晶面選擇性生長對球形氫氧化鎳形貌及電化學活性的影響
唐俊杰 劉燕 田磊 張麗麗 王東興 張廷安
(東北大學多金屬共生礦生態(tài)化冶金教育部重點實驗室,沈陽110819)
晶面選擇性生長對球形氫氧化鎳形貌及電化學活性的影響
唐俊杰 劉燕*田磊 張麗麗 王東興 張廷安
(東北大學多金屬共生礦生態(tài)化冶金教育部重點實驗室,沈陽110819)
在相同的物理化學條件、不同晶化時間的條件下,采用化學沉淀法制備球形氫氧化鎳晶體并應用SEM技術分別考察制得樣品的形貌。研究表明:在相同的物理化學條件下,隨著晶化時間的增加,樣品的微觀形貌由不規(guī)則狀晶體變?yōu)橥暾那驙罹w。結合XRD表征結果分析得出:(001)晶面在晶化時間達到3 h時生長到了一個穩(wěn)定的狀態(tài),并不隨著晶化時間的增加而有明顯的變化,但(100)與(101)晶面隨晶化時間的增加繼續(xù)生長,當晶化時間達到12 h時氫氧化鎳的相對結晶度最大,(100)與(101)衍射峰強度高且峰形尖銳,說明氫氧化鎳晶體的結構規(guī)整性增強。本文還討論了氫氧化鎳生長結晶過程中晶面選擇性生長對晶體形貌及電化學性能的影響。
球形氫氧化鎳;晶面;衍射峰;相對結晶度
Spherical Ni(OH)2is one of the most important battery material which has been widely applied in electroplating,storage battery,spaceflight and so on. Although spherical Ni(OH)2has been industrially produced in large-scale,the structures in different batches always appear different because the growthmechanism of Ni(OH)2crystal has not been researched thoroughly.Wangsummarizedthemechanismof crystal growth defect in detail.He concluded that the sub-step theory could be applied to explain the crystal growth mechanisms of both solution and sublimation processes[1-2].Ma et al.measured and estimated crystal plane growth kinetics.They presented a framework which integrated the various components to achieve the ultimate objective of model-based closed-loop control of the CShD[3-4].Huo et al.proposed a synthetic imageanalysisstrategyforin-situcrystalsize measurement and shape identification for monitoring crystallization processes,based on a real-time imaging system[5-7].
X-ray diffraction plays a key role in the analysis of the crystallization process.Ramesh used XRD to determine the thermal decomposition of Ni(OH)2. Further,he concluded that the decomposition mechanism mainly depended on the preparative conditions[8]. Deschamp presented that continuing advances in all phases of a crystallographic study have expanded the ranges of samples which can be analyzed by X-ray crystallography,including larger molecules,smaller or weakly diffracting crystals,and twinned crystals[9-13]. Xu et al.used X-ray diffraction to analyze the growth and phase transformation of metastable β-HgI2M[14-15].
The crystallization factors of spherical Ni(OH)2on macroscopic perspective were investigated,including temperature,pH(ammonia content),reactant supersaturation,mixing intensity and paddle type as well as many other aspects[16].However,the factors of microcosmic perspective are rarely studied.Therefore,in this study the spherical Ni(OH)2was synthesized by chemical precipitation method with different aging times under the same physical and chemical conditions. The morphologies and the relative crystallinities of the spherical Ni(OH)2were characterized by SEM and XRD.It is found that the crystal growth direction selectivity has a great influence on morphologies and crystallization of spherical Ni(OH)2.This work provides the rules of the growth of crystal plane according to the crystallization of spherical Ni(OH)2.It also offers a theoretical basis and experiences for reducing the structure differences of Ni(OH)2industrial products.
1 Experimental
1.1 Preparation of spherical Ni(OH)2
According to the industrial process of spherical Ni(OH)2preparation,the chemical precipitation method was applied to the preparation of spherical Ni(OH)2. The reaction temperature of the system was controlled within 50~57℃,and the pH value was controlled within 11~11.7.A certain concentration of nickel sulfatesolution,ammoniaandsodiumhydroxide solution in a certain proportion was filled to the bottom of the reactor.Under the same physical and chemical conditions,the samples prepared with the aging times of 3,6,9 and 12 h,and then the samples were filtered out for measurement.
