基于二乙醇胺配體的[Dy2Co8]型配合物的合成、晶體結構和磁性分析
郁有祝 郭玉華 申艷紅 楊立國 牛永生 鄭曉明 張海輝 王 芳
(安陽工學院化學與環境工程學院,安陽 455000)
0 Introduction
The study of 3d-4f heterometallic complexes is an active area of research in contemporary coordination chemistry because oftheirpotential applications in various fields such as non-linear optical materials,luminescence,molecular adsorption and magnetism[1-4].Importantly,some of these 3d-4f heterometallic complexes behave single-molecule magnets(SMMs)properties.Most notable in this vein is the research on polynuclear metal complexes,many of which display fascinating molecular structures and,more importantly,interesting magnetic properties due to the unique exchange interactions between the metal ions[5-10].Over recent years,many examples with SMM behavior of 3d-4f compounds involving cobaltギion have been reported[11-15].It is meaningful to continue to construct and study the magnetism of Co-Ln based molecular cluster.Using bridging ligands to bind 3d and 4f paramagnetic building blocks is the main synthetic plan to develop discrete polynuclear complexes and improve magnetic properties for 3d-4f complexes.Diethanolamine was usefully selected to construct molecular clusters because of its intrinsic nature,such as having more coordination sites and good flexibility[16-18].In this paper,we report the synthesis,structure and preliminary magnetic studies for a new type of[Dy2Co8]complex[Dy2Co8(L)4(HL)4(HCOO)4(OH)2Cl2(CH3OH)2]Cl2·4CH3OH·2H2O using diethanolamine as the ligand.
1 Experimental
1.1 Material and methods
All starting reagents were of A.R.grade and used as purchased without further purification.IR spectrum was recorded as KBr discs on a Shimadzu IR-408 infrared spectrophotometer in the 4 000~600 cm-1range.Analyses of C,H and N were determined on a Perkin-Elmer 240 Elemental analyzer.Thermogravimetric analysis(TGA)experiments were carried out on a NETZSCH STA 449F3 thermal analyzer from 40 to 800℃ under N2at a heating rate of 10℃·min-1.Powder X-ray diffraction (PXRD)determinations was performed on an X-ray diffractometer (D/max 2500 PC,Rigaku)with Cu Kα radiation(λ=0.154 06 nm).The crushed single crystalline powder samples were scanned at 40 kV(generator voltage)and 40 mA(tube current)from 5°to 50°with a step of 0.1°·s-1.The magnetic susceptibility measurements was measured over the temperature range of 2~300 K with a Quantum Design MPMS-XL7 SQUID magnetometer using an applied magnetic field of 2 000 Oe.
1.2 Synthesis of the title complex
To a stirred solution of DyCl3·6H2O(0.3 mmol,113 mg)and CoCl2·6H2O (1 mmol,238 mg)in CH3OH(15 mL)was added diethanolamine(2 mmol,210 mg).After 15 min,CH3ONa (1 mmol,54 mg)were added to the above solution,and the resulting mixture was stirred for 6 h to give a dark-red solution that was filtered.The dark-red filtrate was then left undisturbed for 1 week.Dark-red,cube-shaped crystals were retrieved in 40%yield (based on Dy).Anal.Calcd.for C42H110Cl4Co8Dy2N8O34(%):C,22.83;H,5.02;N,5.07.Found(%):C,22.87;H,5.01;N,5.14.
1.3 X-ray structure determination
Single-crystal X-ray diffraction data of complex 1 was collected on a Bruker SMART APEXⅡdiffractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm)at room temperature by using an ω-2θ scan mode.The structure was solved by directmethods with SHELXS-97 program[19]and refined by full-matrix least-squares techniques on F2with SHELXL-97[20].All non-hydrogen atoms were refined anisotropically.H atoms attached to C were placed geometrically and allowed to rideduring subsequent refinement with an isotropic displacement parameter fixed at 1.2 times Ueqof the parent atoms.H atoms bonded to N or O atoms were first located in difference Fourier maps and then placed in the calculated sites and included in the refinement.The crystallographic data and selected bond lengths and angles are listed in Table 1 and Table 2,respectively.
CCDC:1576526.
