High Efficient Carbon Quantum Dots/BiOCl Nanocomposite for Photocatalytic Pollutant Degradation
ZHANG Zhijie, HUANG Hairui, CHENG Kun, GUO Shaoke
High Efficient Carbon Quantum Dots/BiOCl Nanocomposite for Photocatalytic Pollutant Degradation
ZHANG Zhijie, HUANG Hairui, CHENG Kun, GUO Shaoke
(School of Materials Science and Engineering, Shanghai Institute of Technology, Shanghai 201418, China)
To overcome the limitation of narrow photo-absorption range and high electron-hole recombination rate of pure BiOCl, a nanocomposite of carbon quantum dots (CQDs) and BiOCl with highly efficient photocatalytic activity was fabricated. The photocatalytic decomposition of rhodamine B (RhB) showed that the CQDs/BiOCl nanocomposite displayed superior photocatalytic performance to pure BiOCl, whichwas about 3.4 times higher than that of the latter. The optimal loading amount of CQDs was 7.1wt%, which could completely decolorize RhB within a short period of only 2 min, while the degradation rate of RhB was only 29.5% by pure BiOCl in the same period. UV-Vis diffuse reflectance spectra (UV-Vis DRS), photoelectrochemical measurement, and radicals trapping experiments were performed to elucidate the possible mechanism for the enhanced photocatalytic activity of the CQDs/BiOCl composite. The results show that CQD can expand the visible light absorption range of BiOCl, which is beneficial for light harvesting and generation of electron-hole pairs. Moreover, CQDs has unique up-converted photoluminescence behavior, as well as photo-induced electron transfer ability, which leads to enhanced photocatalytic performance of the CQDs/BiOCl composite.
photocatalysis; CQDs/BiOCl; nanocomposite; up-converted photoluminescence
As a new bismuth-based semiconductor material, BiOCl has recently become the focus of research in the field of photocatalysis and displays enhanced photocatalytic activity than TiO2(P25, Degussa)[1-4]. It possesses a unique layered structure, which can provide large enough space to polarize the related atoms and orbitals[5]. Then the induced dipole can promote the separation of the electron-hole pairs effectively, which accounts for its excellent photocatalytic performance. Nevertheless, BiOCl is a wide bandgap semiconductor (3.6 eV)[2], which means that BiOCl cannot be excited by visible light and can only be excited by UV light. Due to this inherent limitation, the abundant solar energy cannot be utilized efficiently. To expand the excitation wavelength range of BiOCl, many strategies have been applied. For example, Lee,[6]reported that the BiOCl/Bi2O3heterojunction had high efficiency in organic compounds degradation. Zan,[2]prepared black BiOCl by introducing oxygen vacancies, and found that its photocatalytic activity was 20 times higher than that of white BiOCl. Chen,[7]reported a BiOCl/BiOI composite which showed superior photocatalytic performance on degrading Methyl Orange (MO) and RhB.
Another effective method for enhancing photocatalytic performance is to modify the semiconductor with CQDs, which are attracting intense attention due to their environmentally friendly nature, chemical inertness, simple synthetic routes, ease of functionalization, high aqueous solubility, low cost and so on[8-10]. CQDs display strong up-conversion luminescence behavior, which can absorb two or more photons and emit light with wavelengths shorter than the excitation wavelength. Due to this unique photo-physical characteristics, CQDs have wide applications in such fields as light energy conversion, bioimaging, sensors, photovoltaic devices, electrocatalysis and photocatalysis,[11-15]. In addition, CQDs have unique non-localized electron conjugated structure, which can function as an effective electron trap to promote the separation of photo-generated electron-hole pairs.Therefore, the excellent light capturing ability and photo-induced electron transfer capability make CQDs a promising candidate in the field of photocatalysis. Up to date, many CQDs/semiconductor composites with improved visible light photocatalytic performance have been reported, including CQDs/Fe2O3[16], CQDs/Cu2O[17], CQDs/ZnO[18], CQDs/Ag3PO4[19], CQDs/C3N4[20], CQDs/ TiO2[21-23],.These studies indicate that CQDs are efficient components in the construction of composite pho-tocatalysts.
In a previous study, Xia,[24]synthesized CQDs modified BiOCl ultrathin nanosheetsa solvothermal method, employing mannitol as solvent and PVP as surfactant. In this work, a modified solvothermal method was used to synthesize CQDs/BiOCl composites, using a more environment-friendly ethanol as the solvent. By adjusting the solvent and reaction parameters such as solvothermal temperature and time, high-performance BiOCl nanosheets were obtained. By further introducing CQDs as electron trap and light harvester, the photocatalytic performance of BiOCl is enhanced significantly. Furthermore, the mechanism for the improved photocatalytic performance was elucidated detailedly.
