6-甲氧羰基-4,4′-二甲基-2,2′-聯吡啶單核銅雙膦發光配合物
曾雪花 羅燕生 何麗華 陳景林 張夢麗 廖金生 劉遂軍 溫和瑞
曾雪花 羅燕生 何麗華 陳景林*張夢麗 廖金生 劉遂軍 溫和瑞
(江西理工大學冶金與化學工程學院,贛州 341000)
合成并表征了2個新的基于 6-甲氧羰基-4,4′-二甲基-2,2′-聯吡啶的單核銅雙膦配合物[Cu(mmbpy)(dppp)]ClO4(1)和[Cu(mmbpy)(dppb)]ClO4(2)。研究結果表明,銅配合物1和2均表現為扭曲變形的N2P2四面體幾何構型,其P-Cu-P鍵角受輔助雙膦配體控制。在常溫下,這2個銅配合物在固態時均具有發光性質,并且相對于雙膦配體亞甲基鏈的長度,P-Cu-P鍵角對其光物理性質的影響更為顯著。在2,2′-聯吡啶環上引入2個甲基取代基對改善銅配合物的發光性能也被證明是有效的。
銅配合物;甲氧羰基;甲基;雙膦;發光
The emissions of tetrahedral Cucomplexes usually exhibit appreciable metal-to-ligand charge transfer(MLCT)character,which is sensitive to the peripheral ligand and coordination environment[13-21].It is demonstrated that structural modification of the peripheral ligands is an effective way for tuning emission properties of Cucomplexes[19-21].Herein,we report the synthesis,characterization,and photophysical properties of two new mononuclear copperdiphosphine complexes based on 6-methoxycarbonyl-4,4′-dimethyl-2,2′-bipyridine (mmbpy)(Scheme 1),and the effect of the variation of auxiliary diphosphine ligands on the structures and photophysical properties of Cucomplexes.
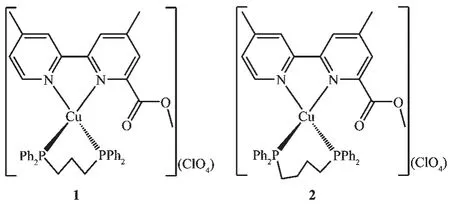
Scheme 1 Molecular structures of complexes 1 and 2
1 Experimental
1.1 Materials and measurements
All reactions were carried out under a N2atmosphere,using anhydrous solvents or solvents treated with an appropriate drying reagent.Commercially available reagents were used without further purification unless otherwise stated.6-Methoxycarbonyl-4,4′-dimethyl-2,2′-bipyridine (mmbpy)was synthesized according to the literature method[22].Infrared(IR)spectra were recorded on a Bruker Optics ALPHA FT-IR spectrometer using KBr pellets.Elemental analyses (C,H and N)were performed on a Perkin-Elmer model 240C elemental analyzer,where all the crystal samples are used after grinding and drying under vacuum.Ultraviolet-visible absorption spectra in CH2Cl2solution were recorded on a Shimadzu UV-2550 spectrometer.The photoluminescence properties in the solid state were determined on an Edinburgh analytical instrument(F900 fluorescence spectrometer)with a thermoelectrically cooled Hamamatsu R3809 photomultiplier tube.An integrating sphere(lab sphere)was applied to measure the emission quantum yield in the solid state.
Caution!Perchlorate salts are potentially explosive and should be handled carefully in small amount.
1.2 Preparations of complexes 1 and 2
1.2.1 [Cu(mmbpy)(dppp)]ClO4(1)
A mixture of[Cu(CH3CN)4]ClO4(19.6 mg,0.060 mmol)and 1,3-bis(diphenylphosphino)propane(dppp)(24.9 mg,0.060 mmol)in CH2Cl2(3 mL)was stirred for 1 h at room temperature;mmbpy(14.6 mg,0.060 mmol)was then added and the mixture was stirred for another 3 h to get a light yellow solution.The solvent was evaporated to dryness under reduced pressure.The resulting residue was then extracted with CH2Cl2,and slow diffusion of petroleum ether into the above mixture gave yellow crystalsof 1(41.8 mg,0.051 mmol,85%).Anal.Calcd.for C41H40ClCuN2O6P2(%):C,60.22;H,4.93;N,3.43.Found(%):C,60.18;H,4.95;N,3.45.IR(KBr,cm-1):3 448(m),3 055(w),2 939(w),1 731(s,-CO2CH3),1 611(s),1 480(w),1 435(s),1 341(m),1 263(m),1 222(m),1 159(w),1 093(vs),833(w),750(m),699(s),624(m),511(s).
