基于PbI2的有機-無機雜化化合物的合成和晶體結構
袁國軍 劉光祥 時 超 邵冬生
基于PbI2的有機-無機雜化化合物的合成和晶體結構
袁國軍*劉光祥 時 超 邵冬生
(南京曉莊學院環境科學學院,南京 211171)
合成得到了2個新的有機-無機雜化化合物{(4-CH3-Bz-4-Ph-Py)[PbI3]}n(1)(其中4-CH3-Bz-4-Ph-Py是4-甲基芐基-4-苯基吡啶陽離子)和{(4-CF3-Bz-4-Ph-Py)[PbI3]}n(2)(其中4-CF3-Bz-4-Ph-Py是4-三氟甲基芐基-4-苯基吡啶陽離子)。對化合物1和2進行了元素分析、粉末X射線衍射等表征,并利用X射線單晶衍射測定了它們的單晶結構。化合物1屬于正交晶系,P21212空間群;化合物2與1同構。結構研究表明,化合物1和2中,鉛碘八面體通過共邊連接方式,形成[Pb3I9]n三鏈,有機陽離子填充在無機碘化鉛鏈空隙中。
碘化鉛;有機-無機雜化化合物;晶體結構
In the context of haloplumbate-based hybrids,the perovskite-type ones have attracted tremendous research interest.The 3D haloplumbate-based perovskites,CH3NH3PbI3-xClx,with much lower exciton binding energies and intense light absorption over the whole visible light region have been employed as absorbers in solar cells.It is remarkable that the records of certified power conversion efficiencies have being constantly updated and over merely a few years,the power conversion efficiency has been enhanced to 20%[19].Most recently,the CH3NH3PbI3-xClxperovskites have been found to show amazing bipolar and bistable resistive switching behavior with small on-off voltage lower than 1.0 V in a simple metal-dielectric-metal capacitor configuration device memory field[22].The 2D haloplumbate-based hybrids,(N-MEDA)-[PbBr4-xClx](N-MEDA=N-methylethane-1,2-diammonium,x=0~1.2),are single-phase white-light emitters,and their broadband emission across the entire visible spectrum arises from corrugated lead halide sheets.Interestingly,the emission is tunable through halide substitution to afford both “warm”and “cold”white light in such haloplumbate-based wide-band gap semiconductors[14].The 1Diodoplumbate-based hybrids were reported to display ferroelectricity,wherein the polarization is switchable under an alternating current electrical field[23].
In addition,a 3D open-framework hybrid,[(EDAMP)2(Pb7I18)·4H2O]n(EDAMP=Et2NHC6H4CH2C6H4NHEt2),in which the inorganic framework is built from purely octahedral PbI6units and behaves as a quantum-wire array,shows a fascinating wavelengthdependent photochromic behavior[24].Its color changes from yellow to olive green under illumination withλ=500 nm light and further to dark green with light of λ<500 nm.Most interestingly,the reversion of the color for the hybrid can be accomplished by heating,indicating that this hybrid possesses switchable photochromic nature.It is well known that a material with switchable functionality through external stimuli,such as thermally-triggered,irradiation-induced and applied pressure,is very useful for application in the fields of sensors,memory and data storage[25-28].
In this study,we report the syntheses,crystal structures and thermal properties of two iodoplumbatebased hybrids,{(4-CH3-Bz-4-Ph-Py)[PbI3]}n(1)and{(4-CF3-Bz-4-Ph-Py)[PbI3]}n(2).
1 Experimental
1.1 Materials and general methods
All chemicals and solvents were reagent grade and used without further purification.Elemental analyses for C,H and N were performed with an Elementar Vario ELⅢanalytic instrument.Powder X-ray diffraction (PXRD)data for 1 and 2 were collected on a Rigaku/max-2550 diffractometer with Cu Kα radiation(λ=0.154 18 nm)at room temperature with acceleration voltage of 40 kV and current of 40 mA.The data were collected in the 2θrange from 5°to 50°.Electric spray ionization mass spectra were performed on LCO Fleet ESI Mass Spectrometer.
