Preparation of Eu2+ Activated Barium Phosphosilicate Phosphors with Two-color Emission by A New Two-step Method
CHENG Zhi-yuan, ZHANG Yan-jie, YU Jing-jie, LI De-sheng, CAO Guan-ying, ZHAO Zuo-ren
(Research Institute of Photonics, Dalian Polytechnic University, Dalian 116034, China)
Preparation of Eu2+Activated Barium Phosphosilicate Phosphors with Two-color Emission by A New Two-step Method
CHENG Zhi-yuan, ZHANG Yan-jie*, YU Jing-jie*, LI De-sheng, CAO Guan-ying, ZHAO Zuo-ren
(Research Institute of Photonics, Dalian Polytechnic University, Dalian 116034, China)
A series of single phase Eu2+-activated barium phosphosilicates phosphors, Ba10-x(PO4)4(SiO4)2∶xEu2+, with apatite structure were successfully synthesized by a novel two-step method and they exhibited interesting two-color emission at 414 nm (blue) and 504 nm (green), respectively. As a significant contrast, the phosphors preparedviatraditional solid state reaction method, only showed single-color emission at 504 nm. The luminescent properties of these as-prepared phosphors are strongly dependent on the substitute sites of Eu2+in apatite crystal structure and the two-color emission was proved due to the occupation of Eu3+on two lattice sites of BaⅠand BaⅡin host material. Furthermore, the relative intensity of two emission peaks can be tuned readilyviaadjusting the substitution of BaⅠsite by Eu2+.
phosphor; single phase; photoluminescence; Ba10(PO4)4(SiO4)2
1 Introduction
Solid-state lighting based on white light-emitting diodes (LEDs) has been dramatically promoted and widely used in illumination fields because of its energy efficiency, long lifetime and non-mercury pollution[1-5]. Currently, phosphor-converted LEDs (pc-LEDs) consisted of a blue chip and a yellow phosphor (YAG∶Ce3+) are dominant in the market because of their low cost and simplicity in manufacturing. However, the lack of red component in blue InGaN-based LEDs results in a white light emitting with low color rendering index (Ra<80) and poor color reproducibility, which cannot meet the requirement of interior lighting[6-7]. Accordingly, a near-ultraviolet (NUV) chip combined with multi-component phosphors (Red, Green and Blue) has attracted much attention recently to achieve high color rendering index (Ra>90) and effectively avoid the uncomfortable glare[8-10]. Consequently, It is necessary to design and develop novel single-component phosphor with two- or three-color emitting due to the serious re-absorption caused by the mixing of multi-component phosphors[8,11-16].
Generally speaking, as for those phosphors with multi-color emission bands, the strategy is to use different rare earth ions and other ions (such as Mn2+) as co-activators. For example, Sr5(PO4)3Cl∶Eu2+,Mn2+was developed and used as a NUV excited LEDs phosphor[17]. A α-Ca2P2O7∶Eu2+,Mn2+phosphor with two emission bands centered at around 416 nm (blue) and 600 nm (orange) under NUV excitation at 400 nm is also reported[18]. In both samples, Mn2+ions are often acted as the red-emitting activator. Nowadays, the development of the phosphor with single activator is a new rising tendency for multi-color emission due to many advantages. For example, the energy loss generated by interaction between various compounds and the non-radioactive transition produced between the activator and different matrices components in multi-matrix phosphors can be reduced[19]. Shindeetal.[20]synthesized novel Dy3+activatedX6AlP5O20(X=Sr, Ba, Ca and Mg) phosphors, which show an efficient blue (485 nm) and yellow (573 nm) band emissions excited by 350 nm NUV light. Jeonetal.[21]developed a new full-color-emitting phosphor (Na2-x-Al2-xSixO4∶Eu2+, 0≤x≤1) based on the variation ofxvalue using a wet chemical reaction. Zhangetal.[22]investigated a novel blue emitting long lasting phosphorescence phosphor Sr5(PO4)3Cl∶Eu2+which showed two emission bands at 426 nm and 467 nm due to the different surrounding coordination of the two Sr2+(Eu2+) sites.
