由聯苯三羧酸配體構筑的零維四核鎳ギ配合物和一維錳ギ配位聚合物的合成、晶體結構及磁性質
黎 彧 溫炳松 鄒訓重 黃 賓 邱文達 張澤敏 游 遨 成曉玲*,
(1廣東輕工職業技術學院生態環境技術學院,佛山市特種功能性建筑材料及其綠色制備技術工程中心,廣州 510300)
(2廣東工業大學輕工化工學院,廣州 510006)
0 Introduction
In recent years,the rational design and assembly of coordination polymers has been of considerable interest due to their potential applications,architectures,and topologies[1-5].Many factors,such as the coordination geometry of the metal centers,type and connectivity of organic ligands,stoichiometry,reaction conditions,template effect,presence ofauxiliary ligands,and pH values can play the key role in the construction of the coordination networks[6-10].The design and selection of the special ligands is very important in the construction of these coordination polymers.
Multi-carboxylate biphenyl ligands have been certified to be of great significance as constructors due to their strong coordination abilities in various modes,which could satisfy different geometric requirements of metal centers[7-9,11-16].In order to extend our research in this field,we chose two biphenyl tricarboxylic acid ligands,biphenyl-2,5,3′-tricarboxylate acid(H3bptc)and 2-(4-carboxypyridin-3-yl)terephalic acid (H3cptc)to construct novel coordination compounds.Both ligands possesses the following features:(1)they have three carboxyl groups that may be completely or partially deprotonated,inducing rich coordination modes and allowing interesting structures with higher dimensionalities; (2)they can act as hydrogen-bond acceptor as well as donor,depending upon the degree of deprotonation;(3)the free rotation of C-C single bonds between two the aromatic rings could form numbers of coordination geometries of metal centers;thus,it may ligate metal centers in different orientation.
Taking into account these factors,we herein report the syntheses,crystal structures,and magnetic properties of two Niギ and Mnギ coordination compounds constructed from biphenyl tricarboxylic acid ligands.
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded usingKBrpelletsandaBrukerEQUINOX 55 spectrometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃·min-1.Magnetic susceptibility data were collected in the 2~300 K temperature range with a Quantum Design SQUID Magnetometer MPMS XL-7 with a field of 0.1 T.A correction was made for the diamagnetic contribution prior to data analysis.
1.2 Synthesis of[Ni2(μ3-Hbptc)(Hbptc)(phen)3(H2O)]2·4H2O(1)
A mixture of NiCl2·6H2O (0.047 g,0.20 mmol),H3bptc(0.057 g,0.2 mmol),phen(0.040 g,0.2 mmol),NaOH (0.016 g,0.40 mmol),and H2O (10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10 ℃·h-1.Green block-shaped crystals of 1 were isolated manually,and washed with distilled water.Yield:45%(based on H3bptc).Anal.Calcd.for C66H46Ni2N6O15(%):C 61.91,H 3.62,N 6.56;Found(%):C 61.75,H 3.60,N 6.61.IR(KBr,cm-1):3 318w,2 924m,1 714w,1 587s,1 564s,1 517m,1 471w,1 424m,1 407w,1 373w,1 216w,1 147w,1 100w,1 042w,985w,927w,852w,805w,776w,724m,643w,516w.
1.3 Synthesisof{[Mn3(μ4-cptc)2(2,2′-bipy)2(H2O)4]·2H2O}n(2)
A mixture of MnCl2·4H2O (0.059 g,0.30 mmol),H3cptc(0.057 g,0.2 mmol),2,2′-bipy(0.047 g,0.3 mmol),NaOH(0.024 g,0.60 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10 ℃·h-1.Yellow block-shaped crystals of 2 were isolated manually,and washed with distilled water.Yield:62% (based on H3cptc).Anal.Calcd.for C48H40Mn3N6O18(%):C 49.97,H 3.49,N 7.28;Found(%):C 50.14,H 3.51,N 7.23.IR(KBr,cm-1):3057w,1 598m,1 569s,1 471w,1 430w,1 373s,1 262w,1 170w,1 153w,1 048w,1 031w,1 014w,933w,904w,868w,852w,810w,765m,730w,707w,649w,551w.The compounds are insoluble in water and common organic solvents,such as methanol,ethanol,acetone,and DMF.
1.4 Structure determinations
Two single crystals with dimensions of 0.25 mm×0.22 mm×0.21 mm(1)and 0.26 mm×0.24 mm×0.23 mm(2)were analyzed at 293(2)K on a Bruker SMART APEXⅡ CCD diffractometer with Mo Kα radiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-2014 program[17].All nonhydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1 and 2 is given in Table 1.The selected bond lengths and angles for compounds 1 and 2 are listed in Table 2.Hydrogen bond parameters of compounds 1 and 2 are given in Table 3 and 4.
CCDC:1588394,1;1588395,2.