1.2.2 [Cu(mmbpy)(dppb)]ClO4(2)
Complex 2 was synthesized according to the procedure for 1 by using[Cu(CH3CN)4]ClO4(21.2 mg,0.065 mmol),1,4-bis(diphenylphosphino)butane(dppb)(27.8 mg,0.065 mmol),and mmbpy(15.8 mg,0.065 mmol).Orange red crystals were afforded by slow diffusion of petroleum ether into a CH2Cl2solution of 2 (40.7 mg,0.049 mmol,75%).Anal.Calcd.for C42H42ClCuN2O6P2(%):C,60.65;H,5.09;N,3.37.Found(%):C,60.69;H,5.06;N,3.35.IR(KBr,cm-1):3 854(w),3 441(s),3 055(w),2 923(w),2 857(w),1 733(s,-CO2CH3),1 611(s),1 481(w),1 436(s),1 344(w),1 265(m),1 221(m),1 096(vs),896(w),839(w),746(m),698(s),625(m),515(m).
1.3 X-ray crystallography
Diffraction data for complexes 1 and 2 were collected on a Bruker D8 QUEST diffractometer at room temperature using graphite-monochromated Mo Kα radiation(λ=0.071 073 nm).The program CrystalClear was used for integration of the diffraction profiles.Structures were solved by direct methods and refined by full-matrix least-squares technique on F2using the SHELXL-97 software package[23].The heavy atoms were located from E-map and other nonhydrogen atoms were found in successive difference Fourier syntheses.All non-hydrogen atoms were refined anisotropically,while the hydrogen atoms were generated geometrically with isotropic thermal parameters.The crystallographic data and structure refinement details of 1 and 2 are summarized in Table 1,and the selected bond lengths and angles are given in Table 2.
CCDC:1543374,1;1543375,2.
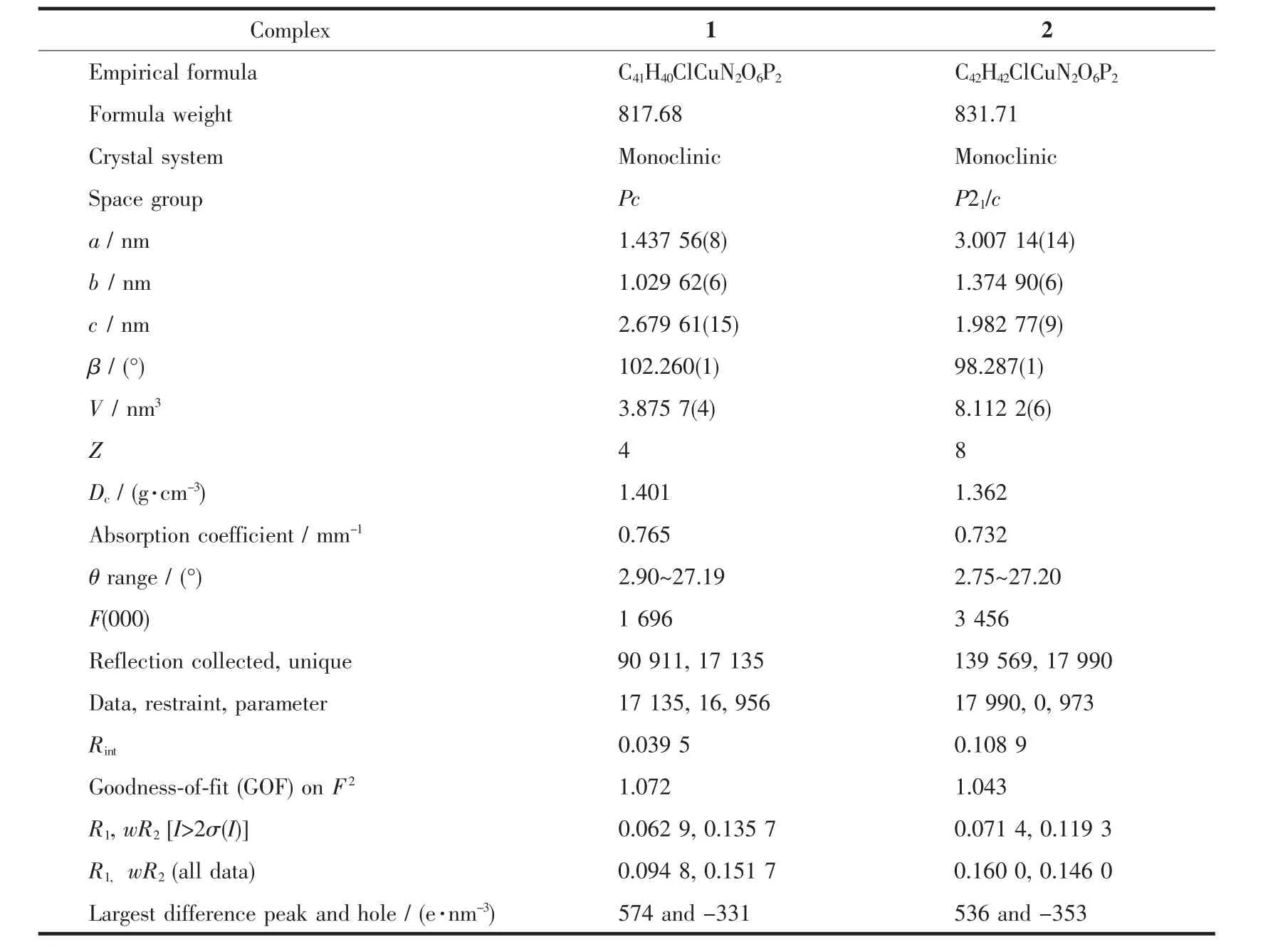
Table 1 Crystal data and structure refinement for 1 and 2
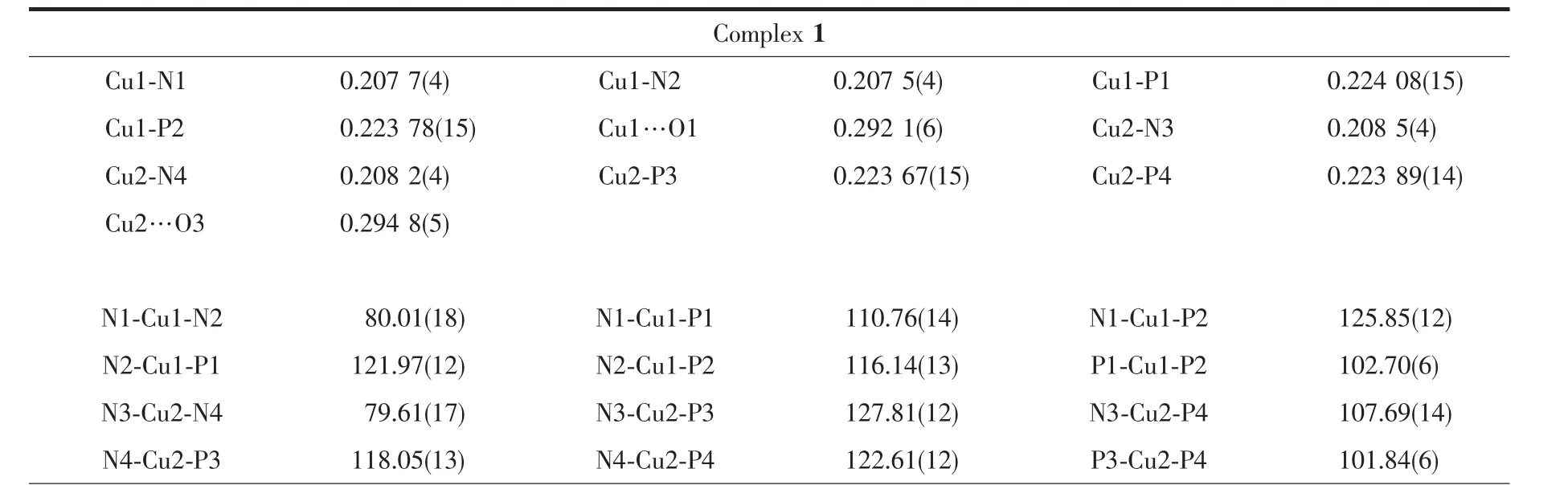
Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2
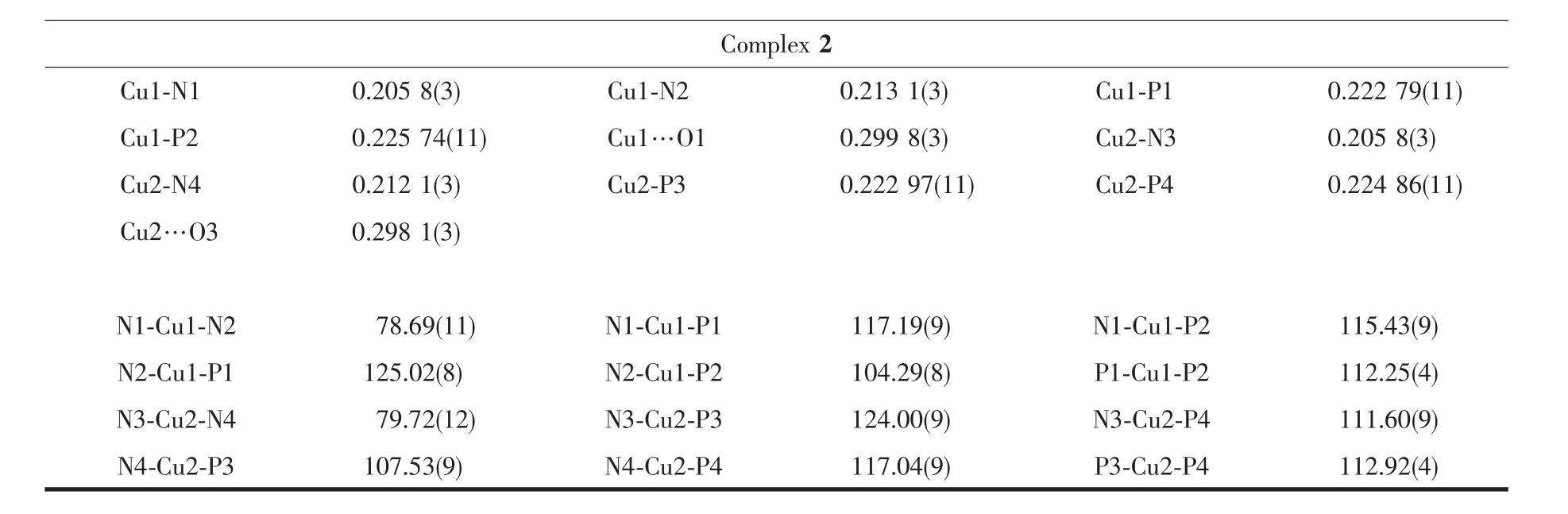
Continued Table 2
2 Results and discussion
2.1 Synthesis and characterization
In order to understand the effect of the methylene chain distance of auxiliary diphosphine ligand on the structures and spectral properties,mononuclear Cuheteroleptic complexes 1 and 2(Scheme 1)were prepared by the reaction of[Cu(CH3CN)4]ClO4with mmbpy and diphosphine(dppp and dppb)in a 1∶1∶1 molar ratio.Complexes 1 and 2 show a strong absorption peak at 1 731 and 1 733 cm-1,respectively,originating from the carbonyl stretching vibration(νC=O)of the methoxycarbonyl group.Another strong absorption peak is observed at 1 093 and 1 096 cm-1for 1 and 2,respectively,attributed to the Cl-O stretching vibration of the perchlorate anion.