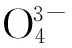
2 Experiments
2.1 Materials and Synthesis
The Ba10-x(PO4)4(SiO4)2∶xEu2+samples were prepared by a two-step solid state reaction method using BaCO3(A.R.), NH4H2PO4(A.R.), H2SiO3(A.R.) and Eu2O3as raw materials. According to the stoichiometric ratios, the raw materials were thoroughly mixed and ground in agate mortar. The two-step solid state reaction method (Two-step method) is described as follows. In the first step, the mixture was transferred into an alumina crucible and then loaded into a tube furnace. The samples were sintered at 1 170 ℃ for 4 h in air. In the second step, the samples obtained after the first step were reduced at 1 170 ℃ for 2 h in an atmosphere of H2(10%) and N2(90%). The influence of temperature was also investigated by a different sintering temperature (800 ℃) in the first step and reducing temperature (850 ℃) in the second step. After the two-step method, the samples were cooled to room temperature in the reducing atmosphere. Then, the products were ground using an agate mortar.
In comparison with the samples prepared by the two-step method, Ba10-x(PO4)4(SiO4)2∶xEu2+phosphors were also synthesized by a traditional solid state reaction method (One-step method) at 1 170 ℃ for 4 h in a reducing atmosphere of H2(10%) and N2(90%).
2.2 Characterizations
The phases of the resulting powders were identified by X-ray powder diffraction (XRD) with a Bruker D8 Advance diffractometer with Cu Kα radiation (λ=0.153 74 nm) operating at 40 mA and 40 kV. Photoluminescence (PL) excitation and emission spectra were recorded by a HITACH F-4500 fluorescence spectrophotometer at room temperature. Morphology of the phosphors were observed by field emission electron microscopy (FESEM, JSM-7800F, JEOL).
3 Results and Discussion
3.1 Phase Component and Structure
Fig.1 presents the X-ray diffraction pattern of typical Ba9.7(PO4)4(SiO4)2∶0.3Eu2+samples obtained by one-step method and two-step method together with the corresponding standard JCPDS card. For both as-synthesized samples, the XRD peaks marked by ◆ in Fig.1 can be well indexed by the standard JCPDS card (JCPDS No. 24-0028) of Ba10(PO4)6(OH)2, which indicates that samples obtained by either way have an isostructure with Ba10-(PO4)6(OH)2. The other XRD peaks are attributed to the replacement of SiO44-on the basis of the JCPDS (JCPDS No.70-2113) of Ba2SiO4. Ba10(PO4)6(OH)2apatite crystallizes in the hexagonal system with the space groupP63/m. According to the crystal structure of Ba10(PO4)6(OH)2, as shown in Fig.2, there are two non-equivalent cationic sites (BaⅠand BaⅡ) for Ba2+with different positions. BaⅠsite (4f,C3point symmetry) and BaⅡsite (6h,Cspoint symmetry) are coordinated with 9 and 7 oxygen atoms, respectively[12].
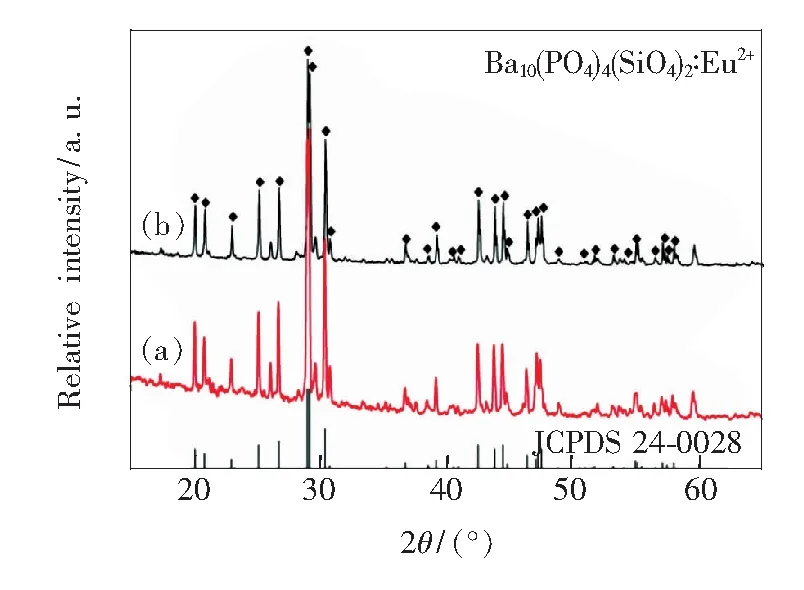
Fig.1 XRD patterns of the typical Ba9.7(PO4)4(SiO4)2∶0.3Eu2+phosphor prepared by one-step method (a) and two-step method (b) compared with the standard patterns of Ba10(PO4)4(SiO4)2(JCPDS No. 24-0028).
The diffraction peaks marked by ◆ are corresponding to Ba10(PO4)6(OH)2.