2 Results and discussion
2.1 Description of the structure
2.1.1 [Ni2(μ3-Hbptc)(Hbptc)(phen)3(H2O)]2·4H2O(1)
Single-crystal X-ray diffraction analysis revealsthat compound 1 crystallizes in the triclinic space group P1.Its asymmetric unit contains two crystallographically unique Niギatoms,two Hbptc2-blocks,three phen moieties,one H2O ligand and two lattice water molecules.As depicted in Fig.1,the sixcoordinated Ni1 atom displays a distorted octahedral{NiN4O2}geometry filled by two carboxylate O atoms from two different μ3-Hbptc2-blocks and four N atoms from two phen ligands.The Ni2 center is coordinated by one carboxylate O atom from one μ3-Hbptc2-block,two carboxylate O atoms from one terminal Hbptc2-block,one O atom from the H2O ligand,and two N atoms from one phen moiety,thus composing distorted octahedral{NiN2O4}geometry.The lengths of the Ni-O bonds range from 0.202 8(4)to 0.221 2(4)nm,whereas the Ni-N distances vary from 0.205 9(5)to 0.212 2(4)nm;these bonding parameters are comparable to those found in other reported Niギcompounds[7,9,11].In 1,the Hbptc2-ligands adopt two different coordination modes(modesⅠ andⅡ,Scheme 1),in which the deprotonated carboxylate groupsshow the monodentate,bidentate or uncoordinated modes.The dihedral angles between two phenyl rings in the Hbptc2-are 52.52°and 39.91°,respectively.Two μ3-Hbptc2-ligands bridge alternately neighboring Niギions to form a discrete tetranuclear nickelギstructure(Fig.2).These Ni4units are assembled to a 3D supramolecular framework through O-H…O hydrogen bond(Fig.3 and Table 3).
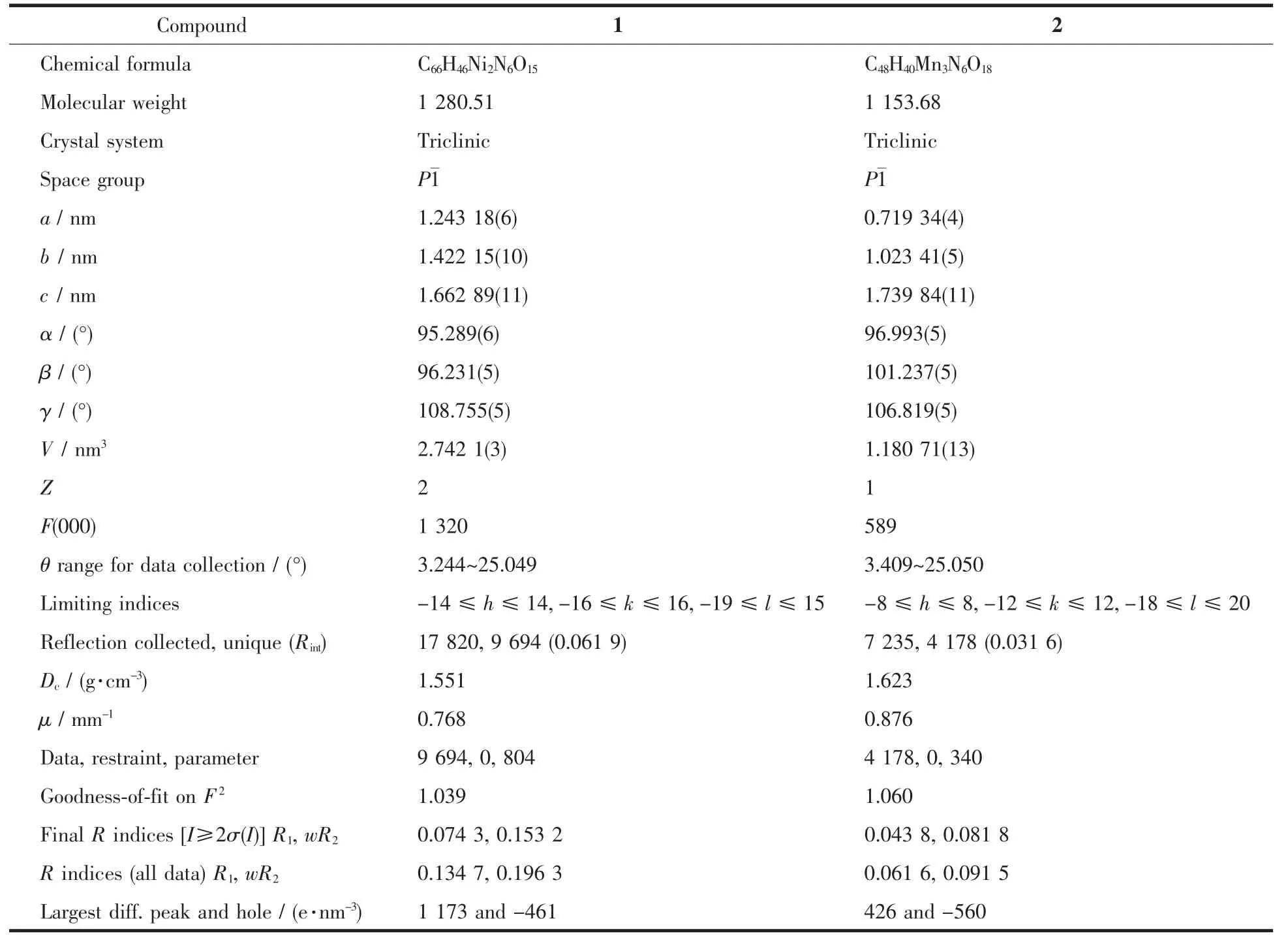
Table 1 Crystal data for compounds 1 and 2
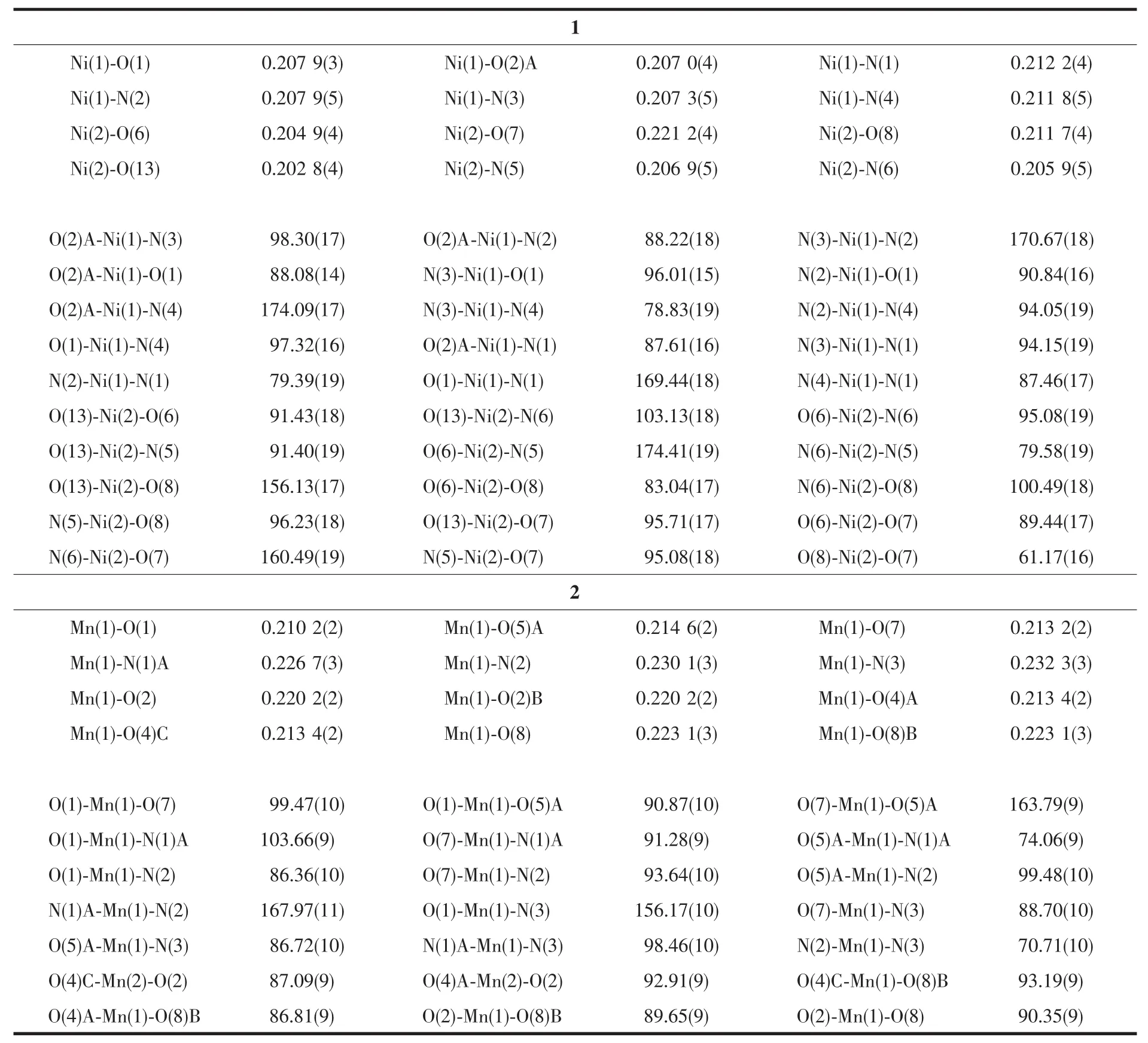
Table 2 Selected bond lengths(nm)and bond angles(°)for compounds 1 and 2
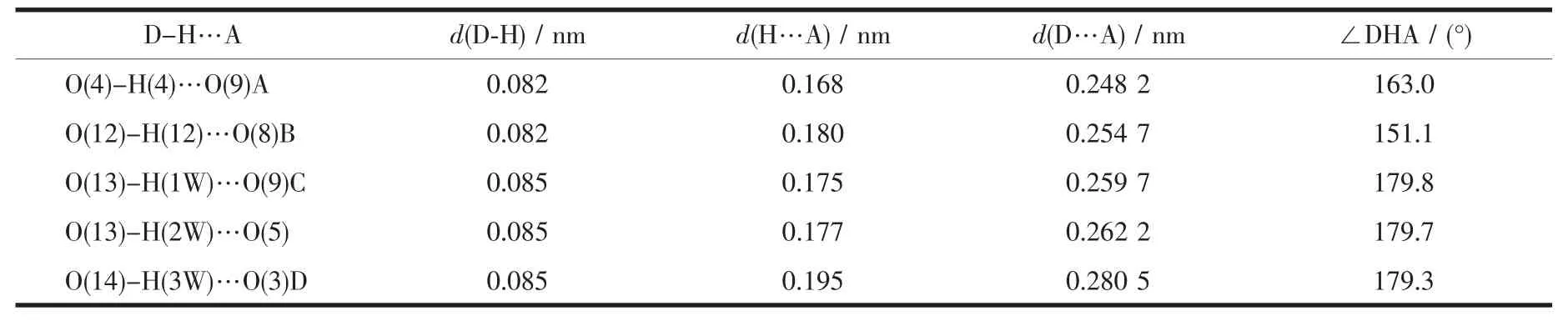
Table 3 Hydrogen bond parameters of compound 1
