basis of pathogenesis of emerging viruses infecting aquatic animals [J].Aquaculture and Fisheries, 2018(3): 1-5.
[44]Zhang Q Y, Gui J F. Aquatic Virology [M]. Beijing:Higher Education Press, 2008: 1-414. [張奇亞, 桂建芳.水生病毒學(xué) [M]. 北京: 中國(guó)高等教育出版社, 2008: 1-414.]
[45]Zhang Q Y, Gui J F. Atlas of Aquatic Viruses and Viral Diseases [M]. Beijing: Science Press, 2012: 1-479. [張奇亞, 桂建芳. 水生病毒及病毒病圖鑒 [M]. 北京: 科學(xué)出版社, 2012: 1-479.]
[46]Gray M J, Chinchar V G. Ranaviruses Lethal Pathogens of Ectothermic Vertebrates [M]. New York: Springer,2015: 1-246.
[47]Deng M, He J G, Zuo T,et al. Infectious spleen and kidney necrosis virus (ISKNV) from Siniperca chuatsi: Development of a PCR detection method and the new evidence of iridovirus [J].Chinese Journal of Virology,2000, 16(4): 365-369. [鄧敏, 何建國(guó), 左濤, 等. 鱖魚傳染性脾腎壞死病毒 (ISKNV)PCR檢測(cè)方法的建立及虹彩病毒新證據(jù) [J]. 病毒學(xué)報(bào), 2000, 16(4): 365-369.]
[48]Ao J, Chen X W. Identification and characterization of a novel gene encoding an RGD-containing protein in large yellow croaker iridovirus [J].Virology, 2006, 355(2):213-222.
[49]Shi C Y, Jia K T, Yang B,et al. Complete genome sequence of a Megalocytivirus (familyIridoviridae) associated with turbot mortality in China [J].Virology Journal, 2010(7): 159.
[50]Song W J, Qin Q W, Qiu J,et al. Functional genomics analysis of Singapore grouper iridovirus: complete sequence determination and proteomic analysis [J].Journal of Virology, 2004, 78(22): 12576-12590.
[51]https://talk.ictvonline.org/ictv-reports/ictv_online_report/dsdna-viruses/w/iridoviridae#Citation.
[52]Chinchar V G, Waltzek T B, Subramaniam K. Ranaviruses and other members of the familyIridoviridae:Their place in the virosphere [J].Virology, 2017(511):259-271.
[53]Boyer M, Yutin N, Pagnier I,et al. Giant Marseillevirus highlights the role of amoebae as a melting pot in emergence of chimeric microorganisms [J].Proceedings of the National Academy of Sciences of The United States of America, 2009, 106(51): 21848-21853.
[54]McNeill W H. Plagues and Peoples [M]. Garden City, N Y, Anchor Press/Doubleday, 1976: 1-369.
[55]Grubaugh N D, Ladner J T, Lemey P,et al. Tracking virus outbreaks in the twenty-first century [J].Nature, 2019,4(1): 10-19.
[56]Lassen K. Virus-Host Interactions [J].Cell, 2011,146(2): 183-185.
[57]Rothenburg S, Brennan G. Species-specific host-virus interactions: implications for viral host range and virulence [J].Trends in Microbiology, 2020, 28(1): 46-56.
[58]World Organisation for Animal Health (OIE). Benefits of aquatic animals are infinite [C]. https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortalAqua-ticAnimals/EN_Brochure%20Aquatic%20Animals_FINAL_LD.pdf, 2019.
[59]Cavalli L S, Brito K C T, Brito B G. One health, one aquaculture: aquaculture under one health umbrella [J].Journal of Marine Biology and Aquaculture, 2015, 1(1):1-2.
[60]Wilson W H, Van Etten JL. Allen M J ThePhycodnaviridae: the story of how tiny giants rule the world [J].Current Topics in Microbiology and Immunology,2009(328): 1-42.
[61]Roitman S, Hornung E, Flores-Uribe J,et al. Cyanophage-encoded lipid desaturases: oceanic distribution,diversity and function [J].The ISME Journal, 2018(12):343-355.
[62]Chinchar V G, Waltzek T B. Ranaviruses: not just for frogs [J].PLoS Pathogens, 2014, 10(1): e1003850.
[63]Li W, Zhang X, Weng S,et al. Virion-associated viral proteins of a Chinese giant salamander (Andrias davidianus) iridovirus (genusRanavirus) and functional study of the major capsid protein (MCP) [J].Veterinary Microbiology, 2014, 172(1-2): 129-139.