1 Experimental procedures
1.1 Preparation of CQDs/BiOCl nanocomposites
CQDs were prepared through a modified literature procedure[25]. By thermolyzing citric acid (100 g) in air at 180 ℃for 40 h, an orange-brown liquid of CQDs capped by carboxylic acid was yielded. Then the high viscosity liquid was stirred with 100 mL of deionized water and 50 mL of NaOH aqueous solution (5 mol/L) to dissolve. Subsequently, approximately 25 mL of NaOH aqueous solution (5 mol/L) was added to neutralize the acidic CQDs, producing an orange-brown solution of sodium carboxylate capped CQDs. After isolating the product by freeze-drying, a yellow-orange powder was obtained.
In order to synthesize CQDs/BiOCl composites, 1 mmol of NaCl was dissolved into 17 mL of distilled water under stirring, then the solution was added into 17 mL of ethanol which contained 1 mmol of Bi(NO3)3·5H2O. After that, 0.5, 1.0, and 1.5 mL of CQDs solution (20 mg/mL) were dropped into the above mixture, respectively. The samples with 0.5, 1.0, and 1.5 mL of CQDs were denoted as C_0.5/BiOCl, C_1.0/BiOCl, and C_1.5/BiOCl, respectively. Correspondingly, the mass percentages of CQDs were calculated to be 3.7wt%, 7.1wt% and 10.3wt% for C_0.5/BiOCl, C_1.0/BiOCl, and C_1.5/BiOCl, respectively. Then the resulting suspension was transferred into an autoclave and kept at 100 ℃ for 18 h. After cooling to room temperature, the product was separated by centrifugation and washed with distilled water for several times, and then dried in air. For comparison, pure BiOCl was synthesized without the addition of CQDs.
1.2 Characterization
X-ray diffraction (XRD) patterns of the products were recorded on an X-ray diffractometer (Rigaku Co. Ltd., Tokyo, Japan) with Cu Kα radiation and in the 2range from 20° to 80°. An FEI tecnaiG2F30 transmission electron microscope (TEM) was used to observe the microstructure of the samples. UV-Vis diffuse reflectance spectra (DRS) of the products were obtained from a PE Lambda 900 UV-Vis spectrophotometer using BaSO4as reference. The photocurrent measurements were conducted on a CHI 650 electrochemical workstation (Shanghai Chenhua, China) using a three-electrode system.
1.3 Photocatalytic test
Photocatalytic performances of the CQDs/BiOCl composites were tested by decomposition of RhB under a 500 W Xe lamp to simulate the solar light. During the experiment, 0.05 g of the photocatalyst was added to 50 mL of RhB (10?5mol/L) solution to get a suspension, which was stirred magnetically in the dark for 1 h in order to establish an adsorption-desorption equilibrium between the pollutant molecules and the photocatalyst powders. 3 mL of suspension was collected at regular intervals, which was centrifuged to obtain a clarified solution for subsequent analysis. The concentration of RhB was analysed by recording the variations of the absorption band maximum (552 nm) on a UV-Vis spectrophotometer (754PC).
2 Results and discussion
2.1 Crystal structure
X-ray diffraction patterns of pure BiOCl and CQDs/BiOCl composites with different contents of CQDs are shown in Fig. 1. It can be seen that all the products are well crystallized, with all diffraction peaks consistent with the tetragonal BiOCl according to JCPDS 06-249. The introduction of CQDs has no obvious influence on the phase purity and crystallinity of the products.No diffraction peaks derived from CQDs can be observed, which is probably due to the low content and poor crystallinity of CQDs.
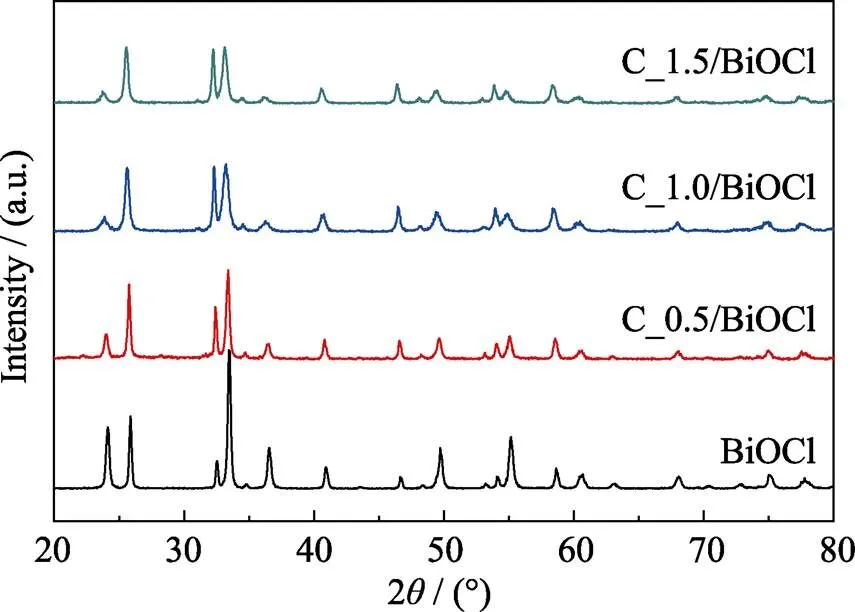
Fig. 1 XRD patterns of the products
2.2 Microstructure
The microstructure of CQDs and the CQDs/BiOCl composite (C_1.0/BiOCl) were observed by the transmission electron microscope (TEM). Fig. 2(A) shows that the as-prepared CQDs are monodisperse with diameters of5 nm. Fig. 2(B, C) show that the CQDs/ BiOCl composite exhibits nanosheet structure of about 200 nm. HRTEM image is further used to investigate the fine structure of the CQDs/BiOCl composite (Fig. 2(D)). The lattice fringes at 0.335 nm in the HRTEM image agrees well with fringe spacing of (101) plane of BiOCl, and the lattice fringes at 0.321 nm coincide with (002) spacing of CQDs. These results further confirm that the CQDs/BiOCl nanocomposite has been successfully constructed with CQDs attached on the surface of BiOCl nanosheets.