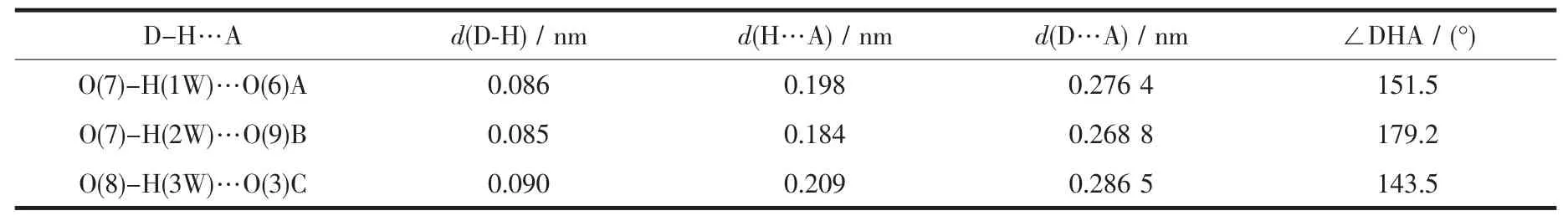
Table 4 Hydrogen bond parameters of compound 2
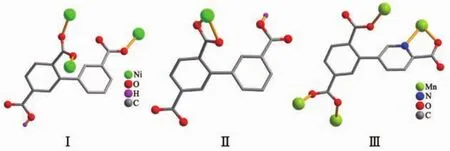
Scheme 1 Coordination modes of Hbptc2-/cptc3-ligands in compounds 1 and 2
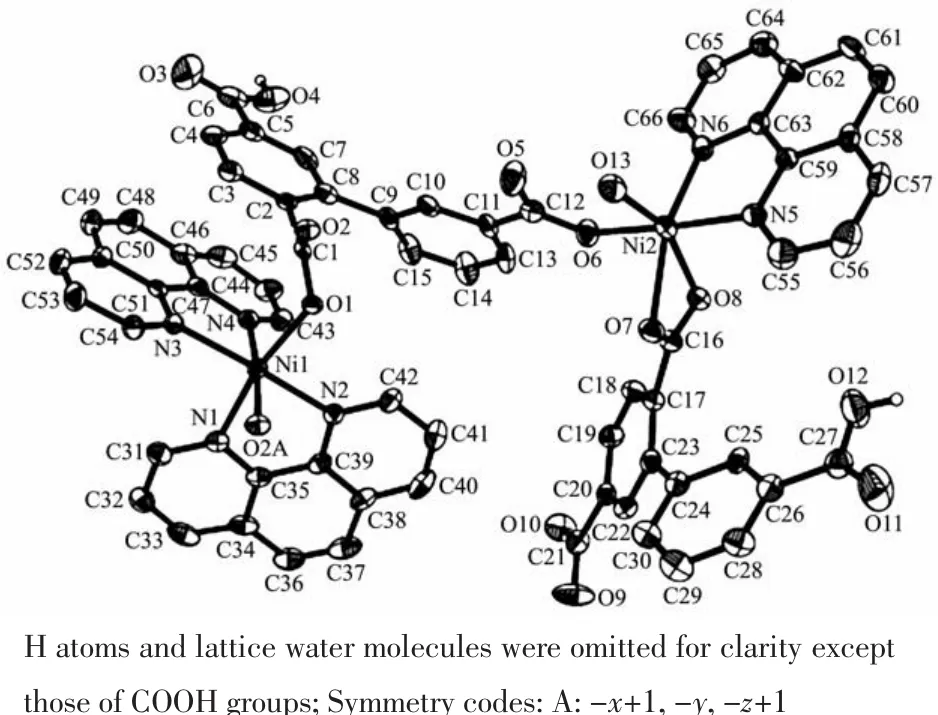
Fig.1 Drawing of the asymmetric unit of compound 1 with 30%probability thermal ellipsoids
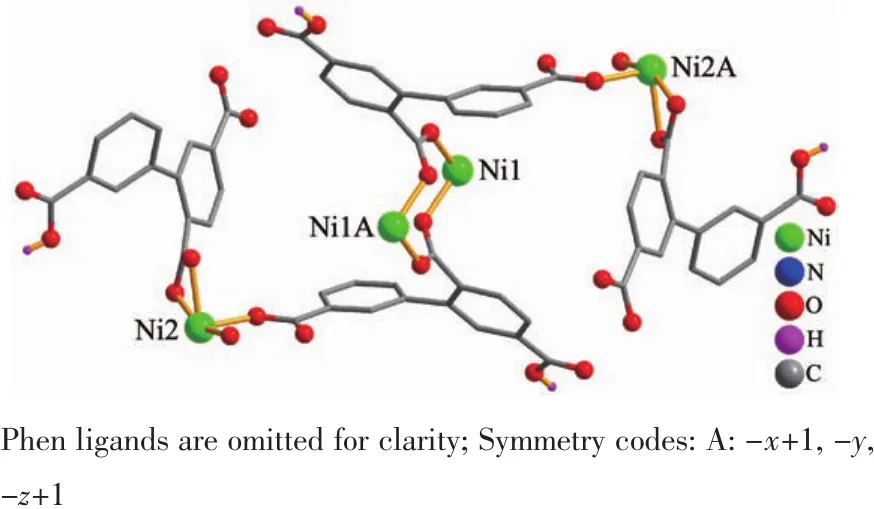
Fig.2 Tetranuclear nickelギunit
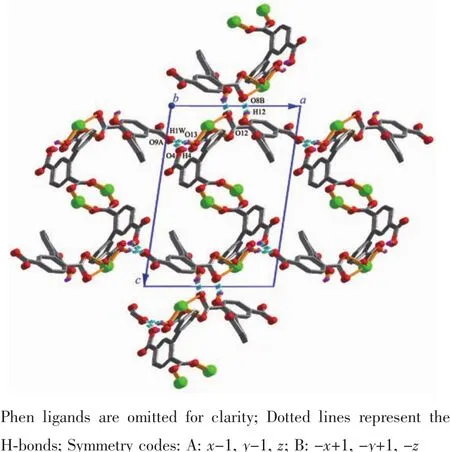
Fig.3 Perspective of 3D supramolecular framework viewed from b axis in 1
2.1.2 {[Mn3(μ4-cptc)2(2,2′-bipy)2(H2O)4]·2H2O}n(2)
The asymmetric unitof2 consistsoftwo crystallographically distinct Mnギ atoms(Mn1 with full occupancy;Mn2 is positioned on a twofold rotation axis),one μ4-cptc3-block,one 2,2′-bipy ligand,two coordinated and one lattice water molecule.As shown in Fig.4,six-coordinate Mn1 atom reveals distorted octahedral{MnN3O3}environment,filled