大鼠視網膜Müller細胞在高糖、氧化應激、缺氧中的表現
李春花 馮朝暉 張雪 田冰玉 雷曉琴 周婷潔 馬為梅
[摘要] 目的 觀察Sprague-Dawley(SD)大鼠視網膜Müller細胞在高糖、氧化應激、缺氧三種病理環境下的特征,為糖尿病視網膜病變的防治提供新的研究依據。 方法 體外培養SD大鼠視網膜Müller細胞,并用谷氨酰胺合成酶(GS)鑒定。將傳2代的細胞隨機分為四組:對照組、高糖組、氧化應激組、缺氧組。倒置顯微鏡觀察細胞形態學改變,免疫熒光染色法觀察細胞GS和α-平滑肌肌動蛋白(α-SMA)的變化,Transwell小室觀察細胞遷移能力。 結果 體外成功培養并鑒定鼠Müller細胞,四組Müller細胞功能均受損,氧化應激組以及缺氧組結構受損明顯,失去正常形態。缺氧組中α-SMA的表達呈陽性,并且在Transwell小室中發生遷移的細胞數明顯多于其他組,差異有統計學意義(P < 0.05)。 結論 高糖、氧化應激、缺氧均可導致Müller細胞功能受損,缺氧可誘導Müller細胞轉化為具有遷移能力的肌纖維樣細胞,提示Müller細胞參與了增生性糖尿病視網膜病變的發病過程。
[關鍵詞] SD大鼠;視網膜Müller細胞;糖尿病視網膜病變;上皮細胞間質轉化;Transwell小室
[中圖分類號] R587.2 ? ? ? ? ?[文獻標識碼] A ? ? ? ? ?[文章編號] 1673-7210(2019)05(c)-0018-04
[Abstract] Objective To observe the characteristics of Sprague-Dawley (SD) rats retinal Müller cells under three pathological conditions including high glucose, oxidative stress and hypoxia, and to provide new research basis for the prevention and treatment of diabetic retinopathy. Methods SD rat retinal Müller cells were cultured in vitro and identified by glutathione synthetase (GS). The second generation of cells were divided into control group, high glucose group, oxidative stress group and hypoxia group randomly. The morphological changes of the cells were observed under inverted microscope, the changes of GS and α-smooth muscle actin (α-SMA) were observed by immunofluorescence staining, and the cell migration ability was observed by Transwell chamber. Results The Müller cells were successfully cultured and identified in vitro. In all groups, the function of Müller cells was impaired, and in oxidative stress group and hypoxia group, the structure was damaged, and the normal morphology was lost. In hypoxia group, the expression of α-SMA was positive, and the number of cells in the Transwell chamber was significantly higher than that in other groups, the differences were statistically