2.3 UV-Vis diffuse reflectance spectra
UV-Visible diffuse absorption spectra (DRS) of pure BiOCl and CQDs/BiOCl composites are compared in Fig. 3. According to Fig. 3, no absorption in the visible region can be observed for pure BiOCl, and it only has photo-absorption at UV light region with absorption edge located at360 nm. However, when the BiOCl nanosheets are modified with CQDs, the absorption edge of CQDs/BiOCl composites are red-shifted to the visible range. Moreover, as the amount of CQDs increases, the absorption edge shifts monotonically to the longer wavelengths. This result indicates that CQDs play a crucial role in harvesting visible light, which implies that more efficient utilization of the sunlight can be realized.
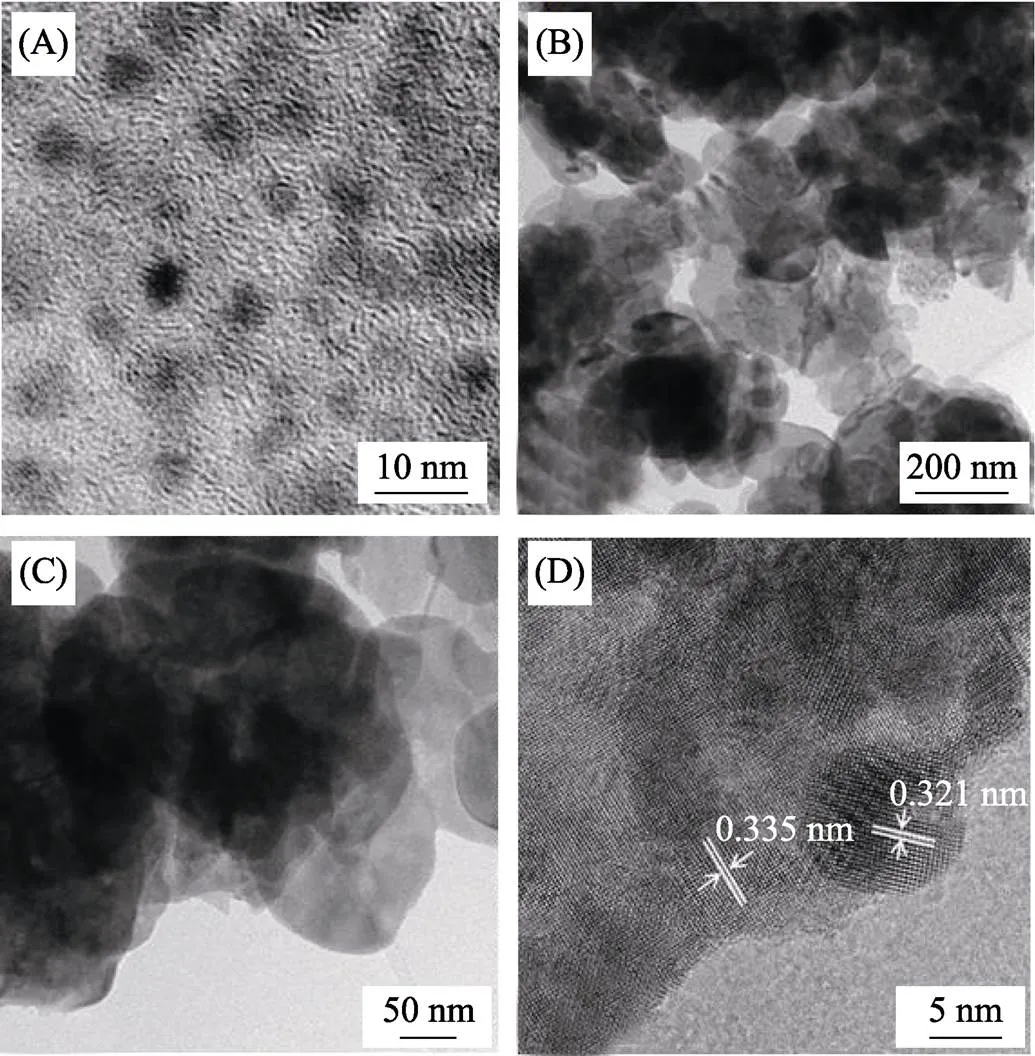
Fig. 2 TEM images of CQDs (A) and CQDs/BiOCl composite (B, C) and high resolution TEM image of CQDs/BiOCl composite (D)
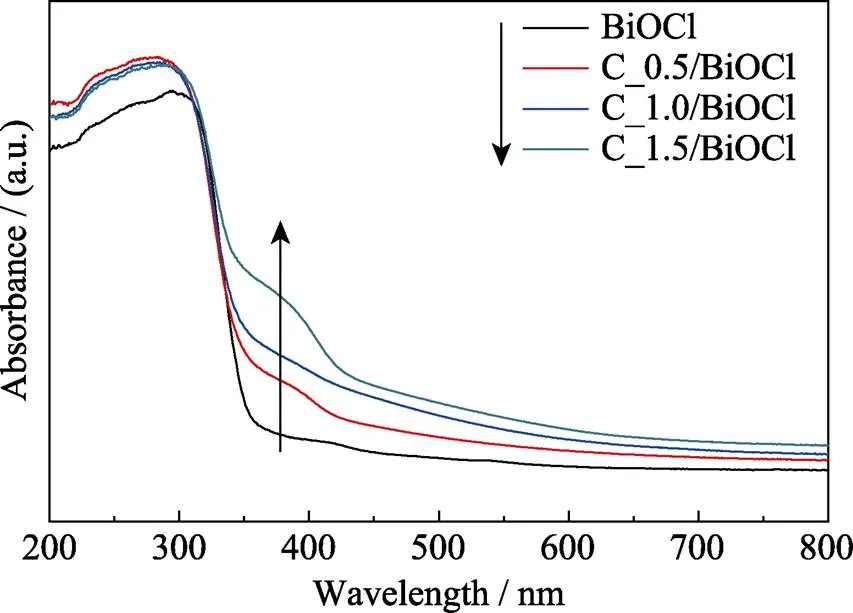
Fig. 3 UV-Vis diffuse re?ectance spectra of pure BiOCl and CQDs/BiOCl composites
2.4 Photocatalytic activity
The photocatalytic activities of the as-prepared products are evaluated by degrading RhB under simulated sunlight irradiation. Fig. 4(A) shows the photo-degradation rates of RhB by pure BiOCl, CQDs and CQDs/BiOCl composites. With the CQDs amount increasing, the photo-degradation rate of RhB incre-ases initially and achieves a maximum at CQDs am-ount of 1 mL. Complete degradation of RhB is realized by sample C_1.0/BiOCl within 2 min under simulated solar light irradiation, while the