1.2 Synthesis of{(4-CH3-Bz-4-Ph-Py)[PbI3]}n(1)
[4-CH3-Bz-4-Ph-Py]Br.4-Methylbenzyl bromide([4-CH3-Bz]Br,370 mg,2 mmol)and 4-Phenylpyridine(4-Ph-Py)(310 mg,2 mmol)were mixed under stirring in acetone (25 mL)at 55℃and the formed purple microcrystal product was filtered off,washed with acetone and then dried in vacuum at room temperature to give 670 mg of[4-CH3-Bz-4-Ph-Py]Br(Yield:92%).Elemental analysis calculated for C19H18NBr(%):C,67.07;H 5.33;N,4.12.Found(%):C,67.02,H,5.30,N,4.09.MS-ESI:m/z=260.25([4-CH3-Bz-4-Ph-Py]+).
{(4-CH3-Bz-4-Ph-Py)[PbI3]}n(1).A mixture of[4-CH3-Bz-4-Ph-Py]Br and KI and PbI2with a molar ratio of 1∶1∶1 in DMF (25 mL)was placed in an oven and slowly evaporated at 55 ℃ for 10~14 days to produce light yellow needle-shaped crystals in ca.95%yield.Elemental analysis calculated for C38H36N2Pb3I8(%):C,23.40;H,1.86;N,1.44.Found(%):C,23.36,H,1.84,N,1.41.
1.3 Synthesis of{(4-CF3-Bz-4-Ph-Py)[PbI3]}n(2)
[4-CF3-Bz-4-Ph-Py]Br.The synthesis of[4-CF3-Bz-4-Ph-Py]Br was similar with[4-CH3-Bz-4-Ph-Py]Br except 4-trifluoromethylbenzyl bromide([4-CF3-Bz]Br,478 mg,2 mmol)was used instead of[4-CH3-Bz]Br.Yield:92%.Elemental analysis calculated for C19H15F3NBr(%):C,57.89;H 3.84;N,3.55.Found(%):C,57.86,H,3.82,N,3.53.MS-ESI:m/z=314.25([4-CF3-Bz-4-Ph-Py]+).
{(4-CF3-Bz-4-Ph-Py)[PbI3]}n(2).The synthesis of 2 was similar with 1 except[4-CF3-Bz-4-Ph-Py]Br was used instead of[4-CH3-Bz-4-Ph-Py]Br.Yield:ca.95%.Elemental analysis calculated for C38H30F6N2Pb3I8(%):C,20.15;H 1.33;N,1.24.Found(%):C,20.12,H,1.30,N,1.21.
1.4 X-ray crystallography
The single-crystal X-ray diffraction data for 1 and 2 were collected at 296(2)K with graphite monochromated Mo Kα (λ=0.071 073 nm)on a CCD area detector(Bruker-SMART).Data reductions and absorption corrections were performed with the SAINT and SADABSsoftware packages[29],respectively.Structures were solved by a direct method using the SHELXL-2014 softwarepackage[30].The non-hydrogen atomswere anisotropically refined using the full-matrix leastsquares method on F2.All hydrogen atoms were placed at the calculated positions and refined riding on the parent atoms.The details about data collection,structure refinement and crystallography are summarized in Table 1.
CCDC:1572362,1;1572361,2.