significant (P < 0.05). Conclusion High glucose, oxidative stress and hypoxia can destroy the function of Müller cells. Hypoxia can induce transforming into myofibroblast cells with migratory ability, which indicates that Müller cells are involved in the pathogenesis of proliferative diabetic retinopathy.
[Key words] SD rat; Retinal Müller cells; Diabetic retinopathy; Epithelial cell interstitial transformation; Transwell chamber
糖尿病視網膜病變(diabetic retinopathy,DR)是糖尿病的主要致盲原因[1-3],其發病機制復雜,近年來研究表明DR的發生發展與視網膜Müller細胞有密切關系[4-8],其通過調節鈉鉀平衡、攝取神經遞質等來維持視網膜細胞外環境的穩定性[9]。糖尿病時的高糖、氧化應激、缺氧可以使Müller細胞功能受損、結構破壞,關于上述3種病理環境中Müller細胞的損傷是否一致的報道甚少,因此本研究在體外模擬了DR的這3個階段,來研究Müller細胞參與DR的發病機制,為延緩或逆轉其結構及功能異常提供探索性研究。
1 材料與方法
1.1 實驗動物
正常出生后7 d的SD大鼠20只,清潔級別,由西安交通大學醫學院動物中心提供,許可證號:SCXK(陜)2018-001,倫理號:2018-191,飼養于無特定病原體級(SPF)動物房。
1.2 儀器與試劑
倒置顯微鏡SZ51型(日本olympus公司);CO2培養箱PYC-16型(美國Scheldon公司);低溫高速離心機5810r型(德國Eppendorf公司,半徑15.4 cm);恒溫水浴箱HH-1型(上海森信研究儀器有限公司)。DMEM培養基(Gibco公司,貨號:C1199550BT);胎牛血清(杭州四季青生物工程公司,貨號:11011-8611);兔抗鼠谷氨酰胺合成酶(GS)抗體(博奧森公司,貨號:BS-1003R);兔抗鼠α-平滑肌肌動蛋白(α-SMA)抗體(博奧森公司,貨號:BS-11665R);熒光素偶聯的山羊抗兔IgG(Abbkine公司,貨號:A22120)。
1.3 實驗方法
1.3.1 大鼠視網膜Müller細胞分離、體外培養、鑒定及分組 ?將出生7 d的SD大乳鼠斷頸處死后浸泡于75%的酒精中,挖出眼球,顯微鏡下分離出視網膜組織,分別加入木瓜蛋白酶(27 U/mL)和0.25%的胰蛋白酶37℃進行消化30 min,后加入含20% FBS的DMEM/F12培養基終止消化。放入37℃、體積分數為5% CO2培養箱培養,2~3 d換液1次。在原代培養5 d時,按1∶3傳代,并使用GS鑒別。將傳2代細胞液隨機分為以下四組:①對照組,細胞未經任何處理。②高糖組,經50 mmol/L的葡萄糖(Glc)溶液處理。③氧化應激組,經100 μmol/L的過氧化氫(H2O2)溶液處理。④缺氧組,經500 μmol/L的氯化鈷(CoCl2)溶液處理。
1.3.2 免疫熒光染色 ?隨機取爬片,20%山羊血清室溫孵育30 min,封閉內源性IgG,加GS、α-SMA抗體工作液,加1∶200稀釋的熒光素標記的二抗,37℃溫室避光孵育1 h,20%甘油封片劑封片,熒光顯微鏡觀察,照相。
1.3.3 細胞移行分析 ?將Müller細胞傳代到8 μm孔徑濾膜的Transwell小室中,待細胞貼壁達70%后,將細胞隨機分為對照組、高糖組、氧化應激組、缺氧組。用預冷的丙酮固定20 min,用細胞刷刮除正面細胞,對透過濾膜小孔移行至濾膜反面的細胞進行蘇木紫著染10 min,自來水沖洗5 min。顯微鏡觀察,照相,隨機取5個區域計數。
1.4 統計學方法
采用SPSS 18.0統計軟件對數據進行分析,計量資料以均數±標準差(x±s)表示,多組間比較采用方差分析,組間兩兩比較采用LSD-t和SNK-q檢驗。以P < 0.05為差異有統計學意義。