by one N and two O atoms from three individual μ4-cptc3-blocks,one O atom from the H2O ligand,and two N atoms from the 2,2′-bipy moiety.The Mn2 center is coordinated by four carboxylate O atoms from four distinct cptc3-moieties and two O atoms from two H2O ligands,thus forming octahedral {MnO6}geometry.The Mn-O distances range from 0.210 2(2)to 0.223 1(3)nm,whereas the Mn-N distances vary from 0.226 7(3)to 0.232 3(3)nm;these bonding parameters are comparable to those observed in other Mnギcompounds[7-9,11].In 2,the cptc3-block acts as a μ4-N,O4-spacer and its COO-groups take a monodentate or bidentate mode(modeⅢ,Scheme 1).In cptc3-,a dihedral angle(between pyridyl and benzene rings)is 52.30°.Three neighboring Mnギions are bridged by four different μ4-cptc3-ligands,giving rise to a centrosymmetric trinuclear Mnギ subunit with the Mn…Mn distance of 0.508 4(6)nm(Fig.5).The adjacent Mn3subunits are further linked by the cptc3-blocks into a 1D chain(Fig.6),having the shortest distance of 1.023 4(5)nm between the neighboring trimanganeseギsubunits.
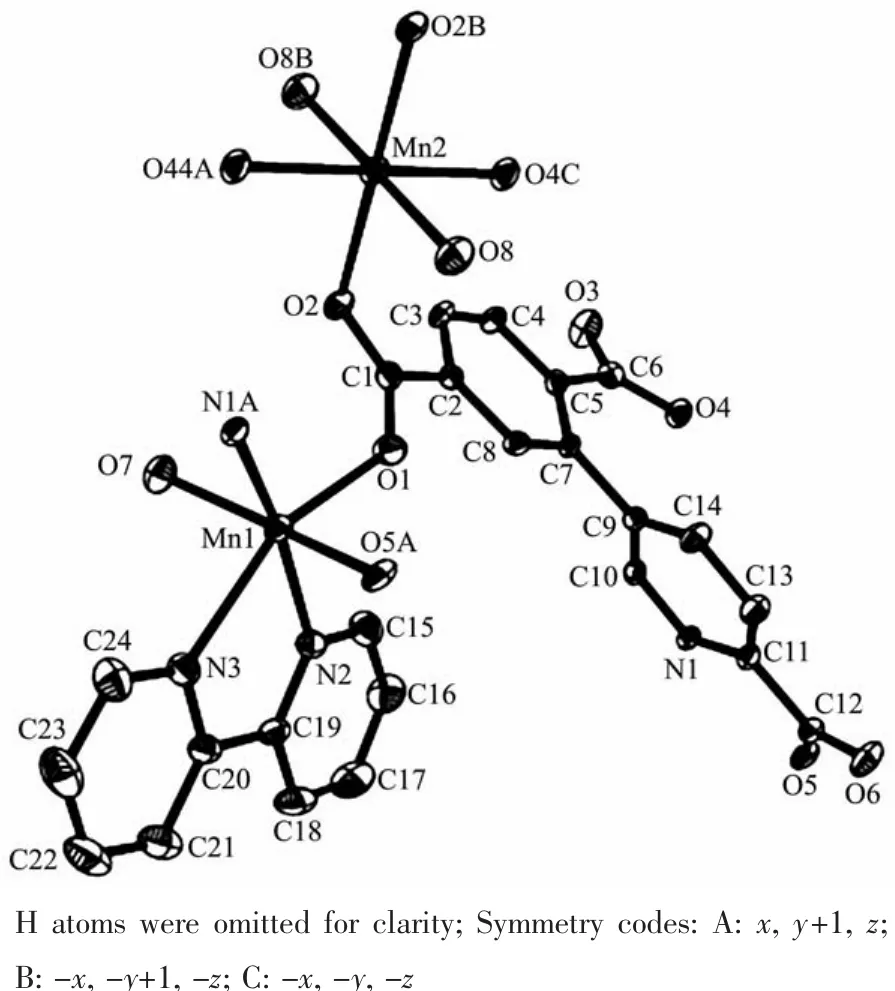
Fig.4 Drawing of the asymmetric unit of compound 2 with 30%probability thermal ellipsoids
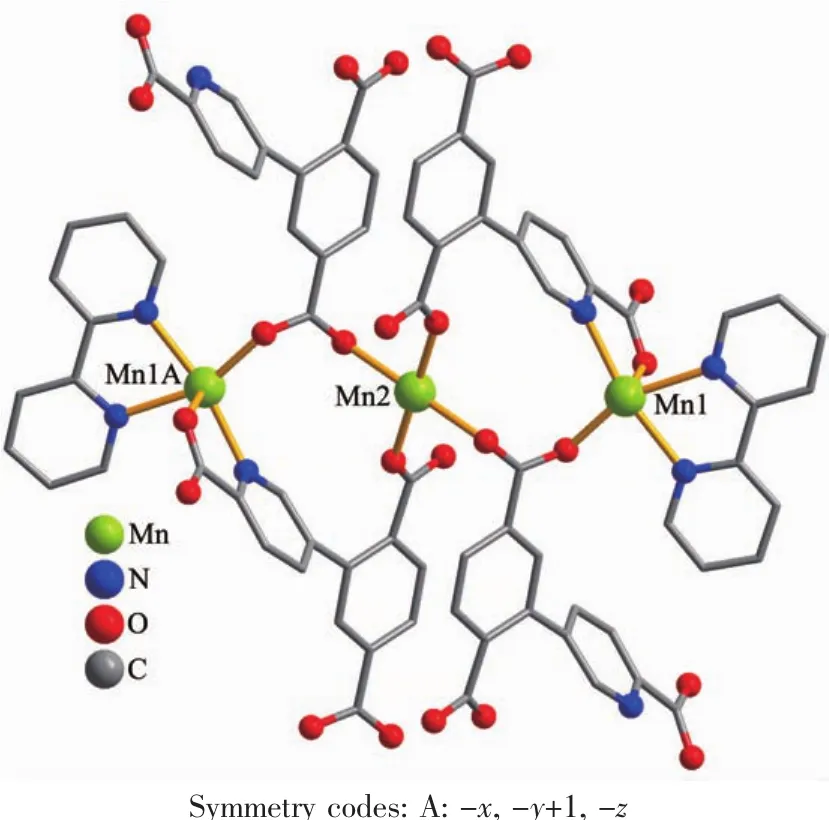
Fig.5 Trinuclear Mnギunit in compound 2
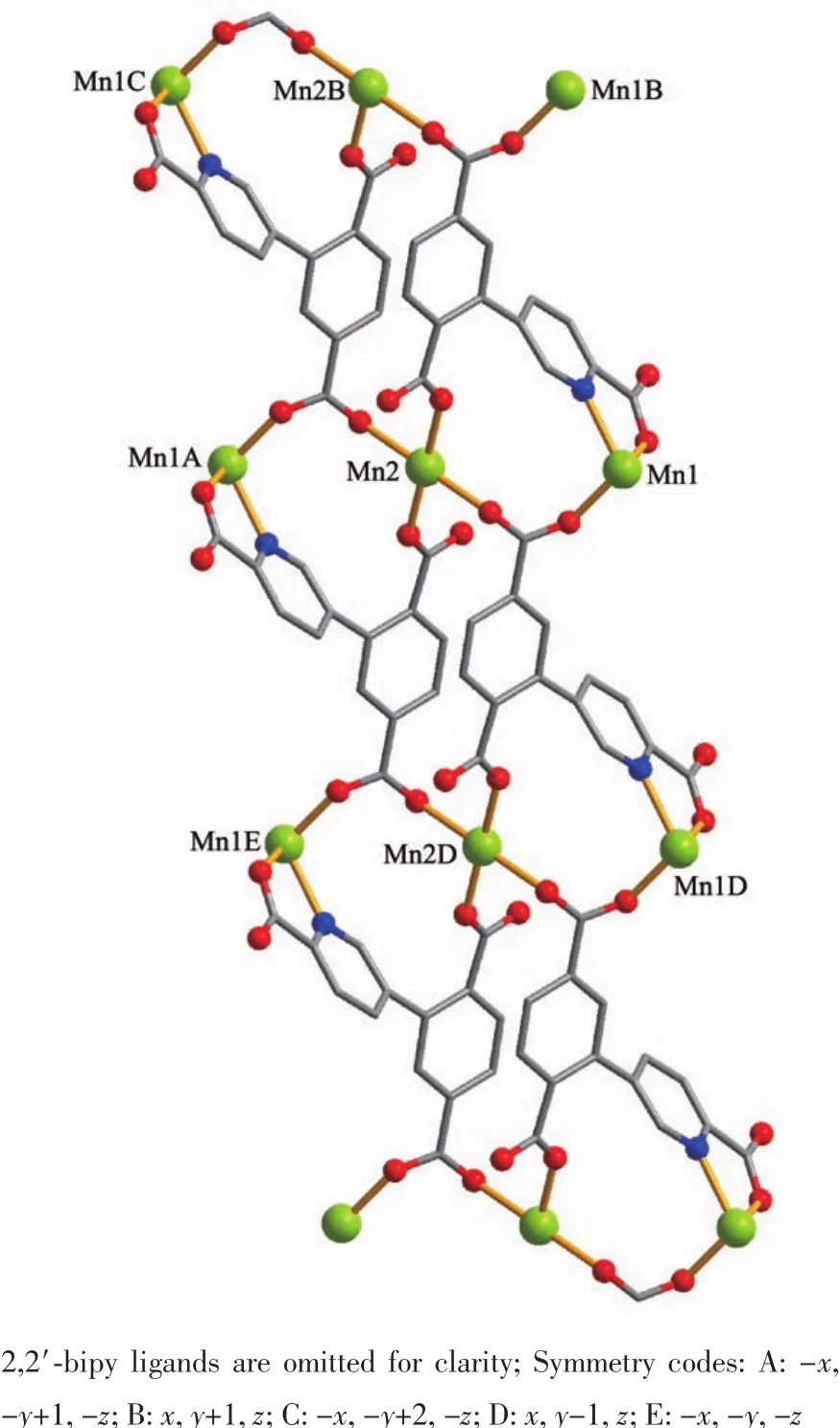
Fig.6 One dimensional chain along a axis in compound 2
2.2 TGA analysis
To determine the thermal stability of compounds 1 and 2,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.7,compound 1 loses its four lattice and two coordinated water molecules in the range of 152~241 ℃ (Obsd.3.9%,Calcd.4.2%),followed by the decomposition at 325℃.The TGA curve of 2 reveals that two lattice and four coordinated water molecules are released between 98~238℃ (Obsd.9.6%,Calcd.9.4%),and the dehydrated solid begins to decompose at 382℃.