degradation rate of RhB is only 29.5% in the presence of pure BiOCl. However, further increasing the amount of CQDs leads to a decreased photocatalytic activity, which implies that there is an optimal loading amount of CQDs. This can be ascribed to that excess amount of CQDs will compete with BiOCl for light harvest, which decreases the absorption of light for degradation of RhB. Moreover, control experiment by using CQDs only under identical conditions is performed, and the result shows that the decolorization of RhB is negligible in the presence of CQDs alone. This result indicates that the decolorization is due to the photocatalysis but not the adsorption of dye molecules on the surface of CQDs.
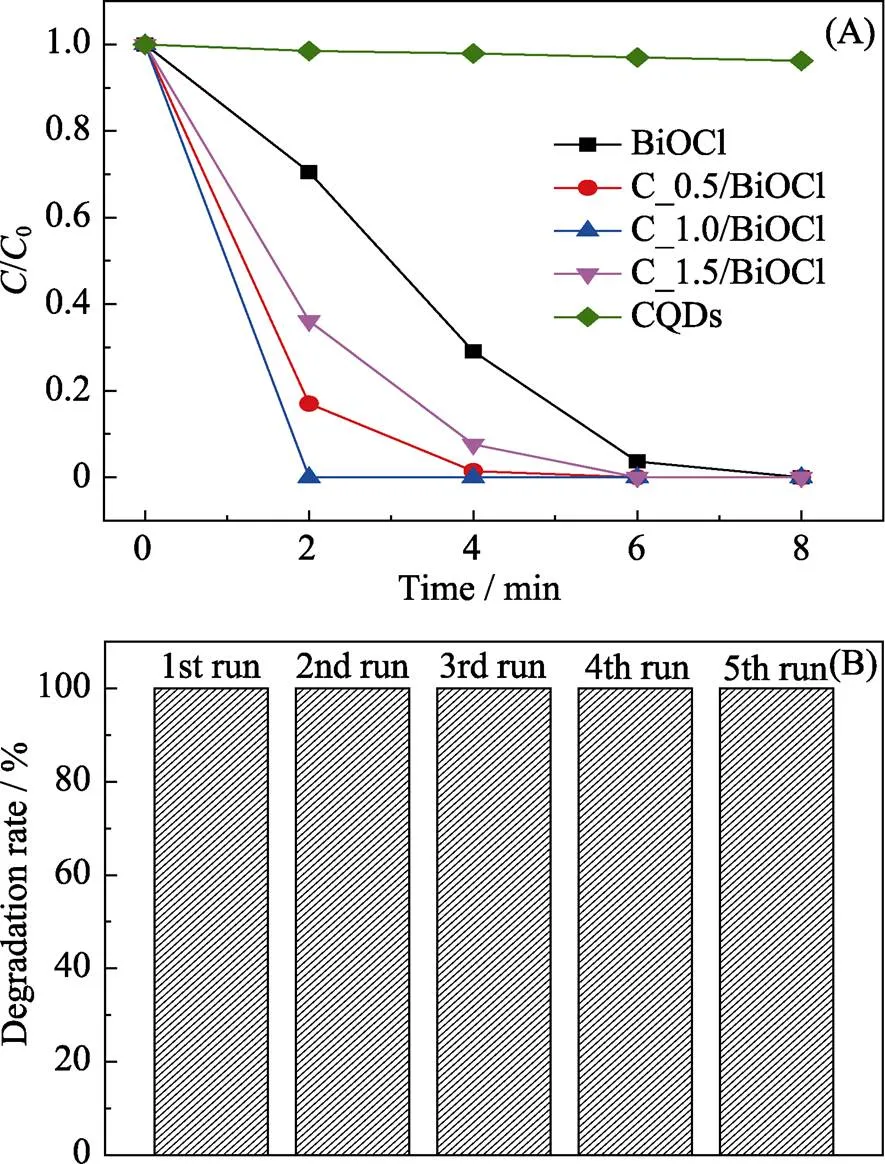
Fig. 4 (A) Photocatalytic degradation of RhB by CQDs, pure BiOCl and CQDs/BiOCl composites under simulated sunlight irradiation, and (B) stability test of the CQDs/BiOCl composite
Moreover, the cycling stability of theCQDs/BiOCl composite is tested through circulating runs. As shown in Fig. 4(B), the photocatalytic activity of the CQDs/BiOCl composite remains basically unchanged after being reused for five runs, which indicates that the CQDs/BiOCl composite has good photocatalytic stability and recyclability.
2.5 Mechanism of the enhanced photo-activity
It has been reported that the upconversion emission of CQDs can enhance the photocatalytic activities of CQDs modified composite photocatalysts under visible light irradiation[26]. Upconversion emission has been frequently cited as an