1.2 Instruments
The scanning electron microscope(SEM)used in this experiment was SU8010 produced by Japan Hitachi Company.The X-ray diffractometer used in this experiment was D8 Advance X Bruker ray analyzer produced by German Bruker Company.Light tube type was Cu target(Kα,λ=0.154 06 nm).The scan range was 10°~90°with scanning speed of 2°·min-1. The electrochemical workstation used in this experiment was Biologic vsp300 produced by France Claix Company.
2 Results and discussion
2.1 SEM analysis
Under the same physical and chemical conditions, when the aging time is 3 h,the Ni(OH)2particles form irregular crystals with different sizes,the surface structure of the crystals is composed of the acicular micro-crystals,as shown in Fig.1.It is because in the early reaction,the Ni(OH)2particles form flocculent precipitatesandmicro-crystalsunderthestrong agglomeration.
When the aging time is 6 h,the Ni(OH)2particles form many agglomerate crystals with similar sizes and shapes,the surface structure of these crystals is composed of many well-defined acicular microcrystals,as shown in Fig.2.It is because the crystalnucleuses gradually form and crystals gradually grow withtheincreaseoftheagingtimeunderthe agglomeration.The flocculent precipitates gradually fill the gaps of agglomerated particles and the quasi spherical crystals are formed.Due to the growth of layers is perpendicular to the spherical surface in the crystallizationprocessofspherical Ni(OH)2,the surface of these quasi spherical crystals is formed by the well-defined acicular micro-crystals.
When the aging time is 9 h,the Ni(OH)2particles form spherical crystals with similar sizes,and the surface structure of these crystals is composed of many grainy micro-crystals,as shown in Fig.3.It is because the precipitates fill the gaps of agglomerated particles and the more compact spherical crystals are formed.Due to the surface of these crystals is attached of grainy micro-crystals,the precipitates continue to adhere to the surface of the crystals and the crystals grow slowly under the agglomeration.
When the aging time is 12 h,the Ni(OH)2particles form many complete spherical crystals with a uniform size,the surface structure of these crystals is composed of many micro-crystals with clear stripes,as shown in Fig.4.It is because the agglomerated particles combine more compact,and the surface structure of these crystals grows more integrated.
2.2 XRD analysis
The XRD patterns of the samples with different aging times are shown in Fig.5.It can be found that the main diffraction peaks are nickel hydroxide,and other crystal items are not observed.When the aging time is 12 h,the characteristic peaks in the XRD patterns are highest,the FWHM is minimum,and the relativecrystallinityisthebest.Iftherelative crystallinity of the samples prepared at 12 h is treated as 100%,according to amorphization formula A=[1-U0Ix/(UxI0)]×100%,the calculation of each samples is plotted in a linear graph,as shown in Fig.6.Moreover, the mathe-matical relationship between aging time and amor-phization is A=-0.007 7T2+0.037 1T+0.653 2, which can be obtained according to Fig.6.
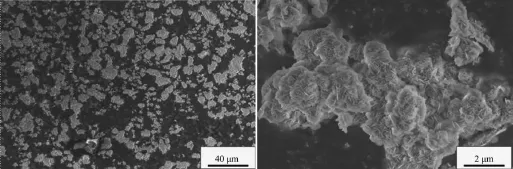
Fig.1SEM images of Ni(OH)2particles(aging time 3 h)