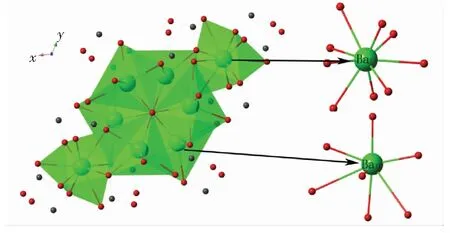
Fig.2 Unit cell structure of Ba10(PO4)6(OH)2and coordination environment of Ba2+ions in the host lattice
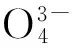
Fig. 3 presents the SEM images of samples obtained by one-step method and two-step method, respectively. No significant difference of morphology can be observed for Fig. 3(a) and 3(b). The particles show an irregular morphology with particle size of 5-20 μm. Therefore, it can be concluded from the results of XRD and SEM that the samples prepared by one-step method and two-step method exhibit similar crystal structure and morphology of Ba10(PO4)4(SiO4)2host.
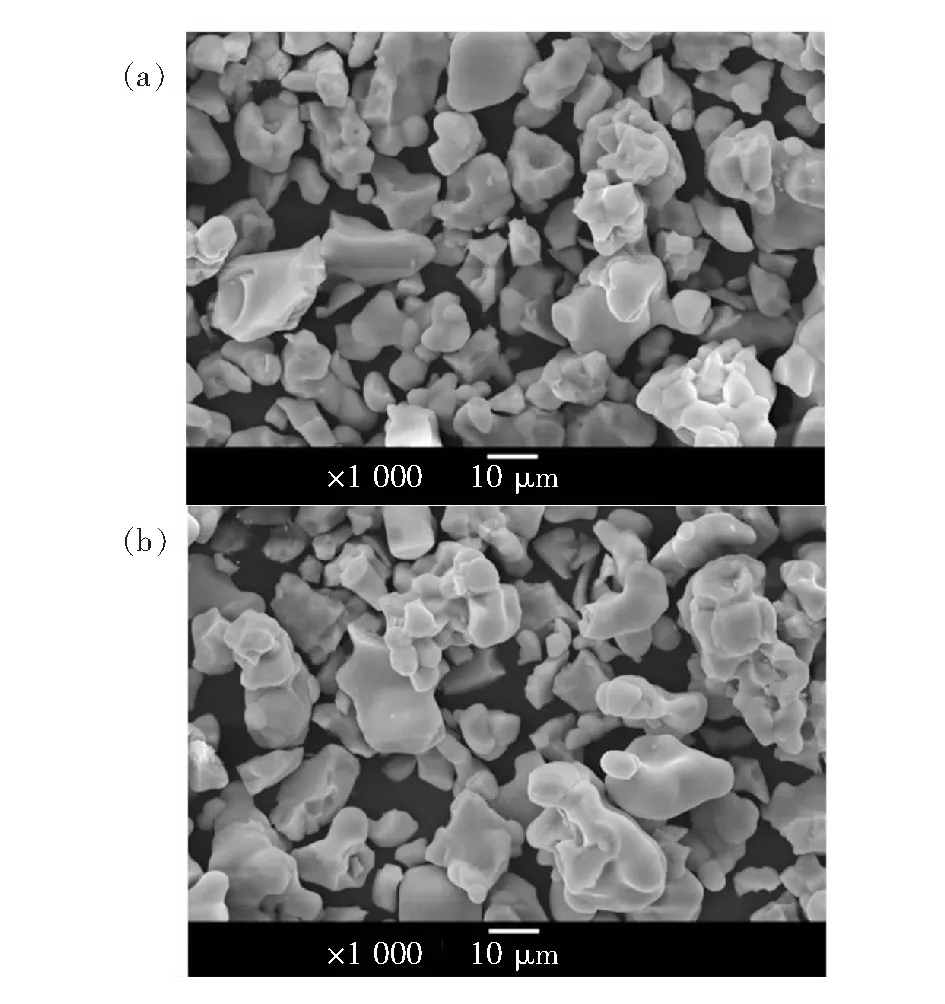
Fig.3 SEM images of the samples obtained by one-step method (a) and two-step method (b), respectively.