important feature in CQDs. For instance, Kang,[27]reported that CQDs can be used as spectrum converters in photoelectro-chemical hydrogen generation systems due to their up-conversion luminescence property. In a previous report[28], we also found that the upconversion emission from CQDs can excite Bi2WO6to produce photo-induced charge carriers, thus increasing the availability of sunlight. When CQDs are introduced into the composite system, a portion of visible light is transformed into ultraviolet light. Then the ultraviolet light excites BiOCl to produce photo-induced charge carriers, which leads to an enhanced photo-activity of the composite.
Another crucial factor that determines the photocatalytic performance is the separation rate of the photo- induced charge carriers, which can be reflected directly by the photocurrent produced by the photocatalyst[29]. The photocurrent generated by pure BiOCl and CQDs/ BiOCl composite (C_1.0/BiOCl) are compared in Fig. 5. Obviously, the introduction of CQDs can significantly enhance the photocurrent of BiOCl. The photocurrent generated by CQDs/BiOCl composite is about 2.1 times higher than that of pure BiOCl electrode, indicating an improved separation rate of charge carriers of the CQDs/ BiOCl composite. The enhancement of photocurrent can be attributed to the contribution of CQDs, which act as electron reservoirs. Electrons from the conduction band of BiOCl can be trapped by the CQDs electron reservoirs, thus suppressing the recombination of the photo-generated charge carriers.