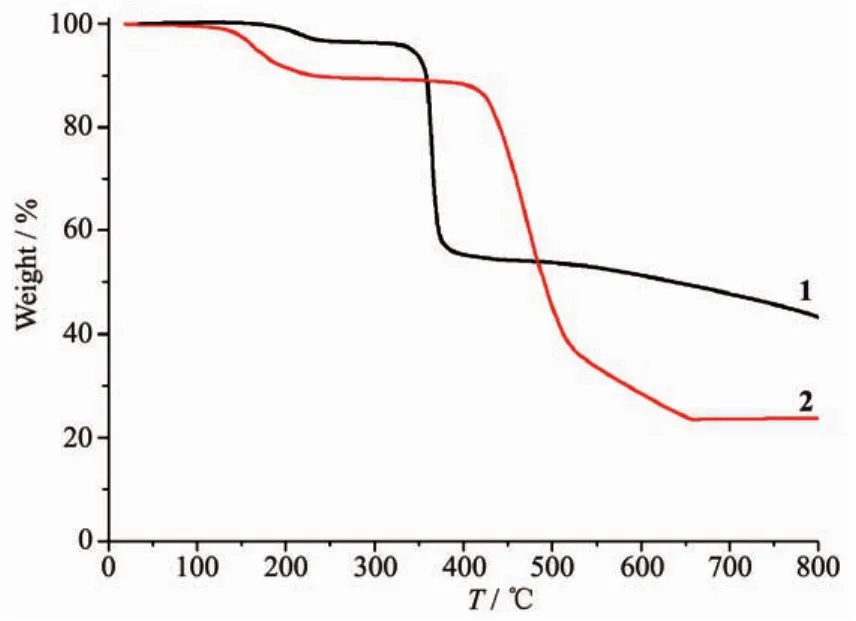
Fig.7 TGA curves of compounds 1 and 2
2.3 Magnetic properties
Variable-temperature magnetic susceptibility studies were carried out on powder samples of 1 and 2 in the 2~300 K temperature range.For 1,the χMT value at 300 K is 4.08 cm3·mol-1·K,which is close to the value of 4.00 cm3·mol-1·K for four magnetically isolated Niギcenter(SNi=1,g=2.0).Upon cooling,the χMT value drops down very slowly from 4.08 cm3·mol-1·K at 300 K to 3.64 cm3·mol-1·K at 60 K,and then decreases steeply to 1.40 cm3·mol-1·K at 2 K(Fig.8).In the 8~300 K interval,thevs T plot for 1 obeys the Curie-Weiss law with a Weiss constant θ of-6.52 K and a Curie constant C of 4.15 cm3·mol-1·K,suggesting a weak antiferromagnetic interaction between the Niギions.
We tried to fit the magnetic data of 1 using the following expression[18]for a dinuclear Niギunit:
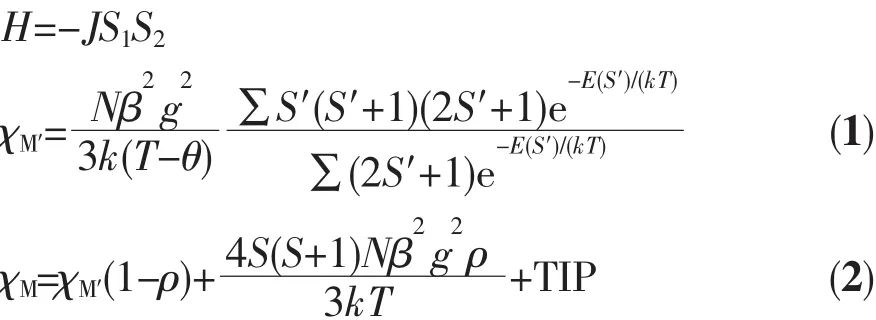
where ρ is a paramagnetic impurity fraction and TIP is temperature independent paramagnetism.Using this model,the susceptibility for 1 above 60 K was simulated,leading to the values of J=-2.25 cm-1,g=2.10,ρ=0.010,and TIP=4.58 ×10-6cm3·mol-1,with theThe negative J parameter confirms that a weak antiferromagnetic exchange coupling exists between the adjacent Niギcenters,which is in agreement with a negative θ value.