2 結果
2.1 Müller細胞的體外培養及鑒定
體外培養的Müller細胞呈扁平的多邊形融合狀生長,形態不規則并有較長的突起。GS是主要鑒別酶,在胞核及胞質中表達。見圖1(封三)。
2.2 各組細胞形態學以及功能觀察
對照組的Müller細胞描述同前,α-SMA表達陰性;高糖組Müller細胞形態基本同對照組,GS、α-SMA表達陰性;氧化應激組Müller細胞數量減少,細胞崩解,GS、α-SMA表達陰性;缺氧組Müller細胞胞體變大,不規則樣突起增多,胞質內可見絲狀纖維,如箭頭所示,GS表達陰性,α-SMA表達陽性。見圖2(封四)。
2.3 各組細胞移行能力觀察
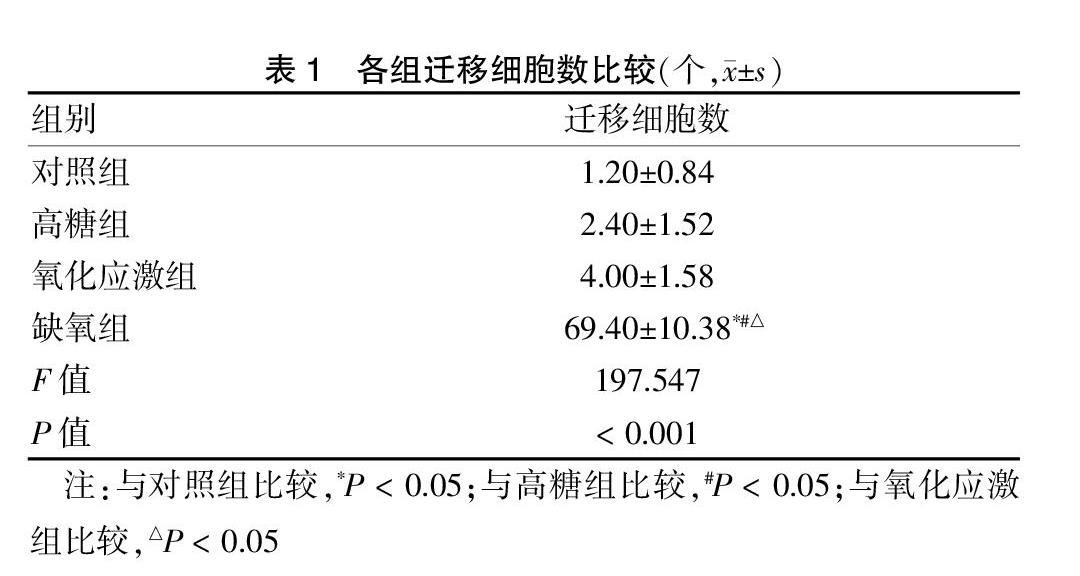
Transwell小室細胞遷移試驗結果顯示高糖組、氧化應激組與對照組細胞遷移能力相近,各組間遷移細胞數比較差異無統計學意義(P > 0.05);缺氧組遷移細胞數明顯多于其他組,差異有統計學意義(P < 0.05)。見圖3(封四)、表1。
3 討論
視網膜膠質細胞以Müller細胞居多[10-12],因此體外培養Müller細胞成功率高。目前培養方法主要有組織塊貼壁培養法和酶解法。本實驗改進了酶解法,采用了木瓜蛋白酶(27 U/mL)和0.25%胰酶的分步消化,減少了離心所帶來的機械損傷。GS是一種表達于Müller細胞,能夠將谷氨酸轉化為谷氨酰胺的關鍵酶,它可以作為Müller細胞的免疫標志物[13-14]。本實驗使用GS鑒別Müller細胞,其主要表達于胞核以及胞質內,這與既往研究一致[15]。
Thompson等[16]的研究表明,糖基化終末產物可以引起Müller細胞功能下降。本研究主要研究了高糖早期(24 h)的變化。體外培養25~30 mmol/L的高糖水平,可近似地等同于糖尿病時體內高糖狀態。因此,本研究選用50 mmol/L葡萄糖模擬體內的絕對高糖條件。以50 mmol/L高糖作用Müller細胞24 h后發現,高糖早期細胞形態未發生明顯改變,而GS表達陰性,分析可能在DR發病的早期,Müller細胞雖然結構完整,但功能已受損。
目前已有研究證明糖尿病時氧化應激的存在[17-18]。在多種細胞中,氧化應激反應都可導致細胞凋亡。Abrahan等[19]使用H2O2模擬體內氧化應激環境。H2O2是氧化應激的產物,來源廣泛,所以本研究使用H2O2造成體外氧化應激環境。本研究使用100 μmol/L的H2O2溶液,作用細胞24 h發現Müller細胞GS表達下降,細胞正常形態破壞,這與之前的研究一致[20]。
本研究選用CoCl2模擬體內缺氧。鈷的一個用途是用于化學模擬低氧,常用化合物是CoCl2(100~250 μmol/L)。本研究選用500 μmol/L CoCl2溶液處理Müller細胞,24 h后發現GS表達減少甚至缺失,細胞體積變大,提示缺氧可損害Müller細胞正常形態和功能,而α-SMA染色陽性,Transwell小室遷移研究提示細胞遷移能力增強。α-SMA是一組表達在多種類型細胞胞質中的具有收縮功能的微管微絲結構,是細胞移行和具有收縮功能的基礎[21]。Transwell小室技術可觀察具有遷移能力的細胞穿過多孔濾膜的情況[22]。本研究使用α-SMA抗體及Transwell小室,提示缺氧可導致Müller細胞表型發生改變,使其轉化為具有遷移、收縮能力的肌纖維樣細胞。因而推測缺氧中的Müller細胞有可能移位于視網膜表面,參與增生性糖尿病視網膜病變的形成。
總之DR是多種因素相互作用的結果。本研究提示高糖、氧化應激及缺氧早期均可造成Müller細胞功能破壞,后兩者對Müller細胞的損害更為嚴重。因此合理的抗氧化及抗缺氧治療可能成為DR治療的新靶點。
[參考文獻]
[1] ?Hendrick AM,Gibson MV,Kulshreshtha A. Diabetic Retinopathy [J]. Prim Care,2015,42(3):451-464.
[2] ?Ola MS,Nawaz MI,Siddiquei MM,et al. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy [J]. J Diabetes Complications,2015,74(1):145-147.