3.2 Influence of Preparation Method on Photoluminescence Properties
For comparison, a traditional solid state reaction method (one-step method) has also been employed to prepare Ba10-x(PO4)4(SiO4)2∶xEu2+phosphors. Fig.4 presents the differences between emission spectra of Ba10-x(PO4)4(SiO4)2∶xEu2+phosphors (x=0.3 and 0.75) preparedviaone-step and two-step method, respectively. In Fig.4(a), two emission bands centered at 414 nm and 505 nm can be observed under the excitation of 350 nm for Ba9.7(PO4)4(SiO4)2∶0.3Eu2+sample prepared by two-step method. The double emission band is consistent with the different substitution sites of Eu2+in host material of Ba10(PO4)4(SiO4)2crystal. Photoluminescence of Eu2+ions generates from the electron transition between 4f7and 4f65d configuration. 4f electrons shielded by 5s and 5p electron cloud can be barely influenced by crystal environment; however, the energy level of unprotected 5d electron can be splitted by the force of crystal field. It is well known that Eu2+located at smaller site will experience a larger crystal field splitting, which leads to the emission band being shifted to a longer wavelength[22]. Therefore, the emission band at 505 nm can be attributed to the substitution of BaⅡsite and another weak one at 414 nm can be assigned to Eu2+occupying BaⅠsite. The sample obtained by one-step method only exhibits one emission band at 502 nm, which shows a large Stokes shift (ΔS≈8 650 cm-1) and full width at half-maximum (FWHM ≈ 2 432 cm-1), indicating that this peak can be attributed to the anomalous fluorescent emission of Eu2+[12,23].
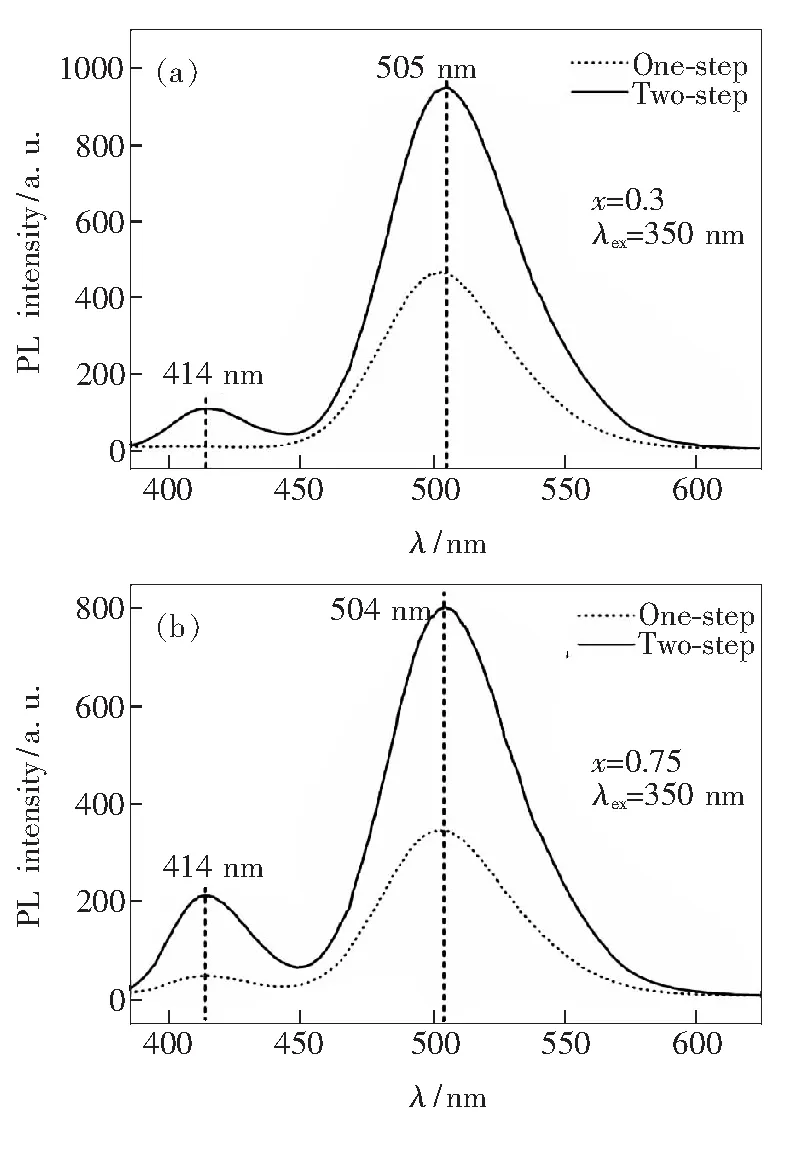
Fig.4 PL emission spectra of Ba9.7(PO4)4(SiO4)2∶0.3Eu2+(a) and Ba9.25(PO4)4(SiO4)2∶0.75Eu2+(b) under the excitation of 350 nm. The samples are synthesized by two-step and one-step methods, respectively.