[64]Wang N, Zhang M, Zhang L,et al. Complete genome sequence of a ranavirus isolated from Chinese giant salamander (Andrias davidianus) [J].Genome Announcements, 2014, 2(1): e1032-e1113.
[65]Stohr A C, Lopez-Bueno A, Blahak S,et al. Phylogeny and differentiation of reptilian and amphibian ranaviruses detected in Europe [J].PLoS One, 2015(10):e118633.
[66]Wirth W, Schwarzkopf L, Skerratt L F,et al. Ranaviruses and reptiles [J].Peer J, 2018(6): e6083.
[67]Garner T W, Stephen I, Wombwell E,et al. The amphibian trade: bans or best practice [J].Ecohealth, 2009(6): 148-151.
[68]Fryer J L, Lannan C N. Three decades of fish cell culture: A current listing of cell lines derived from fishes[J].Journal of Tissue Culture Methods, 1994(16): 87-94.
[69]Lakra W S, Swaminathan, T R, Joy K P. Development,characterization, conservation and storage of fish cell lines: A review [J].Fish Physiology and Biochemistry,2011, 37(1): 1-20.
[70]Pandey G. Overview of fish cell lines and their uses [J].International Journal of Engineering Research and Applications, 2013, 2(3): 580-590.
[71]Sinzelle L, Thuret R, Hwang H Y,et al. Characterization of a novelXenopus tropicaliscell line as a model forin vitrostudies [J].Genesis, 2012(50): 316-324.
[72]Bertin A, Hanna P, Otarola G,et al. Cellular and molecular characterization of a novel primary osteoblast culture from the vertebrate model organismXenopus tropicalis[J].Histochemistry and Cell Biology, 2015,143(4): 431-442.
[73]Mollard R. Culture, cryobanking and passaging of karyotypically validated native Australian amphibian cells[J].Cryobiology, 2018(81): 201-205.
[74]Yuan J D, Chen Z Y, Huang X,et al. Establishment of three cell lines from Chinese giant salamander and their sensitivities to the wild-type and recombinant ranavirus[J].Veterinary Research, 2015, 46(1): 58.
[75]Lei C K, Chen Z Y, Zhang Q Y. Comparative susceptibility of three aquatic animal cell lines to two ranaviruses[J].Journal of Fisheries of China, 2016, 40(10): 1643-1647. [雷存科, 陳中元, 張奇亞. 三種水生動(dòng)物細(xì)胞系對(duì)兩株蛙病毒敏感性的比較 [J]. 水產(chǎn)學(xué)報(bào), 2016,40(10): 1643-1647.]
[76]Chen Q, Ma J, Fan Y,et al. Identification of type I IFN in Chinese giant salamander (Andrias davidianus) and the response to an iridovirus infection [J].Molecular Immunology, 2015, 65(2): 350-359.
[77]Robert J, Jancovich J K. Recombinant ranaviruses for studying evolution of hos. t-pathogen interactions in ectothermic [J].Vertebrates Viruses, 2016, 8(7): E187.
[78]Chen Z Y, Li T, Gao X C,et al. Protective immunity induced by DNA vaccination against ranavirus infection in Chinese giant salamanderAndrias davidianus[J].Viruses, 2018, 10(2): 52.
[79]Luo J, Deng Z L, Luo X,et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system [J].Nature Protocols, 2007, 2(5): 1236-1247.
[80]Zhao Z, Ke F, Gui J F,et al. Characterization of an early gene encoding for dUTPase fromRana gryliovirus [J].Virus Research, 2007, 123(2): 128-137.
[81]Zhao Z, Zhang Q Y. Structure analysis of thymidylate synthase gene from LCDV-C [J].Virologica Sinica,2004, 19(6): 602-606. [趙哲, 張奇亞. 中國(guó)淋巴囊腫病毒胸苷酸合酶基因結(jié)構(gòu)特點(diǎn)及分析 [J]. 中國(guó)病毒學(xué),2004, 19(6): 602-606.]
[82]Chen G, Ward B M, Yu K H,et al. Improved knockout methodology reveals that frogvirus 3 mutants lacking either the 18K immediate-early gene or the truncatedvIF-2alpha gene are defective for replication and growthin vivo[J].Journal of Virology, 2011, 85(2): 11131-11138.
[83]He L B, Ke F, Zhang Q Y.Rana gryliovirus as a vector for foreign gene expression in fish cell [J].Virus Research, 2012, 163(1): 66-73.