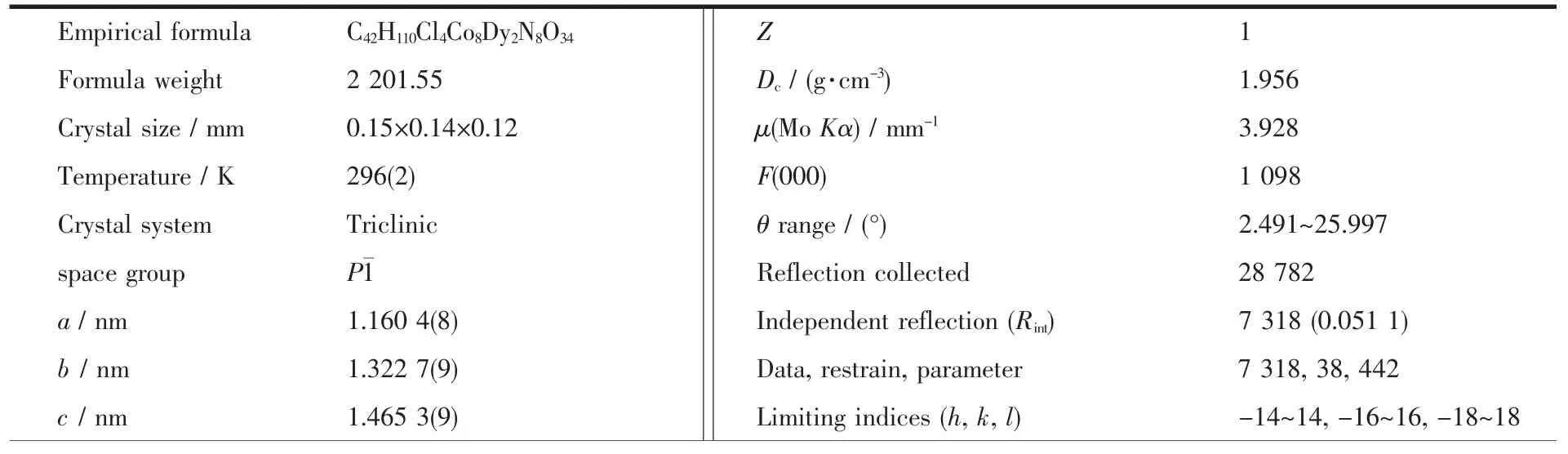
Table 1 Crystal data and structure refinement for 1

續表1
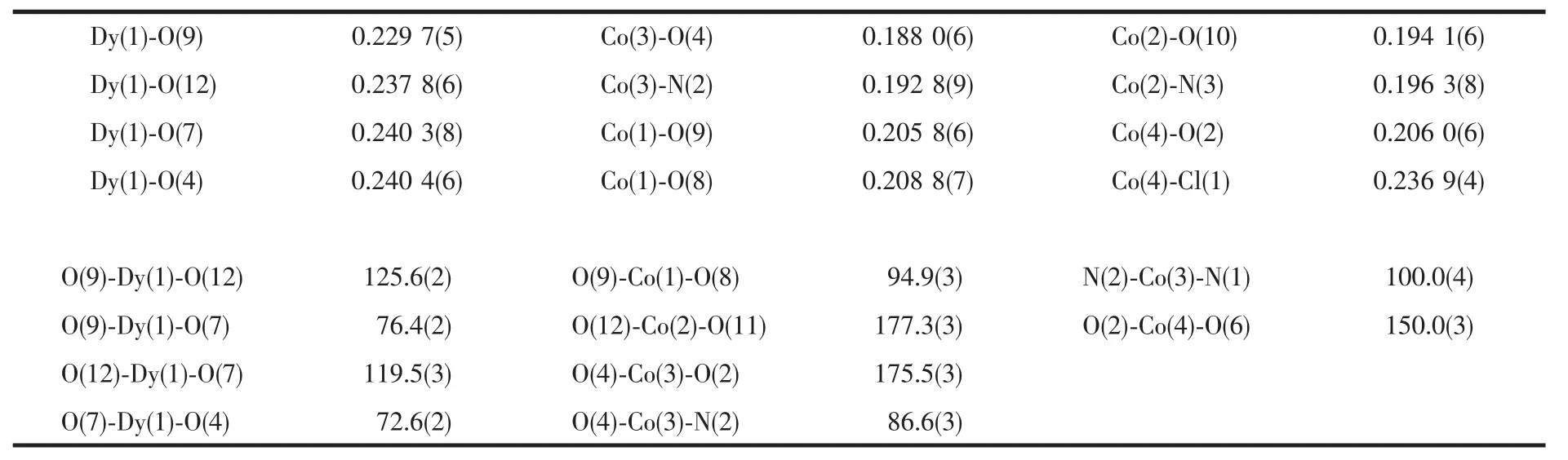
Table 2 Selected bond lengths(nm)and bond angles(°)of 1
2 Results and discussion
2.1 Infrared spectrum
The FT-IR spectra of 1(in KBr)show the bands as follows:3 400(s),3 161(s),2 935(w),2 870(w),1 598(s),1 346(s)and 1 042(s)cm-1.The absorption peak between 1 690 and 1 730 cm-1is not observed,showing all carboxylic groups are deprotonated[21].The strong peaks at 1 598 and 1 346 cm-1are the νas(COO-)and νs(COO-)stretching mode of the coordinated HCOO-ligand.Peak at 1 042 cm-1could be attributed to the stretching vibration of C-O bond.In addition,the strong and broad band centered at 3 400 cm-1for the title compound is attributable to the O-H or N-H stretching vibration on the basis of known structure.
