離子色譜法測定銀耳湯中的硝酸鹽和亞硝酸鹽
林淼 白冰 時(shí)文興

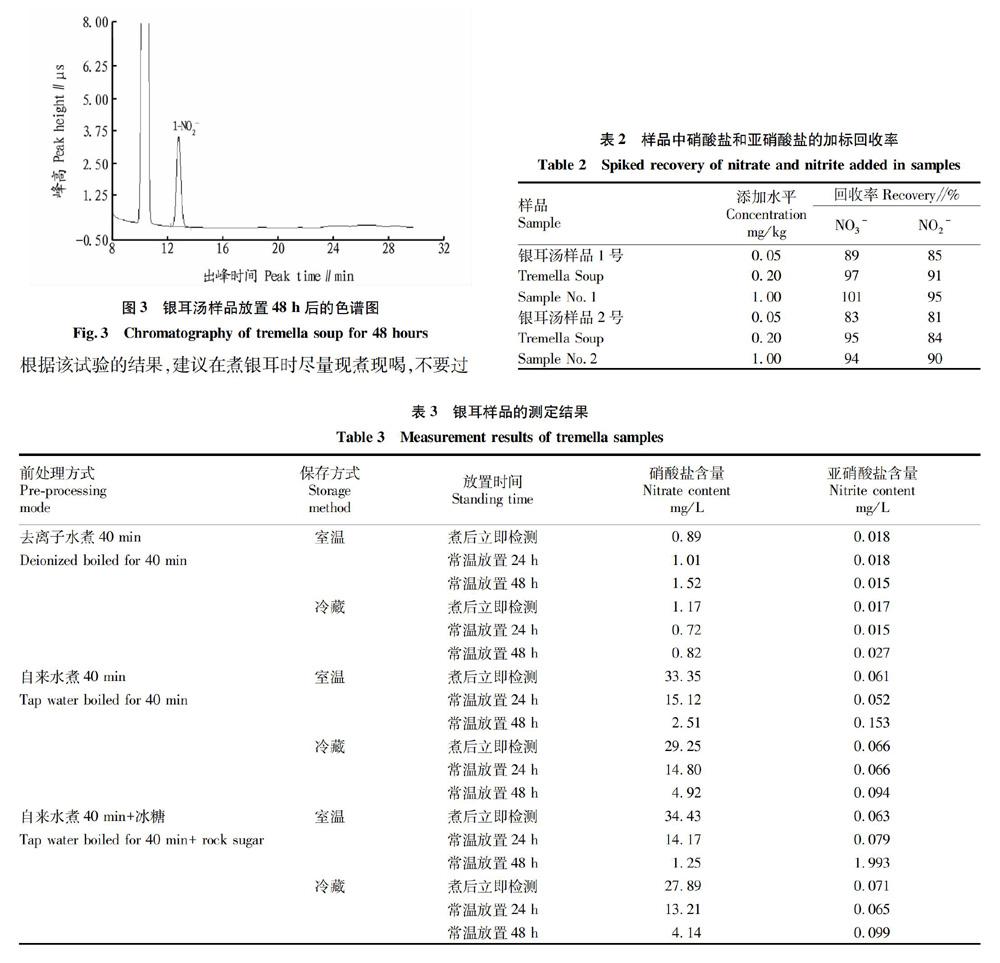

摘要 [目的]建立銀耳湯中硝酸鹽和亞硝酸鹽的離子色譜測定方法。[方法]銀耳樣品經(jīng)純水蒸煮提取,3%乙酸沉淀蛋白質(zhì)后,低溫離心,上清液依次過C18小柱、Ag柱、Na柱,經(jīng)濾膜過濾凈化后,由電導(dǎo)檢測器檢測樣品中的硝酸鹽和亞硝酸鹽含量。[結(jié)果]硝酸鹽(以NO3-計(jì))和亞硝酸鹽(以NO2-計(jì))分別在0.2~2.0和0.02~0.20 mg/L呈良好的線性關(guān)系,決定系數(shù)R2均大于0.999 6。該方法硝酸鹽和亞硝酸鹽檢出限分別為0.4和0.2 mg/kg,硝酸鹽的回收率為89.8%~96.1%,亞硝酸鹽的回收率為85.1%~95.3%。[結(jié)論]該方法結(jié)果準(zhǔn)確可靠,可以用于銀耳湯中硝酸鹽和亞硝酸鹽含量測定。
關(guān)鍵詞 銀耳湯;離子色譜法;硝酸鹽;亞硝酸鹽
中圖分類號 TS207.3文獻(xiàn)標(biāo)識碼 A
文章編號 0517-6611(2019)19-0209-03
Abstract [Objective] The research aimed to establish an ion chromatographic method for the determination of nitrate and nitrite in tremella soup.[Method] After cooking with pure water,protein precipitation using 3% acetic acid,and centrifugaion at low temperature,the supernatant was filtered through C18 column,Ag column and Na column for injection analysis.And then it was analyzed by ion chromatogrephy,and the conductivity dectector was used to detect nitrate and nitrite in tremella soup.[Result] Nitrate (calculated as NO3-) and nitrite (calculated as NO2-) showed good linearity at 0.2-2.0 and 0.02-0.20 mg/L,respectively,and the determination coefficient R2 was greater than 0.999 6.This method detection limit was 0.4 mg/kg for nitrate and 0.2 mg/kg for nitrite.The recovery rate of nitrate was 89.8%-96.1%,and the recovery rate of nitrite was 85.1%-95.3%.