[84]Huang X, Pei C, He L B,et al. The construction of a novel recombinant virus Δ67R-RGV and analysis of 67R gene function [J].Chinese Journal Virology, 2014,30(5): 495-501. [黃星, 裴超, 何利波, 等. 一株新的重組蛙病毒Δ67R-RGV的構(gòu)建及基因67R的功能鑒定 [J].病毒學(xué)報(bào), 2014, 30(5): 495-501.]
[85]He L B, Gao X C, Ke F,et al. A conditional lethal mutation inRana gryliovirus ORF 53R resulted in a marked reduction in virion formation [J].Virus Research, 2013,177(2): 194-200.
[86]Huang X, Fang J, Chen Z Y,et al.Rana gryliovirus TK and DUT gene locus could be simultaneously used for foreign gene expression [J].Virus Research, 2016,214(2): 33-38.
[87]Plemper R K. Cell entry of enveloped viruses [J].Current Opinion in Virology, 2011, 1(2): 92-100.
[88]Zeng X T, Gao X C, Zhang Q Y.Rana gryliovirus 43R encodes an envelope protein involved in virus entry [J].Virus Genes, 2018, 54(6): 779-791.
[89]Eaton H E, Metcalf J, Penny E,et al. Comparative genomic analysis of the familyIridoviridae: re-annotating and defining the core set of iridovirus genes [J].Virology Journal, 2007(4): 11.
[90]Zeng X T, Zhang Q Y. Interaction between two iridovirus core proteins and their effects on ranavirus (RGV)replication in cells from different species [J].Viruses,2019, 11(5): E416.
[91]Zhang R, Zhang Q Y. Adenosine triphosphatase activity and cell growth promotion ofAndrias davidianusranavirus 96L-encoded protein (ADRV-96L) [J].Microbiology China, 2018, 45(5): 1090-1099. [張銳, 張奇亞.大鯢蛙病毒編碼的96L蛋白(ADRV-96L)有ATPase活性和促進(jìn)細(xì)胞生長(zhǎng)的作用 [J]. 微生物學(xué)通報(bào), 2018,45(5): 1090-1099.]
[92]Ming C Y, Ke F, Zhang Q Y. The Expression and immunogenic analysis of ranaviruses homologous proteins RGV-27R and ADRV-85L [J].Chinese Journal of Virology, 2019, 35(6): 926-934. [明成玥, 柯飛. 張奇亞 蛙病毒同源蛋白ADRV-85L和RGV-27R的表達(dá)及其產(chǎn)物免疫原性分析 [J]. 病毒學(xué)報(bào), 2019, 35(6): 926-934.]
[93]Kong B, Moon S, Kim Y,et al. Virucidal nano-perforator of viral membrane trapping viral RNAs in the endosome [J].Nature Communications, 2019, 10(1): 185.
[94]Thompson A J, de Vries R P, Paulson J C. Virus recognition of glycan receptors [J].Current Opinion in Virology, 2019(34): 117-129.
[95]Ke F, Wang Z H, Ming C Y,et al. Ranaviruses bind cells from different species through interaction with heparan sulfate [J].Viruses, 2019, 11(7): E593.
[96]Zhang Q Y Xiao F, Li Z Q,et al. Characterization of an iridovirus form the cultured pig frog (Rana grylio) with lethal syndrome [J].Diseases of Aquatic Organisms,2001, 48(1): 27-36.
[97]Liu Y, Tran B, Wang F,et al. Visualization of assembly intermediates and budding vacuoles of Singapore grouper iridovirus in grouper embryonic cells [J].Scientific Reports, 2016(6): 18696.
[98]Skalsky R L, Cullen B R. Viruses, microRNAs, and host interactions [J].Annual Review of Microbiology,2010(64): 123-141.
[99]Kim Y S, Ke F, Lei X Y,et al. Viral envelope protein 53R genehighly specific silencing and iridovirus resistance in fish cells by amiRNA [J].PLoS One, 2010(5):e10308.
[100]Yuan J D, Chen Z Y, Zhang Q Y. Comparative analysis of serum and skin mucus protein profiles between ranavirus-infectud and normal Chinese giant salamanderAndrias davidianus[J].Acta Hydrobiologica Sinica,2016, 40(3): 594-600. [袁江迪, 陳中元, 張奇亞. 正常和蛙病毒感染后大鯢血清和黏液蛋白圖譜比較分析 [J].水生生物學(xué)報(bào), 2016, 40(3): 594-600.]