With increasing the Eu2+doping content, Ba9.25(PO4)4(SiO4)2∶0.75Eu2+phosphor obtained by one-step method also shows a weak emission band at 414 nm in addition to 505 nm (Fig.4(b)). This indicates that an increase of Eu2+doping content in host can facilitate the substitution of BaⅠsite. In comparison with the phosphor prepared by one-step method, Ba9.25(PO4)4(SiO4)2∶0.75Eu2+phosphor synthesized by two-step method displays much higher emission band intensity at 414 nm and 504 nm (Fig.4(b)), which shows a similar tendency for Ba9.7(PO4)4(SiO4)2∶0.3Eu2+sample (Fig.4(a)). The above results indicate that the luminescence property of the Ba10-x(PO4)4(SiO4)2:xEu2+phosphor in this research is strongly dependent on the preparation method. Two-step method dramatically enhances the emission band intensity, and, in the first step, makes it easier for Eu3+to occupy the BaⅠsite with less O2-ligands because of smaller iron radius (0.101 nm) than Eu2+(0.120 nm), which creates better opportunity for luminescence of Eu2+locating at BaⅠsite after reduction.
3.3 Formation Mechanism of The Two-color Emission by Two-step Method
The major difference between the one-step method and two-step method for preparation of the phosphors is the formation of the intermediates after the first step during two-step method. When substituting Ba2+with Eu3+ions[36-37], charge balance is not disturbed because introduced flux can be used for charge compensation. Fig.5 shows the emission spectra of the intermediates obtained after the first step. The5D0-7FJ(J=0-4) transitions of Eu3+are present under NUV excitation of 394 nm: emissions peaking at 613.4 nm and 624.8 nm can be ascribed to the transition of5D0-7F2and those peaking at 585, 591 and 595.8 nm are related to the transition of5D0-7F1[l]. Obviously, the red emission transition from5D0-7F2is dominant in the spectra, which indicates that Eu3+mainly occupy the asymmetric site (BaⅡsite withCspoint symmetry). The abnormal5D0-7F0transition of Eu3+also appears based on the emission peak at 577 nm in Fig. 5. Normally, the5D0-7F0transition is forbidden and can only be present when Eu3+occupying both the sites ofC3andCs[38]. In our cases, two lattice sites of BaⅠ(C3point symmetry) and BaⅡ(Cspoint symmetry) in host material have been occupied by Eu3+during the first step of two-step solid state method. Furthermore, Eu2+ions will similarly occupy two lattice sites of BaⅠand BaⅡafter Eu3+ions in the intermediates are reduced during the second step process and an expected two-color emission can be finally obtained[23].
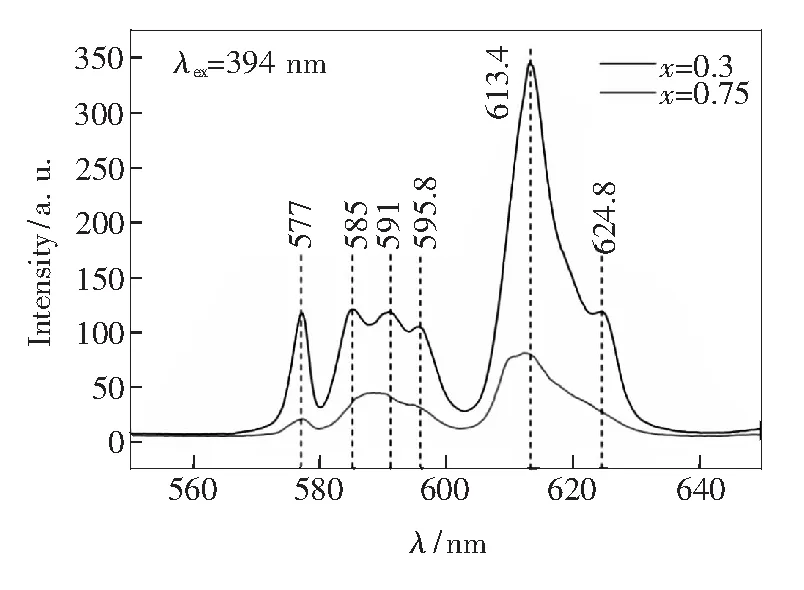
Fig.5 PL emission spectra of the sample after first preparation step forx=0.3 and 0.75 excited by 394 nm
The influence of sintering temperature in the first step and reducing temperature in the second step on luminescence properties of products has also been investigated, as shown in Fig.6. In Fig.6(a), the comparison of a lower sintering temperature of 800 ℃ in the first step gives a lower emission band intensity of Ba9.25(PO4)4(SiO4)2∶0.75Eu2+phosphor, which is probably related to the decreased crystallization of host material. Similarly, a decreased intensity of the emission band can also be observed when a low reducing temperature is employed in the second step (Fig. 6(b)).