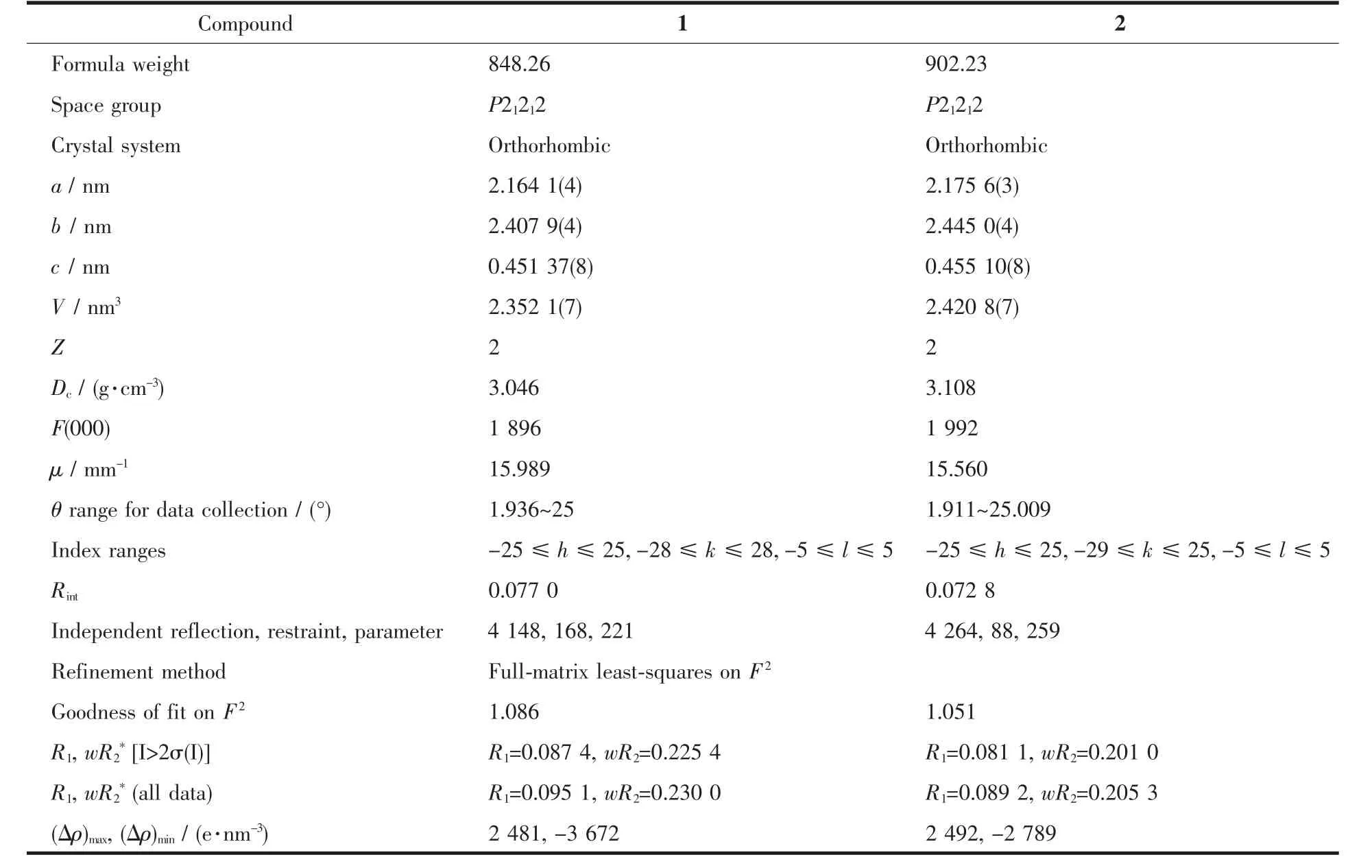
Table 1 Crystallographic and structure refinement data for 1 and 2
2 Results and discussion
2.1 Description of crystal structure
Compound 1 crystallizes in the P21212 space group at room temperature.The asymmetric unit,as shown in Fig.1,consists of one Pb2+ion and three different I-anions together with one 4-CH3-Bz-4-Ph-Py cation.The Pb2+ion locates at an inversion center and is coordinated with six I-to form the slightly distorted PbI6octahedron.The Pb-I lengths range from 0.282 48(46)to 0.358 35(27)nm and the I-Pb-I angles fall within the range of 80.393(74)°~171.711(108)°at 296 K,these geometry parameters within the coordination octahedron are comparable to other iodoplumbates.Three different I-ions adopt theμ3-bridged model to connect three neighboring Pb2+ions.The adjacent PbI6coordination octahedral are connected together via the edge-sharing mode to form a uniform[Pb3I9]nchain along the a-axis direction(Fig.2).

Fig.1 ORTEPview of 1 with thermal ellipsoids at 30%probability level

Fig.2 Edge-sharing octahedral chain of[Pb3I9]n in compounds 1 and 2:(a)Stick and ball model;(b)polyhedron model
The cation is composed of a 4-phenylpyridine and a 4-methylbenzyl,the neighboring cations are aligned into quadrilateral-shaped 1D channels,and the inorganic[Pb3I9]nchains reside in the channels(Fig.3).Charged-assisted H-bonding interactions appear between the CH2groups in the cations and the I-ions in the inorganic chains.Compound 2 (Fig.4~5)is isostructural with compound 1.