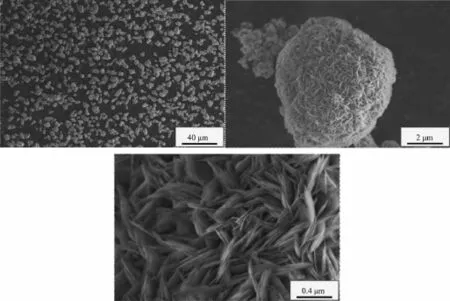
Fig.2SEM images of Ni(OH)2particles(aging time 6 h)
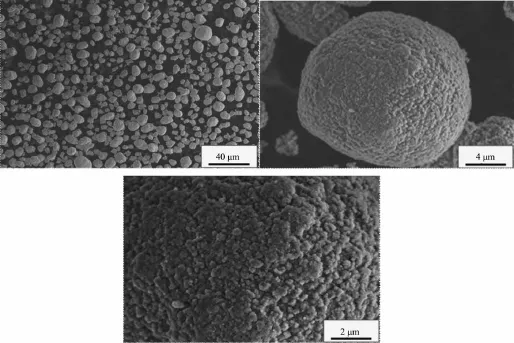
Fig.3SEM images of Ni(OH)2particles(aging time 9 h)
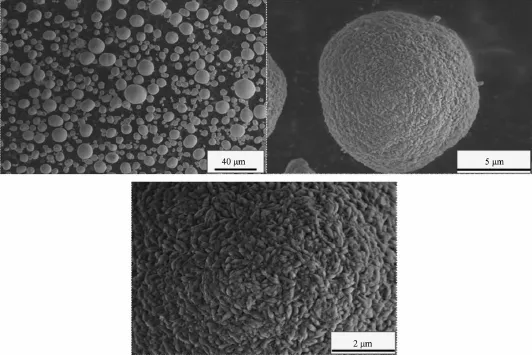
Fig.4SEM images of Ni(OH)2particles(aging time 12 h)
It can be found that the FWHM(001)and the peak intensity on(001)diffraction peak are basically the same with the increase of the aging time from Fig.5. Which means that the growth of(001)crystal plane reaches a steady state when the aging time is 3 h. However,the FWHM(100)and FWHM(101)decrease while the peak intensities are enhanced.Which means that (100)crystal plane and(101)crystal plane continue to grow with the increase of the aging time.SEM images show that when the aging time is increased from 3 to 12 h,the morphology of Ni(OH)2crystals changes from irregular crystals to complete spherical crystals,it can be concluded that the growth of(001)crystal face has not a greater influence on the morphology of Ni(OH)2crystals,but the growths of(100)crystal plane and(101)crystal plane have a greater influence on the morphology.And the FWHM(100)and FWHM(101)of Ni(OH)2crystals with different aging times are given in Table 1.
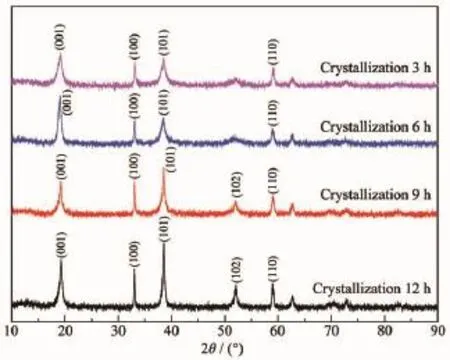
Fig.5XRD patterns of the samples with different aging times
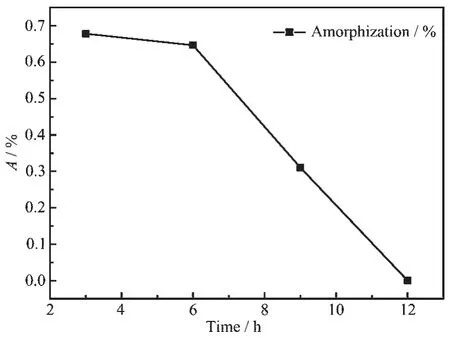
Fig.6Aging time-conversion relation of amorphization
According to Table 1,the selectivity of crystal growth directions on(100)crystal plane and(101) crystal plane with differentagingtimescanbe calculated by Scherrer formula D=Kλ/(Bcosθ),and the results are given in Table 2.From Table 2 it can be seenthatthegrowthrateof(101)crystalplaneis greater than(100)crystal plane when the aging time is 6~9 h, and the growth rate of(100)crystal plane is greater than(101)crystal plane when the aging time is 9~12 h.It shows from SEM images Fig.2~Fig.3 that when the aging time is increased from 6 to 9 h,the morphologies of Ni(OH)2crystals change from agglomerate crystals to spherical crystals.This means that the growth of(101)crystal plane has a greater influence on the sphericity than(100)crystal plane.SEM images Fig.3~Fig.4 show that when the aging time is increased from 9 to 12 h,the surface structures of Ni(OH)2crystals change from grainy micro-crystals to striped micro-crystals.This means that the growth of(100) crystal plane has a greater influence on the surface structure than(101)crystal plane.
2.3 Performance characterization
Theelectrochemicalactivityisthebasic indicator of Ni(OH)2.The Ni(OH)2samples prepared at different aging times were compressed with carbon black and adhesive in proportion as 7∶2∶1 into sheet electrode.CV Scan was tested at the speed of 10 mA/ s in the range of 0~0.6 V,while the Hg/HgO was selected as the reference electrode.The CV curves are shown in Fig.7.According to Fig.7,it can be seenthatwiththeincreaseoftheagingtime,the electrochemically active specific surface area also increases.When the aging time is 12 h,the electrochemically active specific surface area and peak current reach the maximum,which shows the best electrochemicalactivity.Therefore,itcanbe determined that the aging time is proportional to the electrochemical activity of Ni(OH)2crystal.