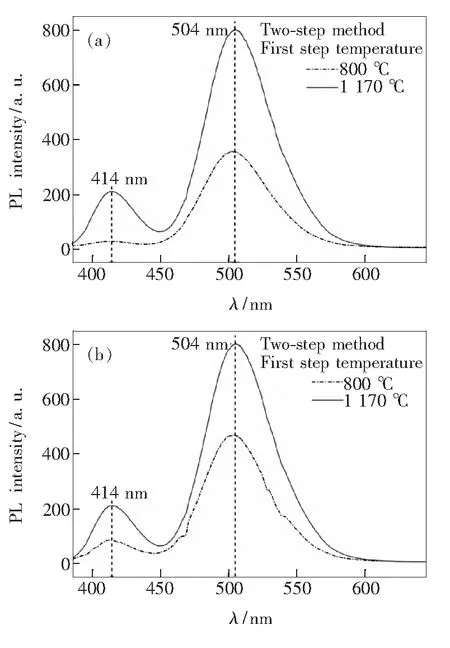
Fig.6 PL emission spectra of Ba9.25(PO4)4(SiO4)2∶0.75Eu2+phosphor under different sintering temperature in the first step (a) and reducing temperature in the second step(b) on luminescence properties of products
3.4 Effect of Eu2+Doping Concentrations on Emission Spectra
In general, energy transfer can only occur if the sensitizer emission spectra overlap the activator adsorption spectra for unlike luminescent centers. In the case of Ba9.0(PO4)4(SiO4)2∶1.0Eu2+, the emission peak at 414 nm from EuⅠsite overlaps the excitation peak of Band Ⅱ from EuⅡsite in Fig.7. Therefore, energy transfer from EuⅠto EuⅡis considered to exist in Ba10-x(PO4)4(SiO4)2∶xEu2+phosphors.
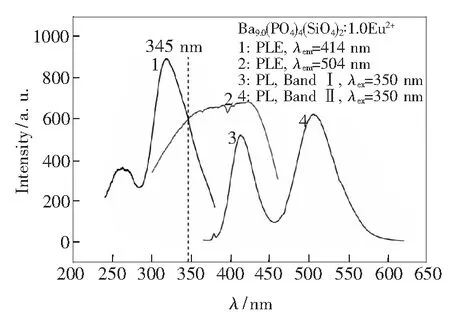
Fig.7 PL emission and excitation spectra of Ba9.0(PO4)4-(SiO4)2∶1.0Eu2+phosphor
Fig.8(a) presents the dependence of emission intensity of Ba10-x(PO4)4(SiO4)2∶xEu2+on Eu2+doping concentration (x=0.2-1.0). The emission intensity at 414 nm (Band Ⅰ) increases with an increased Eu2+doping concentration fromx=0.2 tox=1.0 because of the more substitution of BaⅠsite. While, the intensity of Band Ⅱ shows a peculiar increase whenx=0.3 and then decreases along with the changes ofxvalue (from 0.3 to 1.0) in Fig.8(a). The peculiar increase whenx=0.3 for Band II is attributed to the energy transfer from EuⅠto EuⅡ. The plot of the relative intensity of these two emission band as a function of Eu2+doping concentration is summarized in Fig.8(b). It can be obviously concluded that high Eu2+doping concentration greatly enhances the relative intensity of the band at 414 nm because more BaⅠsites have been occupied by Eu2+in the host material.