[101]Ke F, Gui J F, Chen Z Y,et al. Divergent transcriptomic responses underlying the ranaviruses-amphibian interaction processes on interspecies infection of Chinese giant salamander [J].BMC Genomics, 2018(19): 211.
[102]Ke F, Zhang Q Y. Aquatic animal viruses mediated immune evasion in their host [J].Fish and Shellfish Immunology, 2019(86): 1096-1105.
[103]Jones S, Nelson-Sathi S, Wang Y,et al. Evolutionary,genetic, structural characterization and its functional implications for the influenza A (H1N1) infection outbreak in India from 2009 to 2017 [J].Scientific Reports, 2019,9(1): 14690.
[104]St?hr A C, Blahak S, Heckers K O,et al. Ranavirus infections associated with skin lesions in lizards [J].Veterinary Research, 2013, 44(1): 1-10.
[105]Woo H J, Reifman J. Quantitative modeling of virus evolutionary dynamics and adaptation in serial passages using empirically inferred fitness landscapes [J].Journal of Virology, 2014, 88(2): 1039.
[106]Sawyer S L, Elde N C. A cross-species view on viruses[J].Current Opinion in Virology, 2012, 2(5): 561-568.
[107]McElwee M, Vijayakrishnan S, Rixon F,et al. Structure of the herpes simplex virus portal-vertex [J].PLoS Biology, 2018, 16(6): e2006191.
[108]Davison A J, Eberle R, Ehlers B,et al. The order Herpesvirales [J].Archives of Virology, 2009, 154(1):171-177.
[109]Gui L, Zhang Q Y. A brief review on aquatic animal virology researches in China [J].Journal of Fisheries of China, 2019, 43(1): 168-187. [桂朗, 張奇亞. 中國(guó)水產(chǎn)動(dòng)物病毒學(xué)研究概述 [J]. 水產(chǎn)學(xué)報(bào), 2019, 43(1): 168-187.]
[110]Osterrieder K. Chapter 9 Herpesvirales [M]//James N(Eds.), Fenner’s Veterinary Virology (5th eds.), Elsevier Academic Press, 2017: 189-216.
[111]Fang J, Deng Y S, Wang J,et al. Pathological changes of acute viral hemorrhages in the gills of crucian carp [J].Journal of Fishery Sciences of China, 2016, 23(2): 336-343. [方進(jìn), 鄧院生, 王俊, 等. 急性病毒性鯽鰓出血病的病理變化 [J]. 中國(guó)水產(chǎn)科學(xué), 2016, 23(2): 336-343.]
[112]Zeng X T, Chen Z Y, Deng Y S,et al. Complete genome sequence and architecture of crucian carpCarassius auratusherpesvirus (CaHV) [J].Arch Virology,2016(161): 3577-3581.
[113]Sodhi A, Montaner S. Gutkind J Viral hijacking of Gprotein-coupled-receptor signalling networks [J].Nature Reviews Molecular Cell Biology, 2004(5): 998-1012.
[114]Wang J, Gui L, Chen Z Y,et al. Mutations in the C-terminal region affect subcellular localization of crucian carp herpesvirus (CaHV) GPCR [J].Virus Genes,2016(52): 484-494.
[115]Beilstein F, Cohen G H, Eisenberg R J,et al. Dynamic organization of herpesvirus glycoproteins on the viral envelope revealed by super-resolution microscopy [J].PLoS Pathogens, 2019, 15(12): e1008209.
[116]Zhao Y H, Zeng X T, Zhang Q Y. Fish herpesvirus protein (CaHV-138L) can target to mitochondrial protein FoF1 ATPase [J].Virus Research, 2020(275): 197754.
[117]Wang Z H, Zhang Q Y. Characterization ofCarassius auratusherpesvirus ORF31R (CaHV-31R) and the encoded protein colocalize with cellular organs [J].Journal of Fisheries of China, 2019, 43(5): 1263-1270. [王子豪, 張奇亞. 鯽皰疹病毒ORF31R(CaHV-31R)的特征及其編碼蛋白與細(xì)胞器共定位 [J]. 水產(chǎn)學(xué)報(bào), 2019,43(5): 1263-1270.]
[118]Gao F X, Wang Y, Zhang Q Y,et al. Distinct herpesvirus resistances and immune responses of three gynogenetic clones of gibel carp revealed by comprehensive transcriptome [J].BMC Genomics, 2017(18): 561.