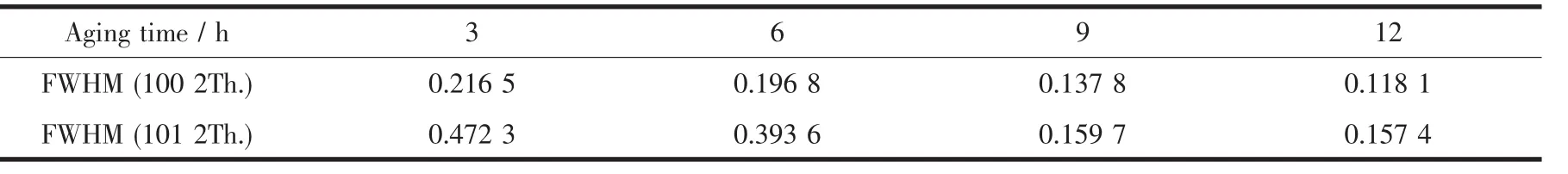
Table 1FWHM for Ni(OH)2crystals with different aging times
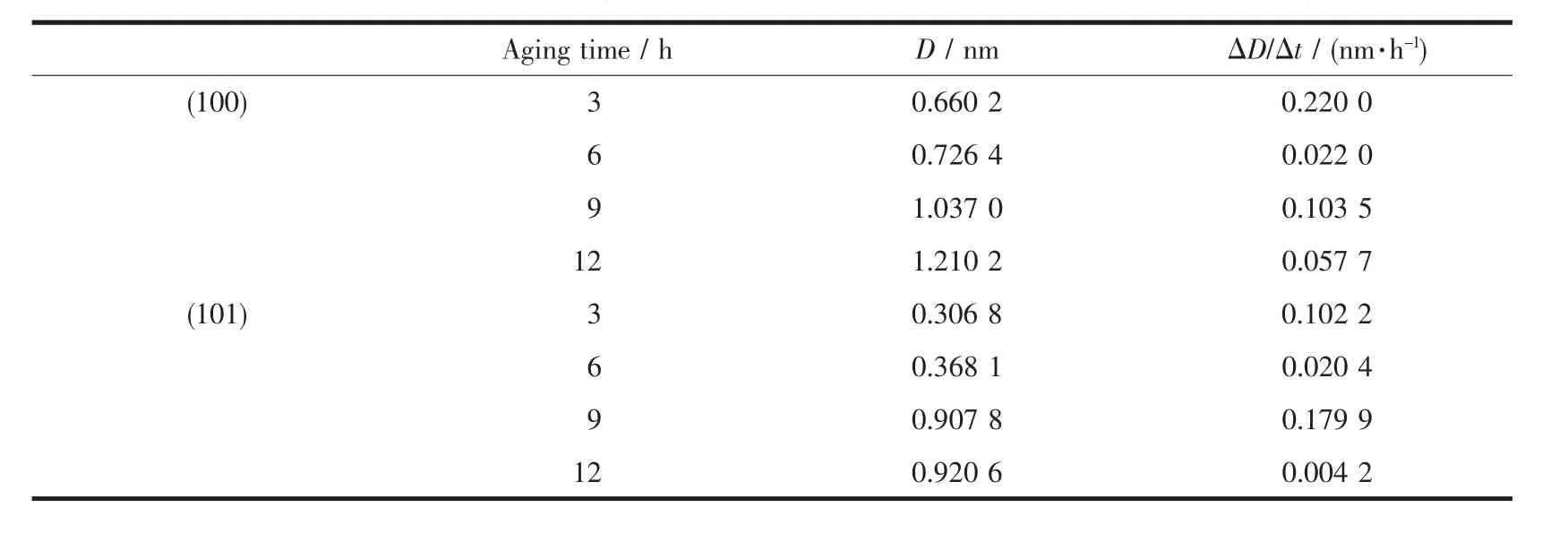
Table 2Selectivity of crystal growth directions for Ni(OH)2with different aging times
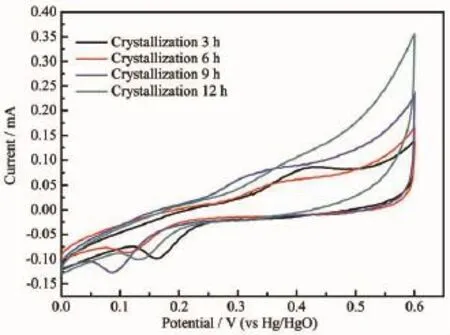
Fig.7CV curves of the samples with different aging times
According to SEM analysis and CV analysis,the aging time has a influence on the morphology and electrochemical activity.Therefore,the relationship between the morphology and electrochemical activity can be determined:the higher sphericity leads to the better electrochemical activity,and the surface structure of striped micro-crystals has a better electrochemical activity than the surface structure of grainy microcrystals.