[Conclusion] This method is accurate and reliable,and suitable for determination of nitrate and nitrite in tremella soup.
Key words Tremella soup;Ion chromatogrephy method;Nitrate;Nitrite
硝酸鹽和亞硝酸鹽是2種經(jīng)常使用的食品添加劑,在肉制品加工業(yè)中常被用作發(fā)色劑,可以使其保持良好的色澤,并起到一定的防腐和增強(qiáng)食品風(fēng)味的功效。即使沒有人工添加,蔬菜在生長過程中也容易富集硝酸鹽[1]。大量攝入硝酸鹽可使人中毒,如搶救不及時(shí)會危及生命。同時(shí),硝酸鹽通過唾液、胃和血管還原成亞硝酸鹽[2],亞硝酸鹽的含量主要受硝酸鹽含量的影響[3]。亞硝酸鹽在人體內(nèi)外與仲胺類作用可形成亞硝胺類物質(zhì),當(dāng)其在人體內(nèi)達(dá)到一定計(jì)量時(shí),就會對人體產(chǎn)生致癌、致畸、致突變的危害[4]。研究發(fā)現(xiàn),攝入硝酸鹽含量較高的食物會增加人們患乳腺癌、腸胃癌、高鐵血紅蛋白癥等疾病的概率[5-7]。當(dāng)患有胃腸道疾病或者胃酸過少時(shí),胃腸道內(nèi)的細(xì)菌會把硝酸鹽還原成亞硝酸鹽而導(dǎo)致中毒,就是人們常說的“腸原性紫紺癥”[8]。……
 安徽農(nóng)業(yè)科學(xué)
2019年19期
安徽農(nóng)業(yè)科學(xué)
2019年19期
- 安徽農(nóng)業(yè)科學(xué)的其它文章
- 雙一流背景下高職動物醫(yī)學(xué)專業(yè)建設(shè)實(shí)踐與探索
- 基于學(xué)生創(chuàng)新能力培養(yǎng)下的高校生物化學(xué)實(shí)驗(yàn)教學(xué)改革
- 《植物生物技術(shù)》全英文授課教學(xué)改革實(shí)踐
- 土壤整段標(biāo)本在課程教學(xué)與實(shí)習(xí)中的應(yīng)用
- “互聯(lián)網(wǎng)+教育”背景下生物統(tǒng)計(jì)學(xué)課程教學(xué)模式的改革與實(shí)踐
- 高校開展傳統(tǒng)農(nóng)耕文化教育的模式創(chuàng)新
