Developmental expression of three prmt genes in Xenopus
DEAR EDITOR,
Protein arginine methyltransferases(PRMTs)are involved in many cellular processes via the arginine methylation of histone or non-histone proteins.We examined the expression patterns of prmt4,prmt7,and prmt9 during embryogenesis in Xenopus using whole-mount in situ hybridization and quantitative reverse transcription polymerase chain reaction(RT-PCR).Xenopus prmt4 and prmt7 were expressed in the neural crest,brain,and spinal cord,and also detected in the eye,branchial arches,and heart at the tailbud stage.Specific prmt9 signals were not detected in Xenopus embryos until the late tailbud stage when weak expression was observed in the branchial arches.Quantitative RT-PCR indicated that the expression of prmt4 and prmt7 was up-regulated during the neurula stage,whereas prmt9 maintained its low expression until the late tailbud stage,consistent with the whole-mount in situ hybridization results.Thus,the developmental expression patterns of these three prmt genes in Xenopus embryos provide a basis for further functional study of such genes.
Post-translational modification plays an essential role in modulating the structure and function of a protein(Walsh& Jefferis,2006). Argininemethylationisacommon post-translational modification in vertebrates,and is mediated by protein arginine methyltransferases(PRMTs)(Biggar&Li,2015;Carr et al.,2015).PRMTs can catalyze the transfer of a methyl group from S-adenosylmethionine(SAM)to the guanidine nitrogen atoms of arginine to form methylarginine(Herrmann et al.,2009).Based on the number and symmetry of the methyl group in methylarginine,PRMTs can be divided into three categories.PRMT4(type I PRMT)and PRMT9(type II PRMT)catalyze the formation of asymmetric and symmetric dimethylarginine,respectively(Cook et al.,2006;Yang&Bedford,2013),whereas PRMT7(type III PRMT)catalyzes the formation of monomethylarginine(Feng et al.,2013;Feng et al.,2014).
The PRMT-mediated arginine methylation of histone or non-histone proteins is involved in many cellular processes,including transcriptional regulation,signal transduction,and RNA splicing(Biggar&Li,2015;Carr et al.,2015;Yang et al.,2015).PRMT4,also known as coactivator associated arginine methyltransferase 1(CARM1),can regulate the cell cycle through arginine methylation of the retinoblastoma protein tumor suppressor(Kim et al.,2015).PRMT9 can methylate SAP145,a component of the U2 snRNP involved in the early stages of splicing,with attenuation of PRMT9 also known to cause gross changes in RNA splicing(Yang et al.,2015).
PRMT7 is required for the maintenance of the regeneration capacity of muscle stem cells by regulating the DNMT3b/p21 axis(Blanc et al.,2016).Specific knockout of PRMT7 in muscle stem cells can cause elevated expression of CDK inhibitor p21CIP1 and reduced expression of its repressor,DNMT3b,leading to cell-cycle arrest and premature cellular senescence,which can be rescued by restoration of DNMT3b(Blanc et al.,2016).Both prmt4 and prmt5 play a combinatorial role during zebra fish myogenesis by controlling fast and slow muscle fi ber formation(Batut et al.,2011). Furthermore,prmt4 is also suggested to regulate the expression of myogenic microRNAs directly(Mallappa et al.,2011).
Although PRMTs are widely involved in various cellular processes via catalyzing the methylation of target proteins,theirrolesin embryonic developmentare notyetwell understood.Limited studies have shown that PRMT4 and PRMT7 are involved in myogenesis(Batut et al.,2011;Blanc et al.,2016;Mallappa et al.,2011).Xenopus is an excellent model in developmental biology(Harland&Grainger,2011),and all prmt genes(prmt1–9)have been identified in the Xenopus tropicalis genome.In this study,we selected three prmt genes,that is,prmt4,prmt7,and prmt9,and studied their spatial and temporal expression patterns during the embryonic development of Xenopus. Our study will provide a basis for further investigations on the functions of prmt genes in Xenopus.
We searched prmt genes of Xenopus tropicalis and other species in the NCBI database(Supplementary Table S1,available online).Protein sequence alignments were performed using Geneious v4.8.5(www.geneious.com/previous-versions/#geneious-4-dot-8),with a dendrogram tree then constructed using neighbor-joining in the same program.