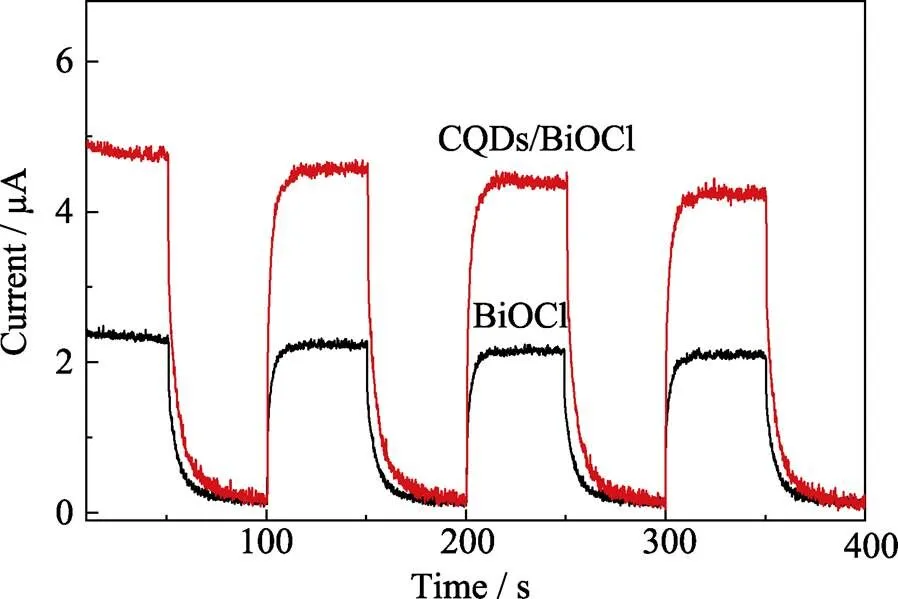
Fig. 5 Photocurrent responses of BiOCl and CQDs/BiOCl composite
In order to further elucidate the photocatalytic mechanism, the main oxidative species are determined by radicals trapping experiments, using benzoquinone as superoxide radical (?O2?) scavenger, EDTA-2Na as holes scavenger and tert-butanol (-BuOH) as hydroxyl radical (?OH) scavenger, respectively[30-31]. As shown in Fig. 6, the additions of EDTA-2Na and benzoquinone cause a severe depression of the photocatalytic activity, which indicates that both holes and ?O2?are the main oxidative species and play crucial roles in the photocatalytic process. On the contrary, the addition of-BuOH has a negligible influence on the photocatalytic activity, implying that ?OH is not the main oxidative species.
Based on the above experimental results, a mechanism is proposed to explain the enhanced photo-activity of the CQDs/BiOCl composite, as illustrated in Fig. 7. Under the irradiation of visible light, CQDs with upconversion luminescence behavior can convert the visible light into ultraviolet light, which can be absorbed by BiOCl to generate electron-hole pairs. On the other hand, CQDs as an excellent electron reservoir can trap electrons from the conduction band of BiOCl, and then transfer the electrons quickly to the surface of the photocatalyst. In thisway, the charge recombination is restricted effectively, while the migration of the charge carriers is promoted significantly. The transferred electrons as good reducing agents can react with the adsorbed O2on the surface ofCQDs to yield superoxide radicals (?O2?), which are highly oxidative agents and can oxidize the pollutant molecules. Moreover, the long-lived holes on the valence band of BiOCl with strong oxidability can react with the RhB molecules directly, as confirmed by the radicals trapping experiments. Above all,due to the efficient harvest of sunlight, as well as the fast separation and migration of the photo-induced electron-hole pairs, the CQDs/BiOCl composite exhibits excellent photocatalytic performance, which can decolorize RhB in a short period of only 2 min.
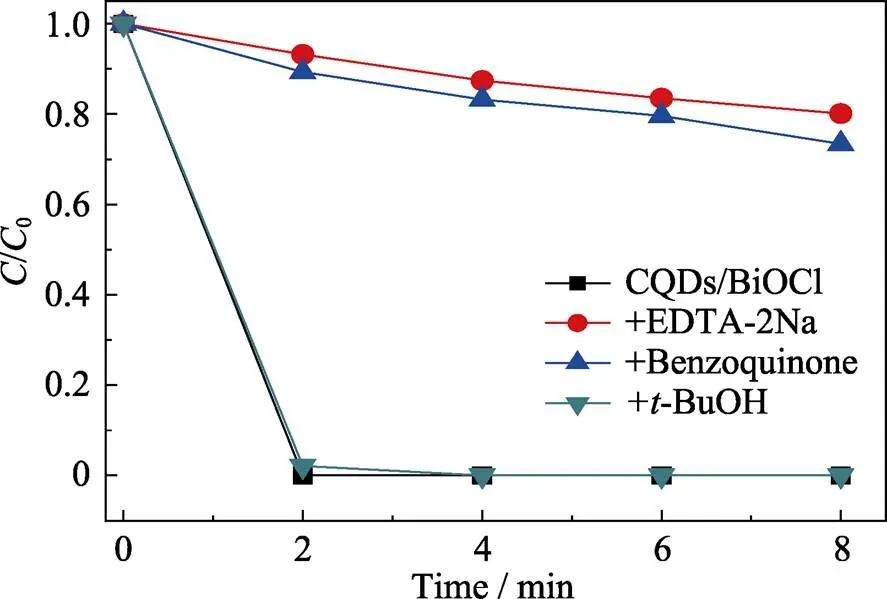
Fig. 6 Trapping experiments of oxidative species during photo-degradation of RhB by CQDs/BiOCl composite
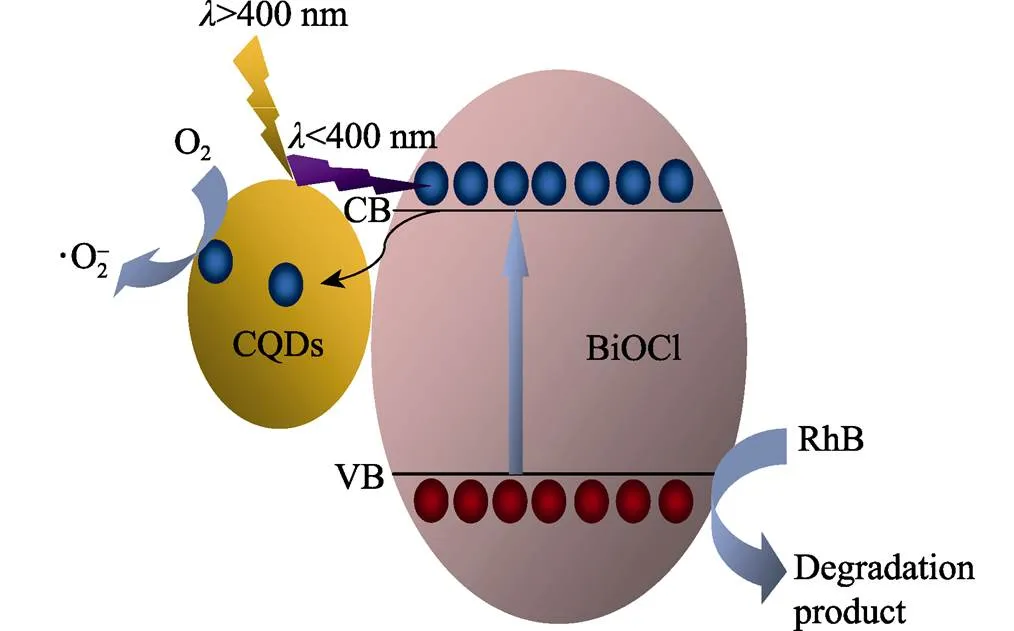
Fig. 7 Schematic illustration for the improved photo-activity of the CQDs/BiOCl composite
3 Conclusions