[119]Lu W J, Gao F X, Wang Y,et al. Differential expression of innate and adaptive immune genes in the survivors of three gibel carp gynogenetic clones after herpesvirus challenge [J].BMC Genomics, 2019(20): 432.
[120]Gao F X, Lu W J, Wang Y,et al. Differential expression and functional diversification of diverse immunoglobulin domain-containing protein (DICP) family in three gynogenetic clones of gibel carp [J].Developmental & Comparative Immunology, 2018(84): 396-407.
[121]Mou C Y, Wang Y, Zhang Q Y,et al. Differential interferon system gene expression profiles in susceptible and resistant gynogenetic clones of gibel carp challenged with herpesvirus CaHV [J].Developmental & Comparative Immunology, 2018(86): 52-64.
[122]Zang Z X, Dan C, Zhou L,et al. Function characterization and expression regulation of two different-sized 3’untranslated region-containing interferon genes from clone F of gibel carpCarassius auratus gibelio[J].Molecular Immunology, 2020(119): 18-26.
[123]Feckaninova A, Koscova J, Mudronova D. The use of probiotic bacteria againstAeromonasinfections in salmonid aquaculture [J].Aquaculture, 2017, 469(20): 1-8.
[124]Zorriehzahra M J, Delshad S T, Adel M,et al. Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review [J].Veterinary Quarterly, 2016, 36(4): 228-241.
[125]Li T, Ke F, Gui J F,et al. Protective effect ofClostridium butyricumagainstCarassius auratusherpesvirus in gibel carp [J].Aquaculture International, 2019, 27(3):905-914.
[126]Ethier V. Monterey Bay Aquarium, Red swamp frayfish[C]. http://seafood.ocean.org/wp-content/uploads/2016/10/Crawfish-Red-Swamp-China. 2013: 1-34.
[127]Longshaw M. Diseases of crayfish: A review [J].Journal of Invertebrate Pathology, 2011(106): 54-70.
[128]Baumgartner W A, Hawke J P, Bowles K,et al. Primary diagnosis and surveillance of white spot syndrome virus in wild and farmed crawfish (Procambarus clarkii,P.zonangulus) in Louisiana, USA [J].Diseases of Aquatic Organisms, 2009, 85(1): 15-22.
[129]Walker P J, Winton J R. Emerging viral diseases of fish and shrimpEmerging viral diseases of fish and shrimp[J].Veterinary Research, 2010, 41(6): 51.
[130]Manfrin C, Souty-Grosset C, Anastácio P,et al. Detection and control of invasive freshwater crayfish: from traditional to innovative methods [J].Diversity, 2019,11(1): 5.
[131]Soowannayan C, Nguyen G T, Pham L N,et al. Australian red claw crayfish (Cherax quadricarinatus) is susceptible to yellow head virus (YHV) infection and can transmit it to the black tiger shrimp (Penaeusmonodon) [J].Aquaculture, 2015(445): 63-69.
[132]Pan Z H, Yang Z L, Lu C P. Diagnosis of white spot syndrome virus in farm crawfish in Anhui province and its epidemiological source [J].Acta Microbiologica Sinica, 2013, 53(5): 492-497. [潘子豪, 楊政霖, 陸承平. 安徽地區(qū)小龍蝦白斑綜合征的診斷及朔源 [J]. 微生物學(xué)報(bào), 2013, 53(5): 492-497.]
[133]Jiang L, Xiao J, Liu L,et al. Characterization and prevalence of a novel white spot syndrome viral genotype in naturally infected wild crayfish,Procambarus clarkii, in Shanghai, China [J].Virusdisease, 2017, 28(3): 250-261.
[134]Shi Z, Huang C, Zhang J,et al. White spot syndrome virus (WSSV) experimental infection of the freshwater crayfish,Cherax quadricarinatus[J].Journal of Fish Diseases, 2000, 23(4): 285-288.
[135]張奇亞, 柯飛. 淡水小龍蝦線頭病毒PCV-87R特異性序列及應(yīng)用的制作方法 [P]. http://www.xjishu.com/zhuanli/27/201910307663.html, 2019.
[136]van Hulten M C W, Witteveldt J, Snippe M,et al. White spot syndrome virus envelope protein VP28 is involved in the systemic infection of shrimp [J].Virology, 2001,285(2): 228-233.
[137]Escobedo-Bonilla C M, Alday-Sanz V, Wille M,et al. A review on the morphology, molecular characterization,morphogenesis and pathogenesis of white spot syndrome virus [J].Journal of Fish Diseases, 2008, 31(1):1-18.