[3] ?Wang SY,Andrews CA,Herman WH,et al. Incidence and Risk Factors for Developing Diabetic Retinopathy among Youths with Type 1 or Type 2 Diabetes throughout the United States [J]. Ophthalmology,2017,124(4):424-430.
[4] ?Luna G,Lewis GP,Banna CD,et al. Expression profiles of nestin and synemin in reactive astrocytes and Müller cells following retinal injury:a comparison with glial fibrillar acidic protein and vimentin [J]. Mol Vis,2010,27(16):2511-2523.
[5] ?Cutler RG. Oxidative stress profiling:part Ⅰ. Its potential importance in the optimization of human health [J]. Ann N Y Acad Sci,2005,1055:93-135.
[6] ?Capozzi ME,Mccollum GW,Cousins DB,et al. Linoleic Acid is a Diabetes-relevant Stimulator of Retinal Inflammation in Human Retinal Muller Cells and Microvascular Endothelial Cells [J]. J Diabetes Metab,2016,7(12):718-723.
[7] ?Kim SJ,Yoo WS,Choi M,et al. Increased O-GlcNAcylation of NF-κB Enhances Retinal Ganglion Cell Death in Streptozotocin-induced Diabetic Retinopathy [J]. Curr Eye Res,2016,41(2):249-257.
[8] ?Zhou T,Che D,Lan Y,et al. Mesenchymal marker expression is elevated in Müller cells exposed to high glucose and in animal models of diabetic retinopathy [J]. Oncotarget,2017,8(3):4582-4594.
[9] ?Bringmann A,Pannicke T,Grosche J,et al. Müller cells in the healthy and diseased retina [J]. Prog Retin Eye Res,2006,25(4):397-424.
[10] ?Reichenbach A,Wurm A,Pannicke T,et al. Muller cells as players in retinal degeneration and edema [J]. Graefes Arch Clin Exp Ophthalmol,2007,245(5):627-636.
[11] ?Enger R,Gundersen GA,Haj-Yasein NN,et al. Molecular scaffolds underpinning macroglial polarization:an analysis of retinal Müller cells and brain astrocytes in mouse [J]. Glia,2012,60(12):2018-2026.
[12] ?Guidry C. The role of Müller cells in fibrocontractive retinal disorders [J]. Prog Retin Eye Res,2005,24(1):75-86.
[13] ?Cui Y,Xu X,Bi H,et al. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose:the role of reactive oxygen species in diabetic retinopathy [J]. Exp Eye Res,2006,83(4):807-816.
[14] ?Zheng L,Du Y,Miller C,et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes [J]. Diabetologia,2007,50(9):1987-1996.
[15] ?Hao C,Weber AJ. Expression of glial fibrillary acidic protein and glutamine synthetase by Müller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factor [J]. Glia,2002,38(2):115-125.
[16] ?Thompson K,Chen J,Luo Q,et al. Advanced glycation end(AGE)product modification of laminin downregulates Kir4.1 in retinal Müller cells [J]. PLoS One,2018,13(2):1-13.
[17] ?Roigrevert MJ,Lleópérez A,Zanónmoreno V,et al. Enhanced Oxidative Stress and Other Potential Biomarkers for Retinopathy in Type 2 Diabetics:Beneficial Effects of the Nutraceutic Supplements [J]. Biomed Res Int,2015,4(12):1-12.
[18] ?Kruk J,Kubasik-Kladna K,Aboul-Enein HY. The role oxidative stress in the pathogenesis of eye diseases:current status and a dual role of physical activity [J]. Mini Rev Med Chem,2015,16(3):241-257.
[19] ?Abrahan CE,Insua MF,Politi LE,et al. Oxidative stress promotes proliferation and dedifferentiation of retina glial cells in vitro [J]. J Neurosci Res,2010,87(4):964-977.
[20] ?Qu C,Cao W,Fan Y,et al. Near-Infrared Light Protect the Photoreceptor from Light-Induced Damage in Rats [J]. Adv Exp Med Bio,2010,664:365-374.
[21] ?Kumawat K,Koopmans T,Menzen MH,et al. Cooperative signaling by TGF-β1 and WNT-11 drives sm-α-actin expression in smooth muscle via Rho kinase-actin-MRTF-A signaling [J]. Am J Physiol Lung Cell Mol Physiol,2016,311(3):529-537.
[22] ?李冬平,原莉莉,張東昌.轉化生長因子β2對人視網膜色素上皮細胞間質轉化的影響[J].山西醫藥雜志,2017, 46(17):2035-2037.
(收稿日期:2018-11-09 ?本文編輯:張瑜杰)