Xenopus laevis prmt4 (NM_001094676), prmt7(NM_001086541),and prmt9(NM_001096961)sequences were obtained by searching the NCBI database.The open reading frames(ORFs)of prmt4,prmt7,and prmt9 were amplified using reverse transcription polymerase chain reaction(RT-PCR).The PCR products were subcloned into the pBluescript II KS(+)vector and verified by sequencing.To prepare probes for in situ hybridizations,plasmids were linearized by cutting with Xho1 and used as templates for the synthesis of digoxigenin-labeled anti-sense probes with T7 RNA polymerase(Roche,Indianapolis,USA).
Xenopus laevis embryos were collected,cultured,and fixed as described previously(Wang et al.,2011).Whole-mount in situ hybridization was performed according to standard methods(Harland,1991). After in situ hybridization,the embryos were embedded and sectioned at a thickness of 50μm. Detailed information on the vibratome sections is described in our previous study(Kam et al.,2010).
Total RNA was extracted from Xenopus tropicalis embryos using TRIzol reagent(Molecular Research Center Inc.,USA).The cDNA was synthesized using the ReverTra Ace?qPCR RT Kit(Toyobo,Japan).Quantitative PCR was performed using the SYBR?Green real-time PCR master mix(Toyobo,Japan).The primer sequences used are listed in Table 1.Ornithine decarboxylase(odc)was used as the internal control.

Table 1 Primers for quantitative RT-PCR
We performed whole-mount in situ hybridization to examine the spatial expression of prmt genes in Xenopus embryos.No evident signals were detected before gastrulation.In the early gastrula stage,the prmt4 signal was mainly expressed in the dorsal ectoderm,which gives rise to the neural ectoderm(Figure 1A).At the early neurula stage,the prmt4 signal was enriched in the anterior region of the neural plate as well as the posterior region around the blastopore(Figure 1B).After this,prmt4 was strongly expressed in the neural plate and neural crest(Figure 1C,D).This expression pattern persisted when neural crest migration began in the later neurula stage(Figure 1E,F).During the tailbud stage,prmt4 was detected throughout the central nervous system,including the brain and spinal cord(Figure 1G,H).Strong expression was also observed in the branchial arches and eye vesicles(Figure 1G,H).In stage 35,prmt4 expression was enriched in the head region,including the forebrain,midbrain,hindbrain,eye,and branchial arches(Figure 1I,J).In stage 40,prmt4 was expressed in the olfactory placode,jaw,and heart,and weak expression in the paraxial mesoderm was also observed(Figure 1K–R).Weak signals were also detected in the eye,brain,dorsal region of the endoderm,and spinal cord(Figure 1N–R).
No apparent expression of prmt7 was detected in the embryos at the early gastrula stage(data not shown). In the early neurula stage,prmt7 was weakly expressed in the neural plate(Figure 2A).The expression of prmt7 intensified and expanded to the migrating neural crest,anterior neural tube,and somites(Figure 2B,C),and then showed strong expression in the branchial arches during the tailbud stage(Figure 2D,E).At stage 25,prmt7 expression was also detected in the intermediate mesoderm(Figure 2D).At stage 29,prmt7 signals were detected in the brain,eye,and somites(Figure 2E–H).Weak expression was also observed in the pronephric tubule(Figure 2I).At stage 40,prmt7 was strongly expressed in the branchial arches,with weak signals in the heart,eye,brain,and olfactory placode(Figure 2J–L).
Whole-mount in situ hybridization could not detect specific signals of prmt9 in Xenopus embryos at almost every stage,though a weak signal was observed in the branchial arches at the late tailbud stage(data not shown).
We studied the temporal expression patterns of Xenopus prmtgenes using quantitative RT-PCR.Differentfrom pseudotetraploid Xenopus laevis, the diploid Xenopus tropicalis species has two sets of chromosomes.Thus,we collected Xenopus tropicalis embryos at different stages and measured the mRNA expression levels of prmt1–4,prmt6,prmt7,and prmt9(Figure 3).Maternal expression of prmt6 and prmt7 was detected,which decreased during the cleavage stage(Figure 3E,F).Apart from prmt7,the expression levels of the detected prmt genes were low during the gastrula stage(Figure 3F).The expression of prmt4,prmt6,and prmt7 were gradually up-regulated from the neurula stage(Figure 3D–F).However,the expression levels of prmt2,prmt3,and prmt9 increased from the tailbud stage(Figure 3B,C,G).During the late tailbud stage,most of the prmt genes maintained their high expression levels,except for prmt2 and prmt9,whose expression continued to increase(Figure 3B,G).The expression of prmt9 remained low before the late tailbud stage(Figure 3G).In contrast,prmt1 expression reached a high level at the early neurula stage(Figure 3A).The up-regulation in the expression of prmt4 and prmt7 during neurulation(Figure 3D,F)accords with their enhanced staining of embryos,as revealed by whole-mount in situ hybridization(Figure 1B–F;Figure 2A–C).The low expression level of prmt9 before the late tailbud stage(Figure 3G)is consistent with the slight staining of embryos after in situ hybridization.