2.2 Structure analysis
The crystal structure of 1 is revealed in Fig.1,and its stacking X-ray structure in Fig.2.The title compound,crystallized as[Dy2Co8(L)4(HL)4(HCOO)4(OH)2Cl2(CH3OH)2]Cl2·4CH3OH·2H2O,belongs to the triclinic system and P1 space group.The structure of this complex is centrosymmetric and contains two Dyバ and eight Coギ ions.Each Dyバ was ninecoordinated by nine oxygen atoms with the average Dy-O bond length 0.244 5 nm.Each Coギion displays distorted octahedral coordination environment.The ligand diethanolamine plays very important role in construction the structure via bridging and chelating metal atoms,and the nitrogen atoms only participate the coordination of cobalt atoms.Four coordination modes were found in the structure.In this case,a,b and c present μ3-η1∶η2∶η3coordination mode,while d presents μ3-η1∶η1∶η3coordination mode(Scheme 1).In the structure two coordination modes of HCOO-were found which mostly come from the oxidation of the solvent methanol(Scheme 2).There are two same μ3-OH groups in the structure which bridges two cobalt atoms and one dysprosium atom.Two kinds of Cl-ions were found in the structure,one of which involved in coordination,and another is used to balance the charge.Also,there are four methanol and two water molecules contained in the stucture as the solvent molecules.The existence of some O-H and N-H bonds makes more hydrogen bonds in the structure(Table 3).
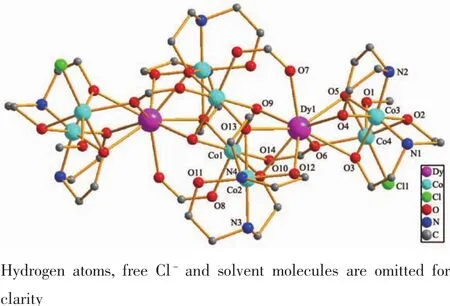
Fig.1 Molecular structure of 1
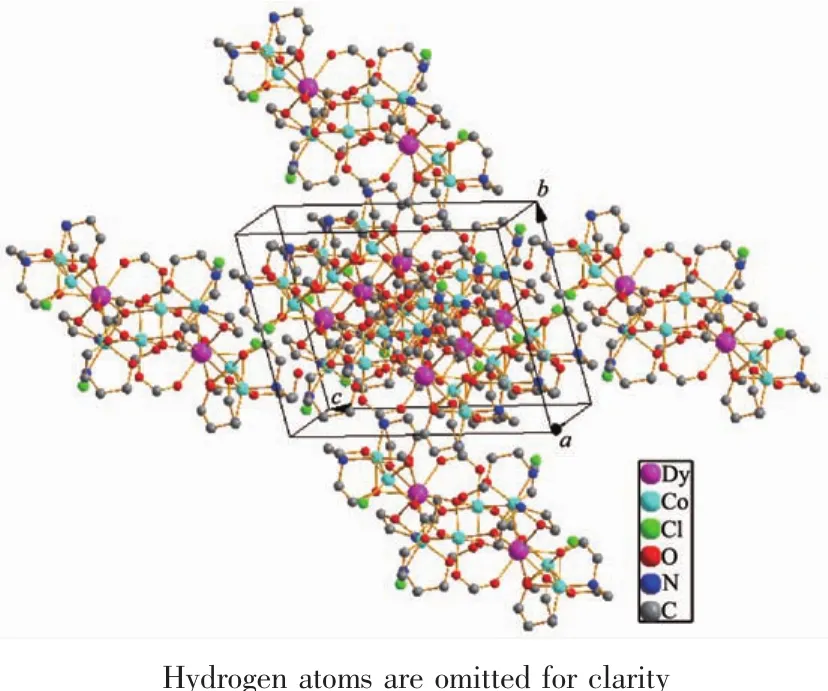
Fig.2 Packing arrangement in a unit cell