[138]Dieu B T, Marks H, Zwart M P,et al. Evaluation of white spot syndrome virus variable DNA loci as molecular markers of virus spread at intermediate spatiotemporal scales [J].Journal of General Virology, 2010, 91(5):1164-1172.
[139]Pradeep B, Shekar M, Karunasagar I,et al. Characterization of variable genomic regions of Indian white spot syndrome virus [J].Virology, 2008, 376(1): 24-30.
[140]Zimmer C. A Planet of Viruses (2nd Edition) [M].Chicago: University of Chicago Press, 2015: 104.
[141]Paez-Espino D, Eloe-Fadrosh E A, Pavlopoulos G A,et al. Uncovering earth’s virome [J].Nature, 2016(536):425-430.
[142]Lavigne R, Ceyssens P J. Family Myoviridae [M]//King A M Q, Adams M J, Carstens E B,et al(Eds.), Virus Taxonomy Classification and Nomenclature of Viruses Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier Academic Press,2012: 46-62.
[143]Zhang Q Y, Gui J F. One kind of strategic bio-resources that cannot be ignored——Freshwater and marine viruses and their roles in the global ecosystem [J].Bulletin of Chinese Academy of Sciences, 2009, 24(4): 520-526. [張奇亞, 桂建芳. 一類不可忽視的戰(zhàn)略生物資源——淡水與海水中的病毒及其在生態(tài)系統(tǒng)中的作用 [J]. 中國(guó)科學(xué)院院刊, 2009, 24(4): 520-526.]
[144]Zimmerman A E, Howard-Varona C, Needham D M,etal. Metabolic and biogeochemical consequences of viral infection in aquatic ecosystems [J].Nature Reviews Microbiology, 2019, 18(41): 1-14.
[145]Guidi L, Chaffron S, Bittner L,et al. Plankton networks driving carbon export in the oligotrophic ocean [J].Nature, 2016(532): 465-470.
[146]Breitbart M, Bonnain C, Malki K,et al. Phage puppet masters of the marine microbial realm [J].Nature Microbiology, 2018, 3(7): 754-766.
[147]Brum J R, Ignacio-Espinoza J C, Roux S,et al. Patterns and ecological drivers of ocean viral communities [J].Science, 2015, 348(6237): 1261498.
[148]Schulz F, Roux S, Paez-Espino D, Jungbluth S,et al.Giant virus diversity and host interactions through global metagenomics [J].Nature, 2020, 578(7795): 432-436.
[149]Levasseur A, Bekliz M, Chabrière E,et al. MIMIVIRE is a defence system in mimivirus that confers resistance to virophage [J].Nature, 2016, 531(7593): 249-252.
[150]Fischer M G, Allen M J, Wilson W H,et al. Giant virus with a remarkable complement of genes infects marine zooplankton [J].Proceedings of the National Academy of Sciences of the United States of America, 2010,107(45): 19508-19513.
[151]Lindell D, Jaffe J D, Coleman M L,et al. Genome-wide expression dynamics of a marine virus and host reveal features of co-evolution [J].Nature, 2007, 449(7158):83-86.
[152]Marston M F, Pierciey F J, Shepard A,et al. Rapid diversification of coevolving marineSynechococcusand a virus [J].Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(12):4544-4549.
[153]Doron S, Fedida A, Hernández-Prieto M A,et al. Transcriptome dynamics of a broad host-range cyanophage and its hosts [J].The ISME Journal, 2016, 10(6): 1437-1455.
[154]Kupczok A, Dagan T. Rates of molecular evolution in a marine synechococcus phage lineage [J].Viruses, 2019,11(8): 720.
[155]Hatzenpichler R, Krukenberg V, Spietz R L,et al. Nextgeneration physiology approaches to study microbiome function at single cell level [J].Nature Reviews Microbiology, 2020, 18(4): 241-256.
[156]Gao E B, Li S H, Lü B,et al. Analysis of the cyanophage (PaV-LD) infection in host cyanobacteria under different culture conditions [J].Acta Hydrobiologica Sinica, 2012, 36(3): 420-425. [高惡斌, 李三華, 呂波,等. 水華藍(lán)藻噬藻體對(duì)不同條件培養(yǎng)的宿主細(xì)胞感染性分析 [J]. 水生生物學(xué)報(bào), 2012, 36(3): 420-425.]