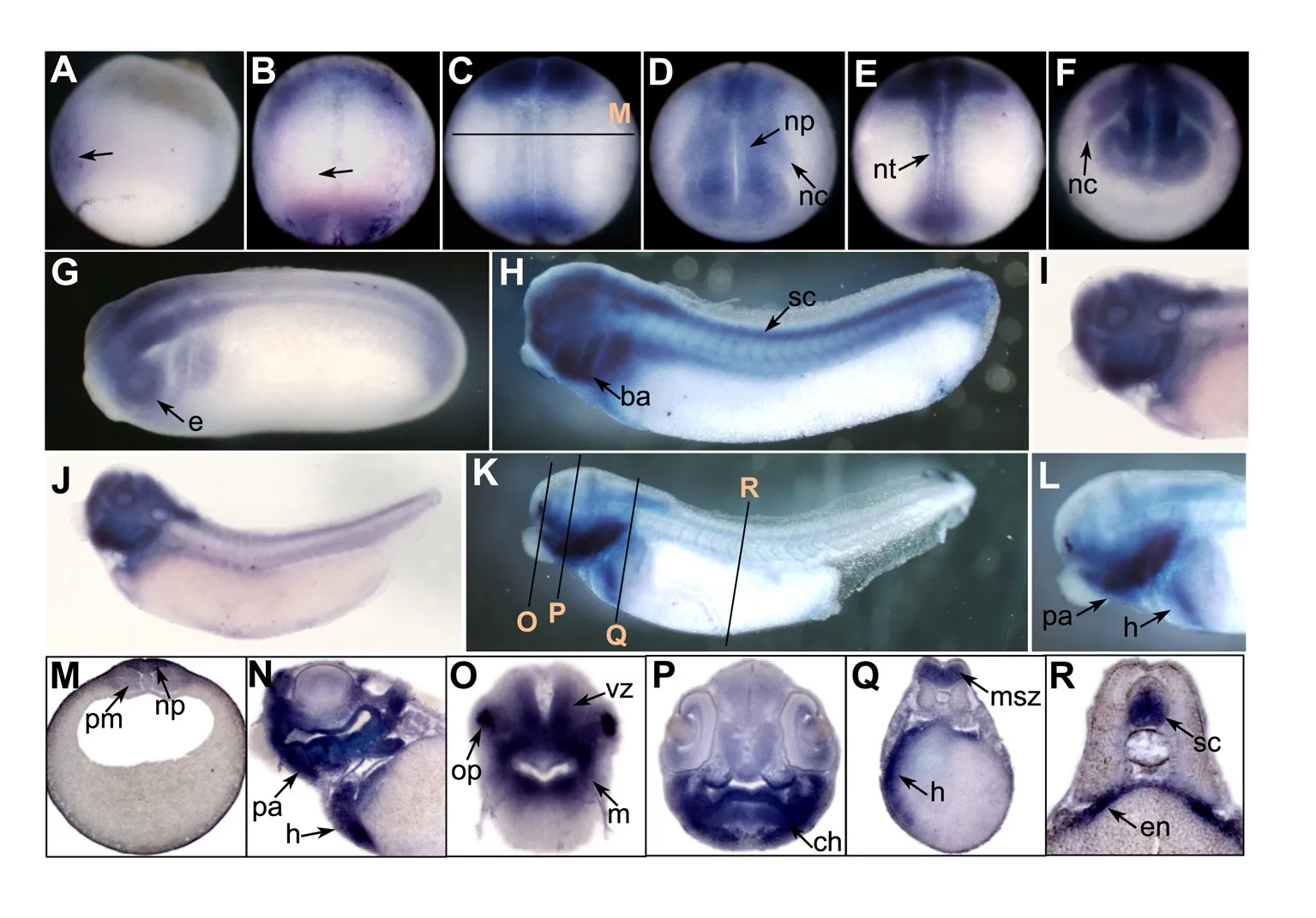
Figure 1 Spatial expression of prmt4 in Xenopus embryos

Figure 2 Expression of prmt7 in Xenopus embryos
Searching the NCBI database we found that all prmt genes,including prmt1–9,have been identified in Xenopus tropicalis.A phylogenetic tree based on their protein sequence alignments was generated(Figure 4A).Results showed that type I PRMTs(prmt1,prmt2,prmt3,prmt4,prmt6,and prmt8)exhibited fewer genetic changes,whereas prmt5 and prmt7 demonstrated more significant genetic variation(Figure 4A).We conducted phylogenetic analysis of the Xenopus prmt4,prmt7,and prmt9 proteins to illustrate their evolutionary distances to humans,mice,chickens,frogs,and zebra fish(Figure 4B).