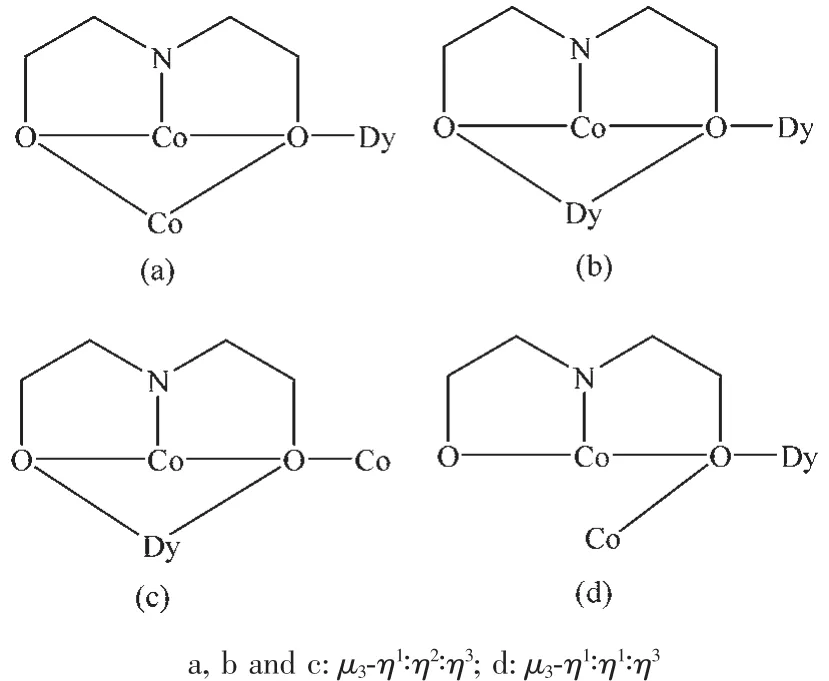
Scheme 1 Different coordination modes of L and HL
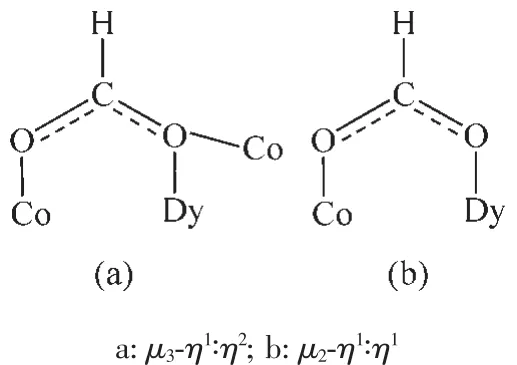
Scheme 2 Different coordination modes of HCOO-
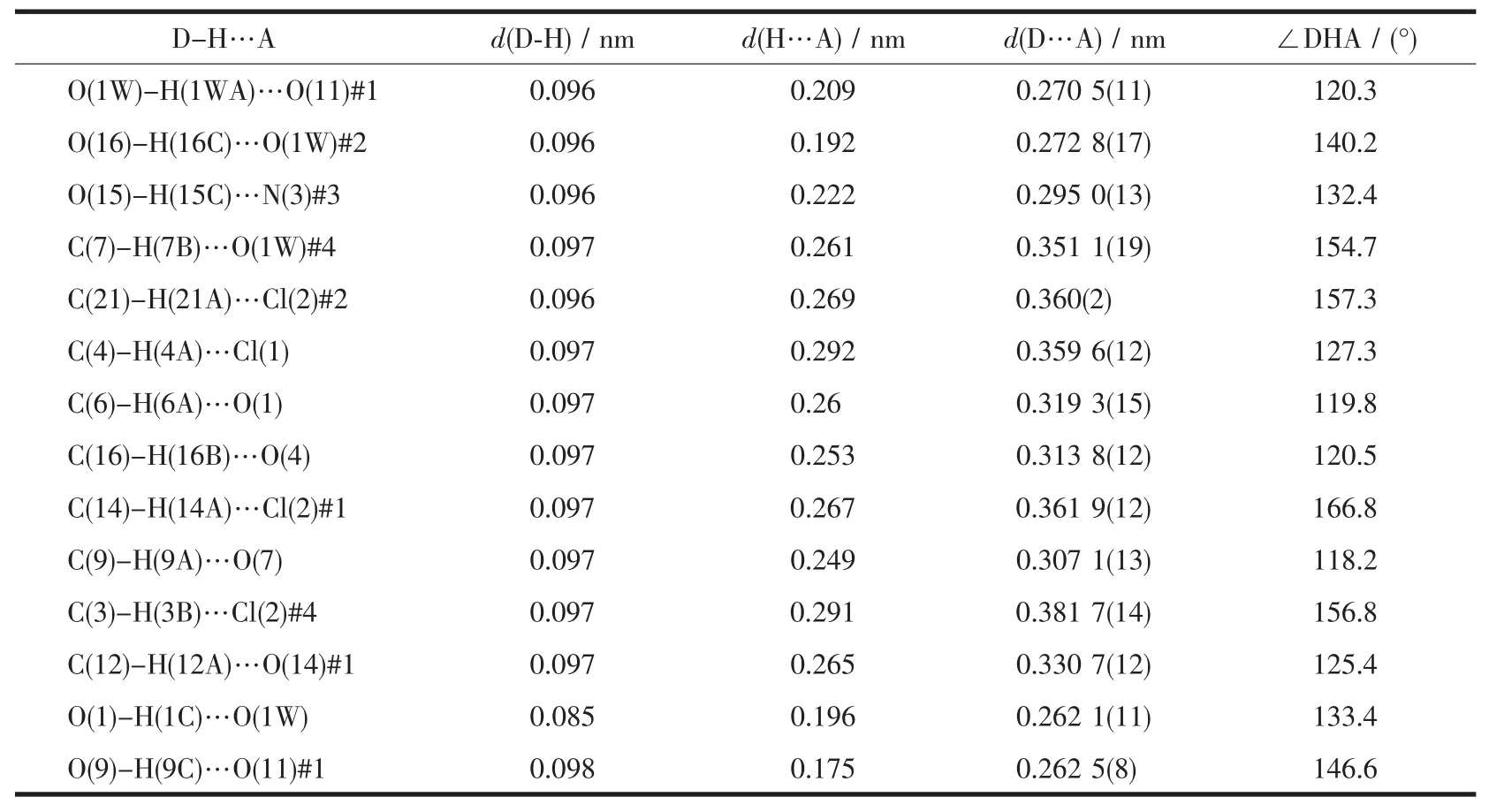
Table 3 Hydrogen bond parameters of 1
2.3 Thermal stability
Thermal stability is an important aspect for the application of coordination compound.Thermogravimetric analysis(TGA)experiments were carried out to determine the thermal stabilities of 1(Fig.3).TG curves showed the first consecutive step of weight loss was observed in the range of 50~180 ℃,corresponding to the release of solvent molecules(Calcd.7.4%;Obsd.6.9%).Then,the continuously weight loss corresponds to the release of ligands(in the range of 180~600 ℃).Finally,the weight loss ends until heating to 600℃and the total weight loss was about 45%.