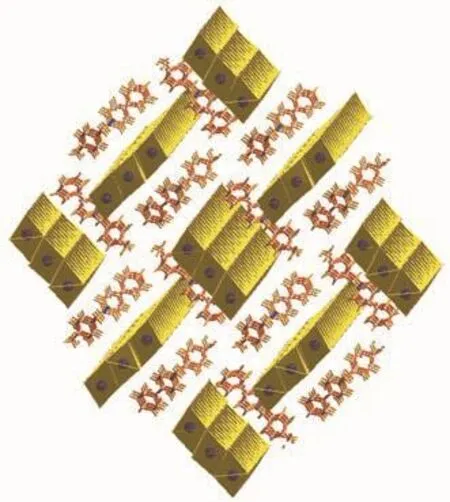
Fig.3 Molecular packing diagram for compound 1

Fig.4 ORTEPview of 2 with thermal ellipsoids at 30%probability level

Fig.5 Molecular packing diagram for compound 2
2.2 Thermal stability and powder X-ray diffraction(PXRD)
To examine the thermal stability of compounds 1 and 2,TG analyses were carried out(Fig.7).The TG study of compound 1 shows a weight loss of 26.09%from 27 to 340℃,corresponding to the loss of cation.Then the compound starts to decomposes at 340℃,corresponding to the loss of anion.In the case of compound 2,a weight loss of 31.47%from 19 to 345℃,corresponding to the loss of cation.Then the compound 2 starts to decompose at 345℃,corresponding to the loss of anion.
Powder X-ray diffraction analysis (PXRD)experiments were carried out for 1 and 2 at room temperature to characterize their purity.As shown in Fig.7,the measured peak positions closely match the simulated peak positions, indicative of pure products.

Fig.6 TGA curves of compounds 1 and 2
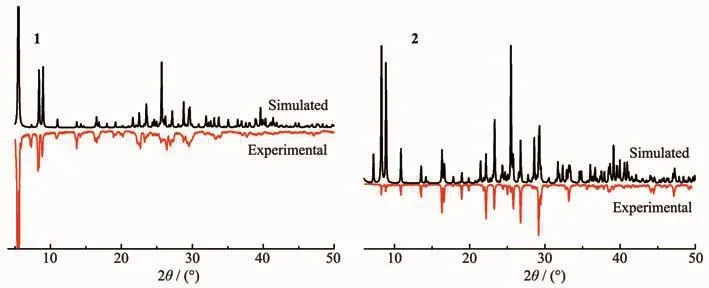
Fig.7 PXRD patterns of compounds 1 and 2
[1]Zhao SP,Ren X M.Dolton Trans.,2011,40:8261-8272
[2]Krautscheid H,Vielsack F.Angew.Chem.Int.Ed.,1995,34:2035-2037
[3]Krautscheid H,Lode C,Vielsack F,et al.J.Chem.Soc.,Dalton Trans.,2001:1099-1104
[4]Tang Z,Guloy A M.J.Am.Chem.Soc.,1999,121:452-453
[5]Liu J J,Guan Y F,Jiao C,et al.Dalton Trans.,2015,44:5957-5960
[6]Zhang Z J,Xiang SC,Zhang Y F,et al.Inorg.Chem.,2006,45:1972-1977
[7]She Y J,Zhao SP,Ren X M,et al.Inorg.Chem.Commun.,2014,46:29-32
[8]Tong Y B,Ren L T,Ren X M,et al.Dalton Trans.,2015,44:17850-17858
[9]Duan H B,Yu SS,Ren X M,et al.Dalton Trans.,2016,45:4810-4818
[10]Lemmerer A,Billing D G.CrystEngComm,2012,14:1954-1966
[11]Willett R D,Maxcy K R,Twamley B.Inorg.Chem.,2002,41:7024-7030
[12]SUN Cai(孫財),WANG Ming-Sheng(王明盛),GUO Guo-Cong(郭國聰).Proceedings of Ninth Chinese Inorganic Chemistry Conference(全國第九屆無機化學化學學術會議論文集).Nanchang:[s.n.],2015:315
[13]Fujian Institute of Research on the Structure of Matter(福建物質結構研究所).Bulletin of the Chinese Ceramic Society(硅酸鹽通報),2016,2:143
[14]Dohner E R,Hoke E T,Karuadasa H I.J.Am.Chem.Soc.,2014,136:1718-1721
[15]Wehrenfenging C,Liu M,Snaith H J,et al.J.Phys.Chem.Lett.,2014,5:1300-1306
[16]Guloy A M,Tang ZJ,Miranda PB,et al.Adv.Mater.,2001,13:833-837
[17]Fujisawa J I,Ishihara T.Phys.Rev.B:Condens.Matter Mater.Phys.,2004,70:113024-113203
[18]Liu G N,Shi J R,Han X J,et al.Dalton Trans.,2015,44:12561-12575