The exact structures of complexes 1 and 2 were established by single-crystal X-ray crystallography.Complexes 1 and 2 crystallize in the monoclinic system,space group Pc and P21/c,respectively.As shown in Fig.1,the Cuions of 1 and 2 are fourcoordinate by two N atoms of the 2,2′-bipyridyl ring and two P atoms from a chelating diphosphine ligand(dppp and dppb),which generate a distorted N2P2tetrahedral arrangement with the N-Cu-N angle(78.69°~80.01°)obviously deviating from the idealized value(109°28′).The average values of P-Cu-P angles are 102.27(6)° and 112.59(4)°for 1 and 2,respectively,which are in reasonable agreement with the methylene chain distances,suggesting that the methylene chain distance of auxiliary diphosphine ligand has an impact on the P-Cu-P bond angle.Moreover,it is noted that the 2,2′-bipyridyl rings of two mmbpy ligands of two adjacent cations are basically parallel and show a favorable pairwise π-π stacking in 1.The inter-bipyridyl separations are in the range of 0.325~0.395 nm,indicating the presence of a weak π-π interaction between intermolecular twoadjacent mmbpy ligands[24].However,similarπ-π stacking interaction is not observed in the crystal lattice of 2.

Fig.1 Molecular structures of the cations of 1(left)and 2(right)with 30%probability ellipsoids
2.2 Photophysical properties
The absorption spectra of the free ligand mmbpy and its Cuderivatives 1 and 2 were investigated in CH2Cl2solution at room temperature.As depicted in Fig.2,the mmbpy ligand gives multiple absorption peaks in the UV region (<325 nm),attributed to the ligand-centered1ππ*transitions.Two mononuclear Cucomplexes 1 and 2 display several strong absorption peaks in the 225~350 nm region;those are assigned to the spin-allowed1ππ* transitions of both mmbpy and diphosphine ligands,which are slightly red-shifted,due to the more-extendedπconjugation,relative to the free mmbpy ligand.In addition,a relatively weak low-energy broad absorption band is also clearly observed at 423 and 403 nm for 1 and 2,respectively.The slightly stronger electron-donating dppb in place of dppp can raise the HOMO level localized at the Cucenter mixed with some contributions of diphosphine and less influence the LUMO level located on the mmbpy ligand,which results in a smaller HOMO-LUMO energy gap and a lower energy of absorption.Besides,it has been reported that a larger P-Cu-P angle can reduce the dσ*interactions and increase the energy required for MLCT excitation[25-26].It is noted that the low-energy absorption(λmax=403 nm)of 2 is blue-shifted(not redshifted)by 20 nm,compared to that(λmax=423 nm)of 1.Therefore,it is suggested that the weak low-energy absorptions of 1 and 2 are more markedly influenced by the P-Cu-P bond angle and not the electrondonating properties of diphosphine,and these lowenergy absorptions can be tentatively attributed to the charge transfer transitions with appreciable metal-toligand charge transfer(1MLCT,Cu→diimine)character[25-27].

Fig.2 Absorption spectra of mmbpy and complexes 1 and 2 in CH2Cl2 solution
The emission properties of 1 and 2 were investigated at room temperature in degassed CH2Cl2solution and using powder samples.Unfortunately,any detectable emission is unobserved for 1 and 2 in CH2Cl2solution at ambient temperature,similar to their Cuderivatives without the methyl substituents on the 2,2′-bipyridyl ring,perhaps due to fast structural relaxation occurring in degassed solution[28-29].It is noteworthy that complexes 1 and 2 display a broad and unstructured solid-state emission profile(Fig.3),maximized at 575 and 561 nm with the quantum yields of 0.176 and 0.094 and the emission lifetimes of 158.4 and 132.7μs,respectively.The solid-state emission of 2(561 nm)is blue-shifted by 14 nm relative to that of 1(575 nm),which is similar to the wavelength trend of weak low-energy absorptions of 1(423 nm)and 2(403 nm)in CH2Cl2solution,indicating that the emissions of 1 and 2 are also affected by both the P-Cu-P angle and the electron-donating properties of diphosphine.Actually,the emission of 1 is also influenced by weak π-π stacking interactions between intermolecular two adjacent mmbpy ligands of 1 revealed via X-ray crystallography,which lower the energy level of its LUMO localized on the mmbpy ligand and less influence its HOMO localized at the Cuand P atoms.Moreover,it is noted that complexes 1 and 2 with two methyl substituents on the 2,2′-bipyridyl ring are all emissive with moderate quantum yields(0.176 and 0.094)in the solid state at ambient temperature,whereas their Cuderivatives with no methyl substituents on the 2,2′-bipyridyl ring are non-emissive or only show very weak solid-state emissions[28-29],suggesting that the introduction of two methyl substituents into the 2,2′-bipyridyl ring is effective for enhancing luminescence properties of Cucomplexes.