Bulk density is oneofthemostimportant electrochemical indices of spherical Ni(OH)2.The bulk density of spherical Ni(OH)2determines the compactness of Ni electrode,and then affects the specific capacity.The international standard GB 1482-84 was applied in this experiment.And the bulk densities of the samples with different aging times are given in Table 3.It can be seen that the bulk density increases with the increase of the aging time,and it suggests that the aging time is proportional to the electrochemical activity of Ni(OH)2crystal,which is in agreement with CV analysis.Moreover,Fig.8 can lead to the mathematical relationship between the bulk density and the aging time:ds=0.174T0.938.
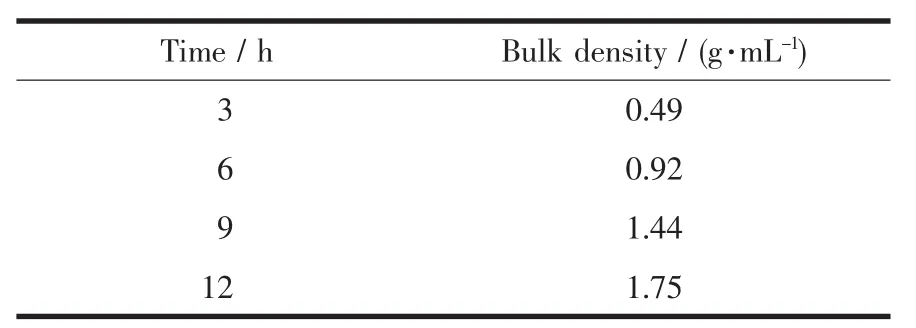
Table 3Bulk densities of the samples with different aging times
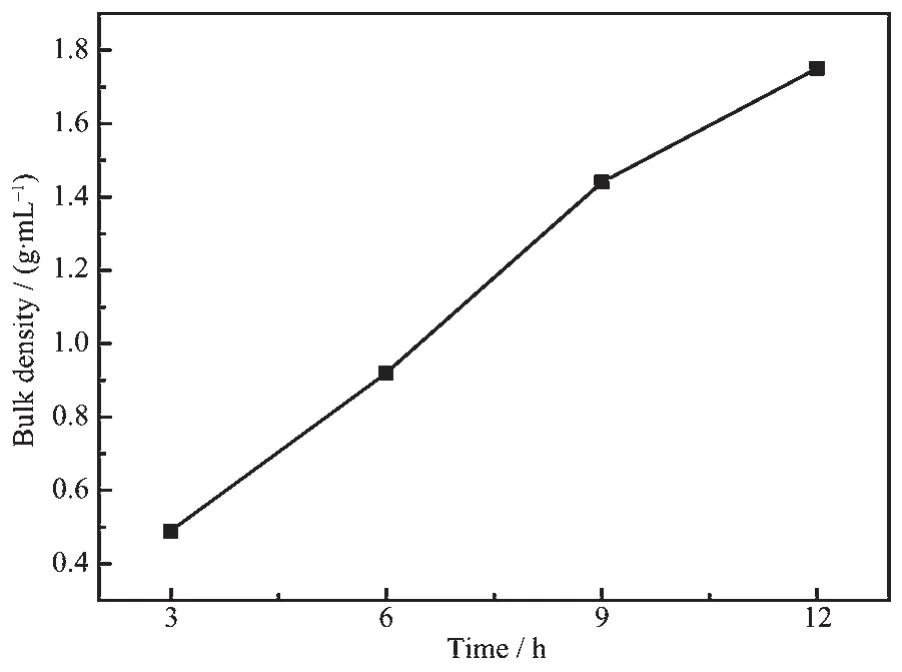
Fig.8Aging time-conversion relation of bulk density
3 Conclusions
(1)Under the same physical and chemical conditions,the sophericity and the relative crystallinity of Ni(OH)2particles are proportional to the aging time. The mathematical relationship between aging time and amorphization is A=-0.007 7T2+0.037 1T+0.653 2.