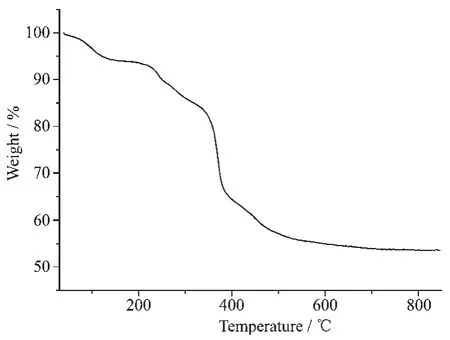
Fig.3 TG curve of complex 1
2.4 PXRD results
Powder X-ray diffraction (PXRD)experiment for complex1 has alsobeen conducted toconfirm whether the crystal structures are truly consistent with the bulk materials.The PXRD computer-simulated and experimental patterns of complex 1 are shown in Fig.4.In comparison with the simulated from crystal mode,the bulk-synthesized material and as-grown crystal can be considered homogeneous for complex 1.
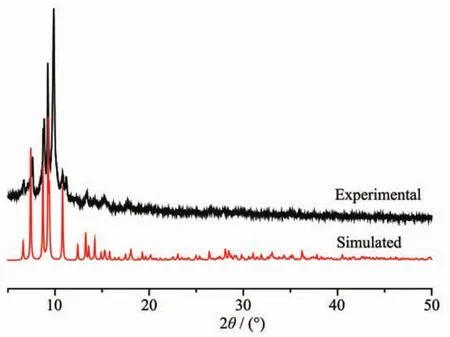
Fig.4 PXRD patterns of 1 measured in air
2.5 Magnetic properties
Temperature-dependent direct current(DC)magnetic susceptibility measurements for 1 was performed at temperatures ranging from 2 to 300 K,under an applied field of 1 000 Oe.The room temperature χMT value(40.81 cm3·mol-1·K)(Fig.5)is slightly smaller than spin only value (43.34 cm3·mol-1·K)for two Dyバ (6H15/2,g=4/3)and eight Coギ (g=2,S=3/2),remaining constant before decreasing from 40.57 cm3·mol-1·K at 100 K to 38.47 cm3·mol-1·K at 15 K indicative of Stark level splitting in the lanthanide ions.Below 15 K,χMT value increases rapidly to 40.05 cm3·mol-1·K at 4.5 K before dropping to 37.09 cm3·mol-1·K at 2 K.The increase of magnetic susceptibility data at low temperature reveals the weak ferromagnetic interactions between metal centers or strong orbital contribution from Coギions.
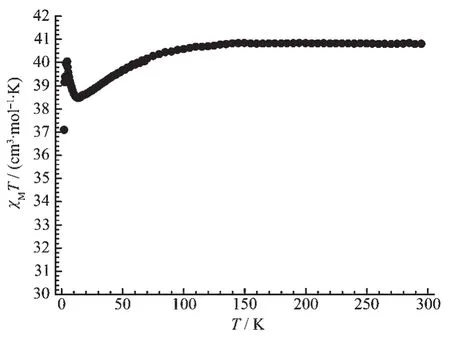
Fig.5 χMT versus T plot of 1 under 2 000 Oe DC field
Field-dependent magnetization(M)dada for 1 was collected in the range of 0~5 T at 2~5 K for the title compound (Fig.6).The curves of M vs H exhibit a gradual increase with increasing field strength reaching a saturation M value of 16.5μBat 2 K and 5 T,smaller than the theoretical saturated value for two Dyバ and eight Coギ ions.The magnetic anisotropy,together with the strong spin-orbital coupling,should be responsible for the magnetic unsaturation even at 5 T.This suggests the presence of magnetic anisotropy and/or considerable crystal-field effects. AC susceptibility measurement has also been conducted(Fig.7).However,no in-phase and out-of-phase signal could be observed.