[157]Pinto D, Santos M A, Chambel L. Thirty years of viable but nonculturable state research: unsolved molecular mechanisms [J].Critical Reviews in Microbiology, 2015,41(1): 61-76.
[158]Li L, Mendis N, Trigui H,et al. The importance of the viable but non-culturable state in human bacterial pathogens [J].Frontiers in Microbiology, 2014(5): 258.
[159]Ayrapetyan M, Williams T, Oliver J D. Relationship between the viable but nonculturable state and antibiotic persister cells [J].Journal of Bacteriology, 2018,200(20): e00249-18.
[160]Ou T, Li S H, Liao X Y,et al. Cultivation and characterization of the MaMV-DC cyanophage that infects bloomforming cyanobacteriumMicrocystis aeruginosa[J].Virologica Sinica, 2013, 28(5): 266-271.
[161]Liao X Y, Ou T, Gao H,et al. Main reason for concentric rings plaque formation of virus infecting cyanobacteria (A-4L) in lawns ofAnabaena variabilis[J].Acta Microbiologica Sinica, 2014, 54(2): 191-199. [廖湘勇,歐銅, 高宏, 等. 藍(lán)細(xì)菌病毒A-4L在魚腥藻 (Anabaena variabilis) 藻苔中形成同心圓噬斑的成因 [J]. 微生物學(xué)報(bào), 2014, 54(2): 191-199.]
[162]Yoshida T, Nagasaki K, Takashima Y,et al. Ma-LMM01 infecting toxicMicrocystis aeruginosailluminates diverse cyanophage genome strategies [J].Journal of Bacteriology, 2008, 190(5): 1762-1772.
[163]Ou T, Gao X C, Li S H,et al. Genome analysis and gene nblA identification ofMicrocystis aeruginosamyovirus(MaMV-DC) reveal the evidence for horizontal gene transfer events between cyanomyovirus and host [J].Journal of General Virology, 2015, 96(12): 3681-3697.
[164]Ou T, Liao X Y, Gao X C,et al. Unraveling the genome structure of cyanobacterial podovirus A-4L with long direct terminal repeats [J].Virus Research, 2015(203): 4-9.
[165]Bertozzi Silva J, Storms Z, Sauvageau D. Host receptors for bacteriophage adsorption [J].FEMS Microbiology Letters, 2016, 363(4): 1-11.
[166]Xiong Z Z, Wang Y L, Dong Y L,et al. Cyanophage A-1(L) Adsorbs to lipopolysaccharides ofAnabaenasp.Strain PCC 7120 via the tail protein lipopolysaccharideinteracting protein (ORF36) [J].Journal of Bacteriology, 2019, 201(3): e516-e518.
[167]Gao E B, Gui J F, Zhang Q Y. A novel cyanophage with cyanobacterial non-bleaching protein a gene in the genome [J].Journal of Virology, 2012, 86(1): 236-245.
[168]Li S H, Gao E B, Ou T,et al. Cloning and expression analysis of major capsid protein gene, endopeptidase and holin gene of cyanophage PaV-LD [J].Acta. Hydrobiologica Sinica, 2013, 37(2): 252-259. [李三華, 高惡斌,歐銅, 等. 噬藻體PaV-LD主要衣殼蛋白、穿孔素和內(nèi)肽酶基因的克隆及表達(dá)分析 [J]. 水生生物學(xué)報(bào), 2013,37(2): 252-259.]
[169]Fridman S, Flores-Uribe J, Larom S,et al. A myovirus encoding both photosystem I and II proteins enhances cyclic electron flow in infected Prochlorococcus cells[J].Nature Microbiology, 2017, 2(10): 1350-1357.
[170]Sawa N, Tatsuke T, Ogawa A,et al. Modification of carbon metabolism inSynechococcus elongatusPCC 7942 by cyanophage-derived sigma factors for bioproduction improvement [J].Journal of Bioscience and Bioengineering, 2019, 127(2): 256-264.
[171]Xiao Y, Luo M, Hayes R P,et al. Structure basis for directional R-loop formation and substrate handover mechanisms in type I CRISPR-Cas system [J].Cell, 2017,170(1): 48-60.
[172]Niewoehner O, Garcia-Doval C, Rost?l J T,et al. Type III CRISPR-Cas systems produce cyclic oligoadenylate second messengers [J].Nature, 2017, 548(7669): 543-548.
[173]Yan W X, Hunnewell P, Alfonse L E,et al. Functionally diverse type V CRISPR-Cas systems [J].Science,2019, 363(6422): 88-91.