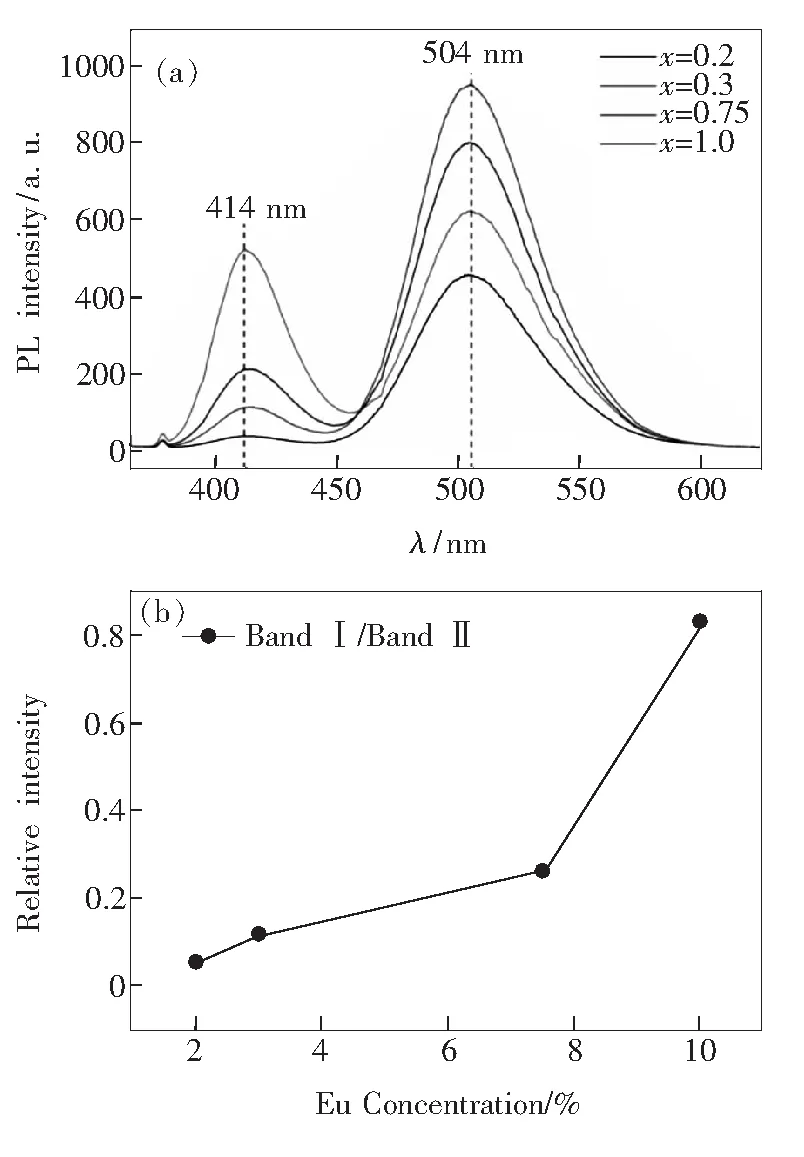
Fig.8 (a) PL emission spectra of Ba10-x(PO4)4(SiO4)2∶xEu2+with different Eu2+doping concentration. (b) Curves of Eu2+doping concentration and relative intensity of Band Ⅰ/Band Ⅱ.
4 Conclusion
In summary, a series of Ba10-x(PO4)4(SiO4)2∶xEu2+phosphors with expected double emission peaks at 414 nm and 504 nm using a 350 nm excitation wavelength were prepared by a new two-step solid state reaction method. The luminescent properties of the phosphors are strongly dependent on the substitute sites of Eu2+in apatite crystal structure. It can be concluded from the PL emission spectra of the sample obtained after first step that Eu3+ions occupied two lattice sites of BaⅠ(C3point symmetry) and BaⅡ(Cspoint symmetry) in host material. The Eu3+ions are reduced during the second step process and the two-color emission can be finally obtained. PL spectra of Ba10-x(PO4)4(SiO4)2∶xEu2+(x=0.2-1.0) also reveal the dependence of the relative intensity of two emission peaks on Eu2+doping. The emission intensity of the band at 414 nm increases whenxis varied from 0.2 to 1.0, which can be attributed to the more substitution of BaⅠsite by Eu2+. The excitation spectra of Ba9.25(PO4)4(SiO4)2∶0.75Eu2+phosphor is located at a large range (250-420 nm) in UV region.
Acknowledgements:We gratefully acknowledge the technical support of Prof. Chun-shan SHI from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences.
[1] H?PPE H A. Recent developments in the field of inorganic phosphors [J].Angew.Chem.Int.Ed., 2009, 48(20):3572-3582.
[2] XIE R J, HIROSAKI N. Silicon-based oxynitride and nitride phosphors for white LEDs—a review [J].Sci.Technol.Adv.Mater., 2007, 8(7-8):588-600.
[3] VANITHAKUMARI S C, NANDA K K. A one-step method for the growth of Ga2O3-nanorod-based white-light-emitting phosphors [J].Adv.Mater., 2009, 21(35):3581-3584.