Figure 3 Quantitative RT-PCR analysis of prmt gene expression in Xenopus tropicalis embryos
We examined the expression patterns of three prmt genes during embryonic development.Our results indicated that prmt4 and prmt7 showed similar expression patterns. Both were expressed in the neural plate during neurulation and were then detected in the brain and spinal cord(Figure 1 and Figure 2).These data suggest that prmt4 and prmt7 function in neural development,similar to that found in other PRMT members(Batut et al.,2005;Hashimoto et al.,2016;Honda et al.,2017;Lee et al.,2005;Lee et al.,2017;Lin et al.,2013).Furthermore,prmt4 and prmt7 were both expressed in the neural crest,which has not been reported previously.Apart from the brain,prmt4 and prmt7 were also expressed in other regions of the head,including the eye and branchial arches.At the late tailbud stage,both were detected in the olfactory placode,pharyngeal arches,and heart regions(Figure 1K,N–R;Figure 2F,J–L).In zebrafish,the expression of prmt8 has also been detected in the heart at the later stages(Lin et al.,2013).Here,weak prmt4 signals were detected in the paraxial mesoderm(Figure 1M),whereas prmt7 was evidently expressed in the somites(Figure 2C,G).These results are in agreement with their potential roles in myogenesis,which have been studied to some extent in zebrafish(Batut et al.,2011).In addition,prmt4 and prmt7 also shared similar temporal expression patterns at the neurula and tailbud stages when their expression levels were gradually up-regulated(Figure 3D,F).The high similarity in expression patterns between prmt4 and prmt7 implies that different members of the PRMT family may have redundant roles in regulating early embryonic development.Compared with other examined prmt genes,prmt9 exhibited a distinct temporal expression pattern.Its expression level was very low before the early tailbud stage but was dramatically elevated from the late tailbud stage(Figure 3G).This is in line with the in situ hybridization results,in which specific prmt9 signals were not detected until the late tailbud stage.Although prmt9 is a non-histone methyltransferase involved in regulating RNA splicing(Yang et al.,2015),whether the special expression pattern of prmt9 is related to its role in RNA splicing remains to be illustrated.This study will facilitate further functional study of prmt genes during embryonic development.