[174]?ul?ius S, ?imoliūnas E, Alzbutas G,et al. Genomic characterization of cyanophage vB_AphaS-CL131 infecting filamentous diazotrophic cyanobacteriumAphanizomenon flos-aquaereveals novel insights into virusbacterium interactions [J].Applied and Environmental Microbiology, 2019, 85(1): 1311-1118.
[175]Shmakov S, Smargon A, Scott D,et al. Diversity and evolution of class 2 CRISPR-Cas systems [J].Nature Reviews Microbiology, 2017(15): 169-182.
[176]Marino N D, Zhang J Y, Borges A L,et al. Discovery of widespread type I and type V CRISPR-Cas inhibitors [J].Science, 2018, 362(6411): 240-242.
[177]Wang J P, Bai P, Li Q,et al. Interaction between cyanophage MaMV-DC and eightMicrocystisstrains, revealed by genetic defense systems [J].Harmful Algae,2019(85): 101699.
[178]Knott G J, Doudna J A. CRISPR-Cas guides the future of genetic engineering [J].Science, 2018, 361(6405):866-869.
[179]Salmond G P, Fineran P C. A century of the phage: past,present and future [J].Nature Reviews Microbiology,2015, 13(12): 777-786.
[180]Liao H K, Gu Y, Diaz A,et al. Use of the CRISPR/Cas9 system as an intracellular defense against HIV 1 infection in human cells [J].Nature Communications,2015(6): 6413.
[181]Gui J F, Bao Z M, Zhang X, J. Development strategy for aquaculture genetic breeding and seed industry [J].Chinese Journal of Engineering Science, 2016, 18(3): 8-14. [桂建芳, 包振民, 張曉娟. 水產(chǎn)遺傳育種與水產(chǎn)種業(yè)發(fā)展戰(zhàn)略研究 [J]. 中國(guó)工程科學(xué), 2016, 18(3): 8-14.]
[182]Kauffman K M, Hussain F A, Yang J,et al. A major lineage of non-tailed dsDNA viruses as unrecognized killers of marine bacteria [J].Nature, 2018, 554(7691):118-122.
[183]Liu J, Yu C, Gui J F,et al. Real-time dissecting the entry and intracellular dynamics of single reovirus particle [J].Frontiers in Microbiology, 2018(9): 2797.
[184]Claussnitzer M, Cho J H, Collins R,et al. A brief history of human disease genetics [J].Nature, 2020,577(7789): 179-189.
[185]Yoshikawa G, Blanc-Mathieu R, Song C,et al. Medusavirus, a novel large DNA virus discovered from hot spring water [J].Journal of Virology, 2019, 93(8): 2130-2218.
[186]Iyer L M, Aravind L, Koonin E V. Common origin of four diverse families of large eukaryotic DNA viruses[J].Journal of Virology, 2001, 75(23): 11720-11734.
[187]Subramaniam K, Behringer D C, Bojko J,et al. A new family of DNA viruses causing disease in crustaceans from diverse aquatic biomes [J].mBio, 2020(11): 2938-3019.
[188]Wessner D R. The Origins of Viruses [J].Nature Education, 2010, 3(9): 37.
[189]Koonin E V, Yutin N. Origin and evolution of eukaryotic large nucleo-cytoplasmic DNA viruses [J].Intervirology, 2010, 53(5): 284-92.
[190]Roossinck M J. The good viruses: viral mutualistic symbioses [J].Nature Reviews Microbiology, 2011(9): 99-108.
[191]Engering A, Hogerwerf L. Pathogen-host-environment interplay and disease emergence [J].Emerging Microbes and Infections, 2013, 2(2): e5.
[192]V?r?smarty C J, McIntyre P B, Gessner M O,et al.Global threats to human water security and river biodiversity [J].Nature, 2010, 468(7321): 555-561.
[193]Song LS. An early warning system for diseases during mollusc mariculture: exploration and utilization [J].Journal of Dalian Ocean University, 2020, 35(1): 1-9.[宋林生. 海水養(yǎng)殖貝類病害預(yù)警預(yù)報(bào)技術(shù)及其應(yīng)用[J]. 大連海洋大學(xué)學(xué)報(bào), 2020, 35(1): 1-9.]
[194]Assefa A, Abunna F. Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish [J].Veterinary Medicine International, 2018, Article ID 5432497: 1-10.
[195]Jeney G. Fish Diseases: Prevention and Control Strategies [M]. London: Academic Press, 2017: 1-278.