[19]Yang W S,Noh J H,Jeon N J,et al.Science,2015,348:1234-1237
[20]Liu M,Johnston M B,Snaith H J.Nature,2013,501:395-398
[21]Xing G,Mathews N,Sun S,et al.Science,2013,18:344-347
[22]Yoo E J,Lyu M,Yun J H,et al.Adv.Mater.,2015,27:6170-6175
[23]Zhao H R,Li D P,Ren X M,et al.J.Am.Chem.Soc.,2010,132:18-19
[24]Zhang Z J,Xiang SC,Guo G C,et al.Angew.Chem.,Int.Ed.,2008,47:4149-4152
[25]Maldonado P,Kanungo S,Saha-Dasgupta T,et al.Phys.Rev.B:Condens.Matter Mater.Phys.,2013,88:020408
[26]Kahn O,Martinez CJ.Science,1999,279:44-48
[27]Liang J,Chen Z,Xu L,et al.J.Mater.Chem.C,2014,2:2243-2250
[28]Dong X Y,Li B,Ma B B,et al.J.Am.Chem.Soc.,2013,135:10214-10217
[29]SMART and SAINT,Siemens Analytical X-ray Instrument Inc.,Madison,WI,1996.
[30]Sheldrick GM.Acta Crystallogr.Sect.C,2015,C71:3-8
Syntheses and Crystal Structures of Two Homogeneous Organic-Inorganic Compounds Based on PbI2
Two homogeneous compounds,{(4-CH3-Bz-4-Ph-Py)[PbI3]}n(1)(4-CH3-Bz-4-Ph-Py=4-methylbenzyl-4-phenylpyridinium)and{(4-CF3-Bz-4-Ph-Py)[PbI3]}n(2)(4-CF3-Bz-4-Ph-Py=4-trifluoromethylbenzyl-4-phenylpyridinium),have been synthesized and characterized by elemental analysis and single-crystal X-ray diffraction.Compound 1 crystallizes in orthorhombic,space group P21212,and compounds 1 and 2 are isostructural.Structure analyses reveal that iodoplumbate ion exhibits octahedron topology in compounds 1 and 2,and all these octahedron topologies form 1D polymeric chain through edge-sharing connecting modes.The organic cation fill in the gap formed by inorganic iodoplumbate polymeric chain.CCDC:1572362,1;1572361,2.
lead iodide;organic-inorganic hybrid compounds;crystal structure
considerable research interests due to their tunable structuresfromthediscretemononuclear or polynuclear species (zero-dimensional,0D)to the infinite variety with higher dimensionality[1-13](one-dimensional[1,3-9],two-dimensional[7-11]or three-dimensional[2];hereafter abbr.as 1D,2D and 3D,respectively)and the wide range of novel physical properties,beneficial in optics[14-18]and electronics[19-21].
O614.43+3
A
1001-4861(2017)10-1855-06
10.11862/CJIC.2017.225
YUAN Guo-Jun*LIU Guang-Xiang SHI Chao SHAO Dong-Sheng
(Department of Applied Chemistry,School of Environmental Science,Nanjing XiaoZhuang University,Nanjing 211171,China)
0 Introduction
2017-04-22。收修改稿日期:2017-08-02。
江蘇省自然科學基金(No.BK20170145)、江蘇省教育廳高校自然科學研究面上項目(No.15KJB150019)和江蘇省高等學校大學生實踐創新訓練計劃(No.201511460027Y)資助。
*通信聯系人。 E-mail:ahchljygj@163.com