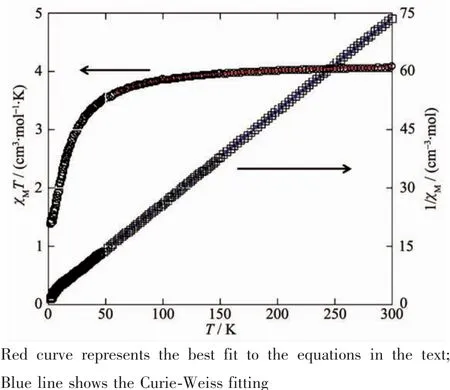
Fig.8 Temperature dependence of χMT(○)and 1/χM(□)vs T for compound 1
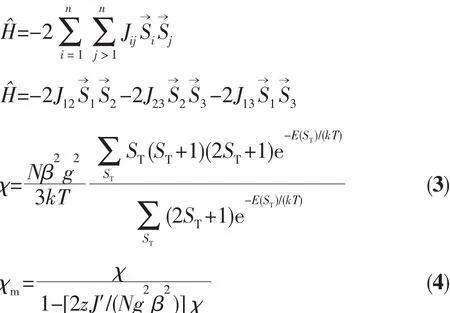
For 2,the χMT value at 300 K is 13.41 cm3·mol-1·K,which is close to the value of 13.12 cm3·mol-1·K expected for three magnetically isolated high-spin Mnギ centers(SMn=5/2,g=2.0).Upon cooling,the χMT value drops down very slowly from 13.41 cm3·mol-1·K at 300 K to 12.60 cm3·mol-1·K at 70 K and then decreases steeply to 2.81 cm3·mol-1·K at 2 K(Fig.9).Thevs T plot for 2 in the 2~300 K interval obeys the Curie-Weiss law with a Weiss constant θ of-4.34 K and a Curie constant C of 13.59 cm3·mol-1·K.The negative value of θ and the decrease of the χMT should be attributed to the overall antiferromagnetic coupling between the Mnギ centers within the Mn3unit.According to the structure of compound 2,there is one set of magnetic exchange pathway within the trinuclear cluster via carboxylate bridge(Fig.5).We tried to fit the magnetic data of 2 using the following expression[19-20]for the linear trinuclear Mnギmotif:where STis tolal spin of the linear trinuclear Mnギmotif;J12=J23=J1,J13=J2(J12and J23are the exchange interactions between the “central” Mnギ and two“outer”Mnギ atoms;J2is the exchange interaction between the “outer”Mnギ ions within a Mn3unit),zJ′refers to the intercluster coupling constant in the 1D chain.This model gives satisfactory results with the superexchange parameters:J1/kB=-1.32 K,J2/kB=-0.41 K,zJ′/kB=-0.20 K,and g=2.02.The agreement factor defined by R=∑(χmTexp- χmTcalc)2/∑(χmTexp)2is 7.54×10-4.These values confirm the presence of antiferromagnetic interaction between the Mnギions within a trinuclear subunit.The inercluster magnetic interaction(zJ′)is rather small,indicating that the exchange interactions between two magnetic clusters are very weak,which is probably due to a long separation(1.023 4(5)nm)of the adjacent magnetic subunits.In compounds 1 and 2,there is one type of the magnetic exchange pathway within the Ni4and Mn3units,namely via double μ2-η1∶η1-carboxylate (syn-syn)bridges(Fig.2 and 5).
3 Conclusions
In summary,two new coordination compounds,namely[Ni2(μ3-Hbptc)(Hbptc)(phen)3(H2O)]2·4H2O(1)and{[Mn3(μ4-cptc)2(2,2′-bipy)2(H2O)4]·2H2O}n(2),have been synthesized under hydrothermal conditions.The compounds feature the 0D tetranuclear and 1D chain structures,respectively.Magnetic studies show an antiferromagnetic coupling between the adjacent metal centers.
[1]Huang Y B,Liang J,Wang X S,et al.Chem.Soc.Rev.,2017,46:126-157
[2]Meng X,Wang H N,Song S Y,et al.Chem.Soc.Rev.,2017,46:464-480
[3]Wen H M,Cui Y J,Zhou W,et al.Adv.Mater.,2016,28:8819-8860
[4]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112:1126-1162
[5]Kuppler R J,Timmons D.J,Fang Q R,et al.Coord.Chem.Rev.,2009,253:3042-3066
[6]Ji P F,Manna K,Lin Z,et al.J.Am.Chem.Soc.,2016,138:12234-12242
[7]Gu J Z,Cui Y H,Liang X X,et al.Cryst.Growth Des.,2016,16:4658-4670
[8]Gu J Z,Gao Z Q,Tang Y.Cryst.Growth Des.,2012,12:3312-3323
[9]Gu J Z,Wu J,Lv D Y,et al.Dalton Trans.,2013,42:4822-4830
[10]Du M,Li C P,Liu C S,et al.Coord.Chem.Rev.,2013,257:1282-1305
[11]Gu J Z,Liang X X,Cui Y H,et al.CrystEngComm,2017,19:117-128
[12]Lee J,Kang Y J,Cho N S,et al.Cryst.Growth Des.,2016,16:996-1004
[13]Li S D,Lu L P,Su F.Chin.J.Struct.Chem.,2016,35:1920-1928
[14]FENG Shang-Fa(馮上發),HE Xin(何鑫),QIN Tao(秦濤),et al.Chinese J.Inorg.Chem.(無機化學學報),2017,33(11):2095-2102
[15]Su F,Lu L P,Feng S S,et al.Dalton Trans.,2015,44:7213-7222
[16]Tian H,Wang K,Jia Q X,et al.Cryst.Growth Des.,2011,11:5167-5170
[17]Spek A L.Acta Crystallogr.Sect.C:Struct.Chem.,2015,C71:9-18
[18]Thompson L K,Niel V,Grove H.Polyhedron,2004,23:1175-1184
[19]Kahn O.Molecular Magnetism.New York:VCH Publishers Inc.,1993:211
[20]Hsu K F,Wang S L.Inorg.Chem.,2000,39:1773-1778