In summary, a highly efficient CQDs/BiOCl composite photocatalyst has been successfully prepared througha modified solvothermal process. Due to upconversion luminescence effect and photo-induced electron transfer ability of CQDs, the photo-absorption range of BiOCl is extended, while the recombination of photo-generated charge carriers is effectively suppressed. Consequently, the as-synthesized CQDs/BiOCl composite shows excellent photocatalytic performance under simulated solar light irradiation, which can decolorize RhB within only 2 min. This study demonstrates that introducing CQDs is an effective way to enhance the photocatalytic activity of the semiconductors.
[1] LEI Y, WANG G, SONG S,. Synthesis, characterization and assembly of BiOCl nanostructure and their photocatalytic properties., 2009, 11: 1857–1862.
[2] YE L Q, DENG K J, XU F,. Increasing visible-light absorption for photocatalysis with black BiOCl., 2012, 14: 82–85.
[3] ZHANG X, AI Z, JIA F,. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X=Cl, Br, I) nanoplate microspheres., 2008, 112: 747–753.
[4] HENLE J, SIMON P, FRENZEL A,. Nanosized BiOX (X=Cl, Br, I) particles synthesized in reverse microemulsions., 2007, 19: 366–373.
[5] ZHANG K L, LIU C M, HUANG F Q,. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst., 2006, 68: 125–129.
[6] CHAI S Y, KIM Y J, JUNG M H,. Heterojunctioned BiOCl/Bi2O3, a new visible light photocatalyst., 2009, 262: 144–149.
[7] LI T B, CHEN G, ZHOU C,. New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances., 2011, 40: 6751–6758.
[8] LI H T, HE X D, LIU Y,. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties., 2011, 49: 605–609.
[9] TANG L B, JI R B, CAO X K,. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots., 2012, 6: 5102–5110.
[10] RAY S C, SAHA A, JANA N R,. Fluorescent carbon nanoparticles: synthesis, characterization, and bioimaging application., 2009, 113: 18546–18551.
[11] ZONG J, ZHU Y H, YANG X L,. Synthesis of photoluminescent carbogenic dots using mesoporous silica spheres as nanoreactors., 2011, 47: 764–766.
[12] SHEN J H, ZHU Y H, YANG X L,. One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light., 2012, 36: 97–101.
[13] LI Y, HU Y, ZHAO Y,. An electrochemical avenue green- luminescent graphene quantum dots potential electron-acceptors photovoltaics., 2011, 23: 776–780.
[14] SHI W, LI X H, MA H M. A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells., 2012, 51: 6432–6435.
[15] BAKER S N, BAKER G A. Luminescent carbon nanodots: emergent nanolights., 2010, 49: 6726–6744.
[16] ZHANG H C, MING H, LIAN S Y,. Fe2O3/carbon quantum dots complex photocatalysts and their enhanced photocatalytic activity under visible light., 2011, 40: 10822–10825.
[17] LI H T, LIU R H, LIU Y,. Carbon quantum dots/Cu2O composites with protruding nanostructures and their highly efficient (near) infrared photocatalytic behavior., 2012, 22: 17470–17475.