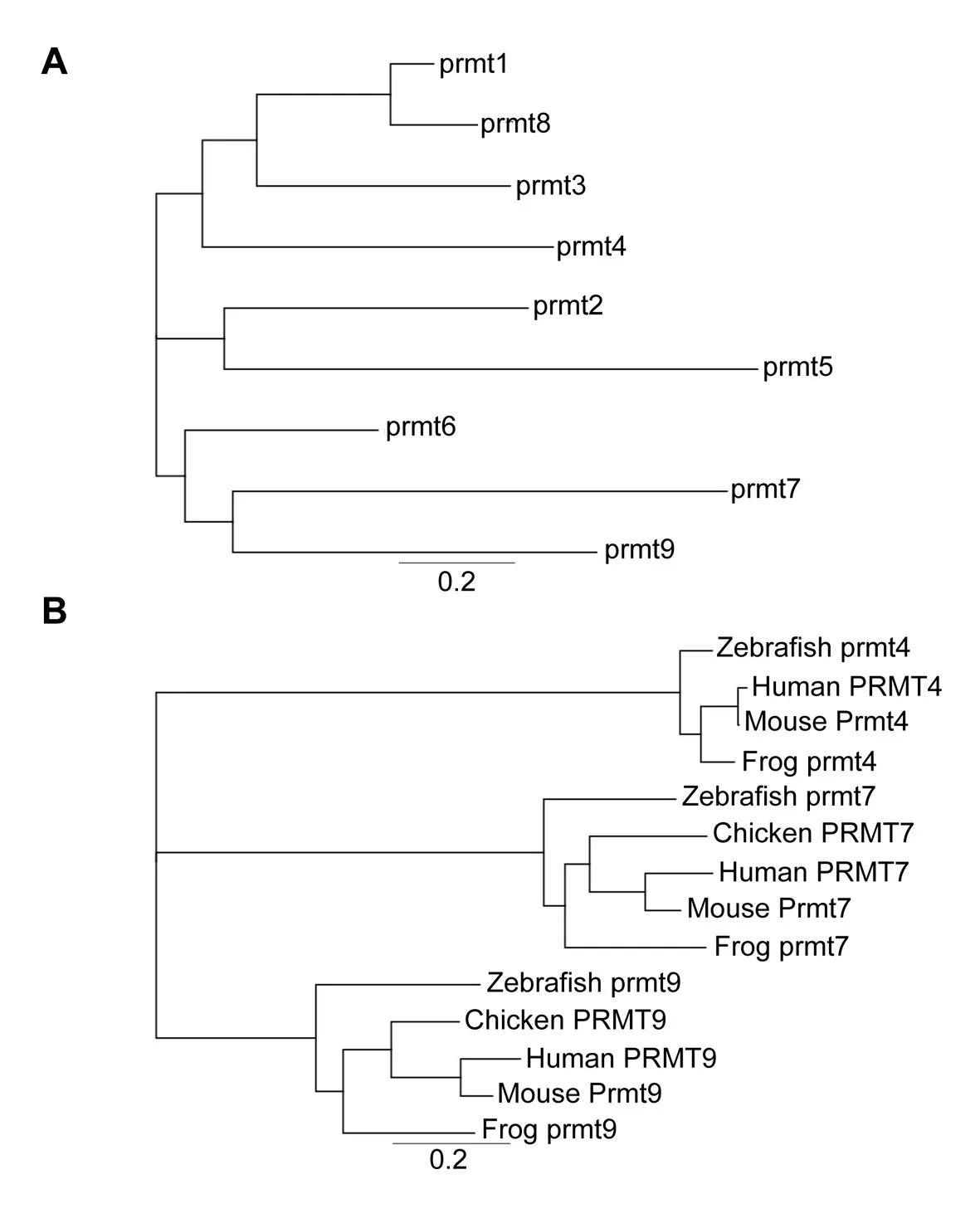
Figure 4 Phylogenetic analysis of prmt proteins
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’CONTRIBUTIONS
H.Z.designed the study and supervised the project.C.D.W.,X.F.G.,T.C.B.W.and H.W.performed the whole-mount in situ hybridization.T.C.B.W.did vibratome sectioning on the stained embryos.C.D.W.made phylogenic analysis on prmt members. X.F.G did quantitative RT-PCR to analyze expression of prmt genes.C.D.W.,X.F.G and H.Z.analyzed the results.C.D.W and X.F.G wrote the manuscript with input from all authors.X.F.Q.,D.Q.C.,Y.D.,and H.Z.revised the manuscript.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank our laboratory colleagues for helpful discussion on this project.
Cheng-Dong Wang1,#,Xiao-Fang Guo2,5,#,Thomas Chi Bun Wong1,Hui Wang1,Xu-Feng Qi2,5,Dong-Qing Cai2,5,Yi Deng3,Hui Zhao1,4,*1Key Laboratory for Regenerative Medicine,Ministry of Education,School of Biomedical Sciences,Faculty of Medicine,The Chinese University of Hong Kong,Hong Kong SAR,China
2Key Laboratory for Regenerative Medicine of Ministry of Education,Jinan University,Guangzhou Guangdong 510632,China
3Guangdong Provincial Key Laboratory of Cell Microenvironment,Department of Biology,South University of Science and Technology of China,Shenzhen Guangdong 518055,China
4Kunming Institute of Zoology,Chinese Academy of Sciences-The Chinese University of Hong Kong Joint Laboratory of Bioresources and Molecular Research of Common Diseases,Hong Kong SAR,China
5Department of Developmental&Regenerative Biology,College of Life Science and Technology,Jinan University,Guangzhou Guangdong 510632,China
#Authors contributed equally to this work
*Corresponding author,E-mail:zhaohui@cuhk.edu.hk
- Zoological Research的其它文章
- Interchange between grooming and infant handling in female Tibetan macaques(Macaca thibetana)
- Effects of age,sex and manual task on hand preference in wild Rhinopithecus roxellana
- Home range variation of two different-sized groups of golden snub-nosed monkeys(Rhinopithecus roxellana)in Shennongjia,China:implications for feeding competition
- Social functions of relaxed open-mouth display in golden snub-nosed monkeys(Rhinopithecus roxellana)
- Marker-assisted selection of YY supermales from a genetically improved farmed tilapia-derived strain
- Identification and characterization of two novel cathelicidins from the frog Odorrana livida