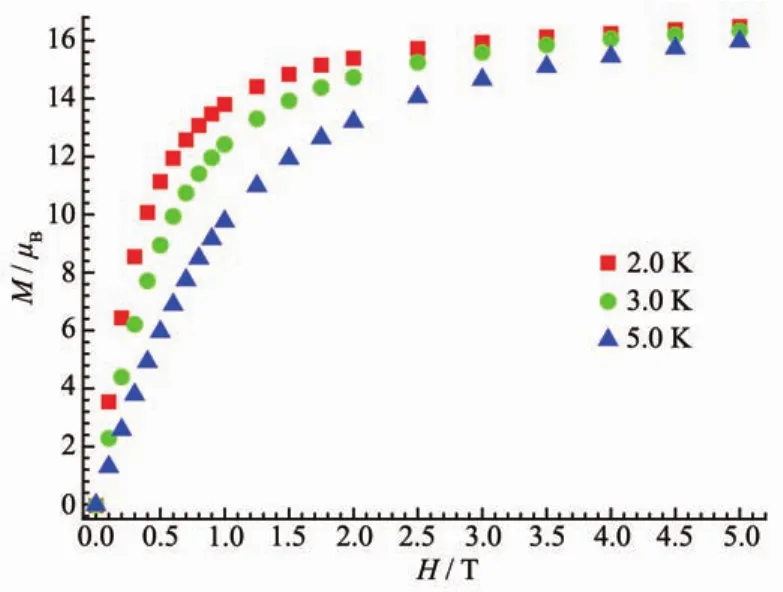
Fig.6 Isothermal magnetization curves for 1 collected from 2 to 5 K
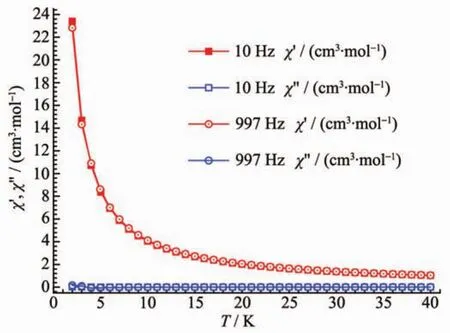
Fig.7 AC susceptibility measurement for 1
3 Conclusions
In summary,we have successfully prepared an unprecedented[Dy2Co8]core complex with diethanolamine as ligand,which have been structurally characterized.In particular,magnetic properties of the title complex was investigated and AC susceptibility measurement revealed that in-phase and out-of-phase signal could not be observed.
[1]Sessoli R,Powell A K.Coord.Chem.Rev.,2009,253(19):2328-2341
[2]Andruh M.Chem.Commun.,2007(25):2565-2577
[3]Sorace L,Benelli C,Gatteschi D.Chem.Soc.Rev.,2011,40(6):3092-3104
[4]Chen W P,Liao P Q,Yu Y,et al.Angew.Chem.,Int.Ed.,2016,55(32):9375-9379
[5]Kong X J,Ren Y P,Long L S,et al.J.Am.Chem.Soc.,2007,129(22):7016-7017
[6]Kong X J,Wu Y,Long L S,et al.J.Am.Chem.Soc.,2009,131(20):6918-6919
[7]Kong X J,Long L S,Zheng Z,et al.Acc.Chem.Res.,2009,43(2):201-209
[8]Zheng Y Z,Evangelisti M,Tuna F,et al.J.Am.Chem.Soc.,2012,134(2):1057-1065
[9]Peng J B,Zhang Q C,Kong X J,et al.J.Am.Chem.Soc.,2012,134(7):3314-3317
[10]Zheng Y Z,Evangelisti M,Winpenny R E P.Chem.Sci.,2011,2(1):99-102
[11]Abtab S M T,Majee M C,Maity M,et al.Inorg.Chem.,2014,53(3):1295-1306
[12]Ungur L,Thewissen M,Costes J P,et al.Inorg.Chem.,2013,52(11):6328-6337
[13]Li J,Wei R M,Pu T C,et al.Inorg.Chem.Front.,2017,4(1):114-122
[14]Liu J L,Wu J Y,Huang G Z,et al.Sci.Rep.,2015,5:16621[15]Mondal K C,Sundt A,Lan Y,et al.Angew.Chem.,Int.Ed.,2012,51(30):7550-7554
[16]Ferguson A,Lawrence J,Parkin A,et al.Dalton Trans.,2008(45):6409-6414
[17]Ako A M,Mereacre V,Clérac R,et al.Inorg.Chem.,2009,48(14):6713-6723
[18]Peng Y,Tian C B,Lan Y H,et al.Eur.J.Inorg Chem.,2013,2013(32):5534-5540
[19]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Sturcture,University of G?ttingen,Germany,1997.
[20]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Sturcture,University of G?ttingen,Germany,1997.
[21]REN Yan-Qiu(任艷秋),HAN Wei(韓偉),CHENG Mei-Ling(程美令),et al.Chinese J.Inorg.Chem.(無機化學學報),2014,30(11):2635-2644