[18] YU H, ZHANG H C, HUANG H,. ZnO/carbon quantum dots nanocomposites: one-step fabrication and superior photocatalytic ability for toxic gas degradation under visible light at room temperature., 2012, 36: 1031–1035.
[19] ZHANG H, HUANG H, MING H,. Carbon quantum dots/Ag3PO4complex photocatalysts with enhanced photocatalytic activity and stability under visible light., 2012, 22: 10501–10506.
[20] LIU J Y, LIU N Y, HAN Y Z,. Metal-free efficient photocatalyst for stable visible water splittinga two-electron pathway., 2015, 347: 970–974.
[21] YU H J, SHI R, ZHAO Y F,. Smart utilization of carbon dots in semiconductor photocatalysis.s, 2016, 28: 9454–9477.
[22] KE J, LI X Y, ZHAO Q D,. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance., 2017, 496: 425–433.
[23] HU Y D, XIE X F, WANG X,. Visible-light upconversion carbon quantum dots decorated TiO2for the photodegradation of flowing gaseous acetaldehyde., 2018, 440: 266–274.
[24] DI J, XIA J X, JI M X,. Carbon quantum dots modified BiOCl ultrathin nanosheets with enhanced molecular oxygen activation ability for broad spectrum photocatalytic properties and mechanism insight., 2015, 7: 20111?20123.
[25] GUO C X, ZHAO D, ZHAO Q,. Na+-functionalized carbon quantum dots: a new draw solute in forward osmosis for seawater desalination., 2014, 50: 7318?7321.
[26] LI H, HE X, KANG Z,. Water-soluble fluorescent carbon quantum dots and photocatalyst design., 2010, 49: 4430–4434.
[27] ZHANG X, HUANG H, LIU J,. Carbon quantum dots serving as spectral converters through broadband upconversion of near- infrared photons for photoelectrochemical hydrogen generation., 2013, 1: 11529–11533.
[28] ZHANG Z J, ZHENG T T, XU J Y,. Carbon quantum dots/Bi2WO6composites for efficient photocatalytic pollutant degradation and hydrogen evolution., 2017, 12: 1750082.
[29] KIM H G, BORSE P H, CHOI W Y,. Photocatalytic nanodiodes for visible light photocatalysis., 2005, 44: 4585–4589.
[30] ZHU Y Y, LIU Y F, LV Y H,. Enhancement of photocatalytic activity for BiPO4phase junction., 2014, 2: 13041–13048.
[31] YUE D, CHEN D M, WANG Z H,. Enhancement of visible photocatalytic performances of a Bi2MoO6-BiOCl nanocomposite with plate-on-plate heterojunction structure., 2014, 16: 26314–26321.
高效碳量子點/BiOCl納米復合材料用于光催化污染物降解
張志潔, 黃海瑞, 程昆, 郭少柯
(上海應用技術大學 材料科學與工程學院, 上海 201418)
為了克服單純BiOCl光譜吸收范圍窄和載流子復合幾率高的缺點, 本研究制備了一種具有高效光催化活性的碳量子點(CQDs)/BiOCl納米復合材料。光催化降解羅丹明B染料實驗表明CQDs/BiOCl納米復合材料的光催化性能遠優于單純的BiOCl, 其光催化性能約為后者的3.4倍。當CQDs的復合量為7.1wt%時, 樣品的光催化性能最佳, 能夠在2 min之內將羅丹明B完全脫色, 而單純的BiOCl在相同時間內對羅丹明B的降解率僅為29.5%。通過紫外-可見漫反射譜、光電化學測試以及自由基捕獲實驗揭示了CQDs/BiOCl納米復合材料的光催化性能提升機理, 結果表明CQDs可以拓展BiOCl的可見光吸收范圍, 這有利于增強其光捕獲能力以及促進電子–空穴對的產生。除此之外, CQDs獨特的上轉換發光行為, 以及光誘導的電子轉移能力提升了CQDs/BiOCl納米復合材料光催化性能。
光催化; CQDs/BiOCl; 納米復合材料; 上轉換發光
O641
A
2019-05-09;
2019-06-20
National Natural Science Foundation of China (51402194)
ZHANG Zhijie(1984–), female, PhD. E-mail: zjzhang@sit.edu.cn
張志潔(1984–),女,博士. E-mail: zjzhang@sit.edu.cn
1000-324X(2020)04-0491-06
10.15541/jim20190211
- 無機材料學報的其它文章
- 鋅在林格氏液中的體外長期腐蝕降解行為
- BaTiO3-ZnNb2O6陶瓷介電及儲能性能研究
- 高強度預應力陶瓷的發展與探索
- Synthesis, Crystal Structure, and Electrical Conductivity of Pd-intercalated NbSe2
- SnS2 Nanoplates: Synthesis and NO2 Sensing Property
- Removal of Volatile Organic Compounds Driven by Platinum Supported on Amorphous Phosphated Titanium Oxide