(2)Under the same physical and chemical conditions,the growth of(101)crystal plane has a greater influence on the sphericity of Ni(OH)2crystal than(100) crystal plane and contrary to the surface structure.
(3)Under the same physical and chemical conditions,the bulk densities increase with the increase of the aging time.The mathematical relationship between the bulk density and the aging time is ds=0.174T0.938.
(4)The aging time is proportional to the electrochemical activity of spherical Ni(OH)2.
[1]WANG Ji-Yang(王繼洋).Physics(物理),2001,6:332-339
[2]FENG Shi-Hong(馮世宏),JIA Tai-Xuan(賈太軒),DU Hui-Ling(杜慧玲),et al.Non-Ferrous Min.Metall.(有色礦冶), 2004,20(5):48-50
[3]Ma C Y,Liu J J,Wang X Z.Particuology,2016,2:1-18
[4]Lee C H,Lee C H.Korean J.Chem.Eng.,2005,22:712-716
[5]Huo Y,Liu T,Liu H,et al.Chem.Eng.Sci.,2016:126-139
[6]Bagheri G H,Bonadonna C,Manzella I,et al.Powder Technol., 2015,270:141-153
[7]Borchsert C,Sundmacher K.Chem.Eng.Technol.,2011,34: 545-556
[8]Ramesh T N.J.Phys.Chem.B,2009,113:13014-13017
[9]Deschamps J R.Life Sci.,2010,86:585-589
[10]Deschamps J R,George C.Trends Anal.Chem.,2003,22: 561-564
[11]Cachau R E,Podjarny A D.J.Mol.Recognit.,2005,18:196-202
[12]Wouters J,Ooms F.Curr.Pharm.Des.,2001,7:529-545
[13]Blundell T L,Jhoti H,Abell C.Nat.Rev.Drug Discovery, 2002,1:45-54
[14]XU Gang(許崗),LI Ying-Jun(李英俊),GU Zhi(谷智),et al. Chinese J.Inorg.Chem.(無機化學學報),2016,32(7):1135-1140
[15]Su Q F,Shi W M,Li D M,et al.Nucl.Instrum.Methods Phys.Res.Sect.A,2011,659:299-301
[16]TANG Jun-Jie(唐俊杰),LIU Yan(劉燕),TIAN Lei(田磊), et al.Chinese J.Inorg.Chem.(無機化學學報),2016,32(7): 1127-1134
Influence of Crystal Growth Direction Selectivity on Morphology and Electrochemical Activity of Spherical Nickel Hydroxide
TANG Jun-JieLIU Yan*TIAN LeiZHANG Li-LiWANG Dong-XingZHANG Ting-An
(Key Laboratory for Ecological Utilization of Multimetallic Mineral,Ministry of Education, Northeastern University,Shenyang 110819,China)
The spherical nickel hydroxide was synthesized by chemical precipitation method under the same physical and chemical conditions but different aging times.The morphologies of the spherical nickel hydroxide were characterized by SEM.It was found that with the increase of the aging time,the morphology of the spherical nickel hydroxidechanged from irregular crystals to regular spherical crystals.XRD patterns showed that when the aging time was 3 h,the growth of(001)crystal plane reached a steady state,and it did not transform with the increase of the aging time.However,(100)crystal plane and(101)crystal plane continued to grow with the increase of the aging time,and the relative crystallinity reached a maximum value.The shapes of the diffraction peaks were sharp and high when the aging time was 12 h,which indicated that the structural regularity strengthened.The influence of crystal growth direction selectivity on the morphology and electrochemical activity were discussed in the crystallization process of spherical nickel hydroxide.
spherical nickel hydroxide;crystal plane;diffraction peak;relative crystallinity
O614.81+3
A
1001-4861(2017)02-0354-07
10.11862/CJIC.2017.049
2016-09-07。收修改稿日期:2016-12-08。
國家自然科學基金云南聯合重點基金(No.U1402271,U1202274)資助項目。*
。E-mail:liuyan@smm.neu.edu.cn