Fig.3 Emission spectra of 1 and 2 in the solid state
3 Conclusions
We have synthesized and characterized two new mononuclear Cudiphosphine complexes with 6-methoxycarbonyl-4,4′-dimethyl-2,2′-bipyridine.It is revealed that two Cucomplexes all show distorted N2P2tetrahedral geometries with varied P-Cu-P angles regulated by auxiliary diphosphine ligands.The two Cucomplexes are all emissive in the solid state at ambient temperature,and their photophysical properties are more remarkably influenced by the P-Cu-P angle than the methylene chain length of diphosphine.It is also shown that the introduction of two methyl substituents into the 2,2′-bipyridyl ring is effective for improving luminescence properties of Cucomplexes.We believe that the results presented herein might provide new insight into the design and synthesis of highly efficient Cuphosphine complexes with functionalized 6-alkoxycarbonyl-2,2′-bipyridine ligands.
Acknowledgements:We thank the financial supports from the National Natural Science Foundation of China(Grants No.21561013,21501077),the Major Program of Jiangxi Provincial Natural Science Foundation of China (Grants No.20143ACB21017,20161ACB21013),the Natural Science Foundation of Jiangxi Province of China (Grants No.20171BAB203005,20171BCB23066,20151BAB213003),the Project of Education Department of Jiangxi Province(Grant No.GJJ160597),and the Program for Qingjiang Excellent Young Talents,JXUST.
[1]Zhang J,Xiong R G,Zuo JL,et al.Chem.Commun.,2000,36:1495-1496
[2]Wang JH,Li M,Li D.Chem.Sci.,2013,4:1793-1801
[3]He L H,Chen J L,Zhang F,et al.Inorg.Chem.Commun.,2012,21:125-128
[4]Chen Y Q,Li G R,Chang Z,et al.Chem.Sci.,2013,4:3678-3682
[5]He L H,Chen JL,Wang JY,et al.Chin.Chem.Lett.,2012,23:1169-1172
[6]Armaroli N,Accorsi G,Holler M,et al.Adv.Mater.,2006,18:1313-1316
[7]Li JC,Li H X,Li H Y,et al.Cryst.Growth Des.,2016,16:1617-1625
[8]He L H,Luo Y S,Di B S,et al.Inorg.Chem.,2017,56:10311-10324
[9]Barbieri A,Accorsi G,Armaroli N.Chem.Comm.,2008,44:2185-2193
[10]JIN Dou-Man(金斗滿),YANG Rui-Na(楊瑞娜),WANG Dong-Mei(王冬梅),et al.Chinese J.Inorg.Chem.(無機化學學報),2000,16(2):335-340
[11]ZHANG Zhong(張眾),LIU Ji-Zhong(劉積中),GAO Peng(高鵬),et al.Chinese J.Inorg.Chem.(無機化學學報),2012,28(1):195-200
[12]HUANGYan-Hong(黃廷洪),YAN Jie(顏杰),YANG Hu(楊虎),et al.Chinese J.Inorg.Chem.(無機化學學報),2015,31(12):2393-2400
[13]Chen JL,Cao X F,Wang JY,et al.Inorg.Chem.,2013,52:9727-9740
[14]Hsu C W,Lin C C,Chung M W,et al.J.Am.Chem.Soc.,2011,133:12085-12099
[15]Chen X L,Yu R,Zhang Q K,et al.Chem.Mater.,2013,25:3910-3920
[16]Chen JL,Guo ZH,Luo Y S,et al.New J.Chem.,2016,40:5325-5332
[17]Armaroli N,Accorsi G,Cardinali F,et al.Top.Curr.Chem.,2007,280:69-115
[18]Chen J L,Cao X F,Gu W,et al.Inorg.Chem.Commun.,2012,15:65-68
[19]Chen JL,Guo Z H,Yu H G,et al.Dalton Trans.,2016,45:696-705
[20]Zhang Q,Chen X L,Chen J,et al.Dalton Trans.,2015,44:10022-10029
[21]Zhang Q,Zhou Q,Cheng Y,et al.Adv.Mater.,2004,16:432-436
[22]Norrby T,B?rje A,Zhang L,et al.Acta Chem.Scand.,1998,52:77-85
[23]Sheldrick G M.SHELXTL-97,Program for the Refinement of Crystal Structures,University of G?ttingen,Germany,1997.
[24]Hunter CA,Meah M N,Sanders JK M.J.Am.Chem.Soc.,1990,112:5773-5780
[25]Cuttell D G,Kuang SM,Fanwick P E,et al.J.Am.Chem.Soc.,2002,124:6-7
[26]Kuang SM,Cuttell D G,McMillin D R,et al.Inorg.Chem.,2002,41:3313-3322
[27]McCormick T,Jia W L,Wang S.Inorg.Chem.,2006,45:147-155
[28]XIA Yong(夏勇),ZENG Xue-Hua(曾雪花),LUO Yan-Sheng(羅燕生),et al.Chinese J.Inorg.Chem.(無機化學學報),2016,32(11):2012-2016
[29]Chen JL,Fu X F,Wang JY,et al.Inorg.Chem.Commun.,2015,53:88-91
Luminescent Mononuclear CopperDiphosphine Complexes with 6-Methoxycarbonyl-4,4′-dimethyl-2,2′-bipyridine
Two new mononuclear copperdiphosphine complexes based on 6-methoxycarbonyl-4,4′-dimethyl-2,2′-bipyridine(mmbpy),[Cu(mmbpy)(dppp)]ClO4(1)and[Cu(mmbpy)(dppb)]ClO4(2),have been synthesized and characterized.It is revealed that Cucomplexes 1 and 2 all exhibit distorted N2P2tetrahedral geometries with varied P-Cu-Pangles regulated by auxiliary diphosphine ligands.The two Cucomplexes are all emissive in the solid state at ambient temperature,and their photophysical properties are more markedly influenced by the P-Cu-P angle than the methylene chain length of diphosphine.It is also demonstrated that the introduction of two methyl substituents into the 2,2′-bipyridyl ring is effective for improving luminescence properties of Cucomplexes.CCDC:1543374,1;1543375,2.
Cucomplex;methoxycarbonyl;methyl;diphosphine;luminescence
considerable research attention,because of their promising potential applications in materials science and the high relative abundance and environmental friendliness of copper[1-12].Recently,a rapidly increasing interest has been paid to tetrahedral Cucomplexes,especially mononuclear Cuheteroleptic complexes with one diimine plus one diphosphine or two monophosphine,due to the markedly enhanced emission properties[13-16].
O614.121
A
1001-4861(2017)10-1816-07
10.11862/CJIC.2017.234
ZENG Xue-Hua LUO Yan-Sheng HE Li-Hua CHEN Jing-Lin*ZHANGMeng-Li LIAO Jin-Sheng LIU Sui-Jun WEN He-Rui
(School of Metallurgy and Chemical Engineering,Jiangxi University of Science and Technology,Ganzhou,Jiangxi 341000,China)
0 Introduction
2017-04-13。收修改稿日期:2017-08-26。
國家自然科學基金(No.21561013,21501077)、江西省青年科學基金重大項目(No.20143ACB21017,20161ACB21013)、江西省自然科學基金(No.20171BAB203005,20171BCB23066,20151BAB213003)、江西省教育廳科技項目(No.GJJ160697)和江西理工大學清江青年英才支持計劃資助。
*通信聯系人。 E-mail:gzchenjinglin@126.com