[4] LIN C C, LIU R S. Advances in phosphors for light-emitting diodes [J].J.Phys.Chem.Lett., 2011, 2(11):1268-1277.
[5] KIM J S, JEON P E, CHOI J C,etal.. Warm-white-light emitting diode utilizing a single-phase full-color Ba3MgSi2O8∶Eu2+, Mn2+phosphor [J].Appl.Phys.Lett., 2004, 84(15):2931-2933.
[6] DAICHO H, IWASAKI T, ENOMOTO K,etal.. A novel phosphor for glareless white light-emitting diodes [J].Nat.Commun., 2012, 3:1132.
[7] WATANABE H, WADA H, SEKI K,etal.. Synthetic method and luminescence properties of SrxCa1-xAlSiN3∶Eu2+mixed nitride phosphors [J].J.Electrochem.Soc., 2008, 155(3):F31-F36.
[8] NISHIDA T, BAN T, KOBAYASHI N. High-color-rendering light sources consisting of a 350-nm ultraviolet light-emitting diode and three-basal-color phosphors [J].Appl.Phys.Lett., 2003, 82(22):3817-3819.
[9] TAKAHASHI K, HIROSAKI N, XIE R J,etal.. Luminescence properties of blue La1-xCexAl(Si6-zAlz)(N10-zOz)(z~1) oxynitride phosphors and their application in white light-emitting diode [J].Appl.Phys.Lett., 2007, 91(9):091923-1-3.
[10] QIAN F J, FU R L, AGATHOPOULOS S,etal.. Synthesis and luminescence properties of a broad-band red phosphor Ca3Si2O7∶Eu2+for warm white light-emitting diodes [J].J.Lumin., 2012, 132(1):71-75.
[11] LI K, GENG D L, SHANG M M,etal.. Color-tunable luminescence and energy transfer properties of Ca9Mg(PO4)6F2∶Eu2+, Mn2+phosphors for UV-LEDs [J].J.Phys.Chem. C, 2014, 118(20):11026-11034.
[12] YU J J, GONG W T, ZHANG Y J,etal.. White-light-emitting diode using a single-phase full-color (Ba, Sr)10(PO4)4(SiO4)2∶Eu2+phosphor [J].J.Lumin., 2014, 147:250-252.
[13] RAVINDRANADH K, BABU B, PUSHPA MANJARI V,etal.. Optical and structural properties of undoped and Mn2+doped Ca-Li hydroxyapatite nanopowders using mechanochemical synthesis [J].J.Lumin., 2015, 159:119-127.
[14] CHEN X, DAI P P, ZHANG X T,etal.. A highly efficient white light (Sr3, Ca, Ba)(PO4)3Cl∶Eu2+, Tb3+, Mn2+phosphorviadual energy transfers for white light-emitting diodes [J].Inorg.Chem., 2014, 53(7):3441-3448.
[15] JIAO M M, JIA Y C, Lü W,etal.. Structure and photoluminescence properties of novel Ca2NaSiO4F:Re(Re=Eu2+, Ce3+, Tb3+) phosphors with energy transfer for white emitting LEDs [J].J.Mater.Chem. C, 2014, 2(21):4304-4311.
[16] HUANG C H, CHEN T M. A novel single-composition trichromatic white-light Ca3Y(GaO)3(BO3)4∶Ce3+, Mn2+, Tb3+phosphor for UV-light emitting diodes [J].J.Phys.Chem. C, 2011, 115(5):2349-2355.
[17] GUO C, LUAN L, DING X,etal.. Luminescent properties of Sr5(PO4)3Cl∶Eu2+, Mn2+as a potential phosphor for UV-LED-based white LEDs [J].Appl.Phys. B, 2009, 95(4):779-785.
[18] HAO Z D, ZHANG J H, ZHANG X,etal.. White light emitting diode by usingα-Ca2P2O7∶Eu2+, Mn2+phosphor [J].Appl.Phys.Lett., 2007, 90(26):261113-1-3.
[19] YANG W J, CHEN T M. Ce3+/Eu2+codoped Ba2ZnS3: a blue radiation-converting phosphor for white light-emitting diodes [J].Appl.Phys.Lett., 2007, 90(17):171908-1-3.
[20] K. N. SHINDE K N, DHOBLE S J, KUMAR A. Eu3+activatedM6AlP5O20(M=Sr/Ba/Mg) novel red phosphors [J].J.Lumin., 2011, 131(9):1939-1944.
[21] HAN J Y, IM W B, KIM D,etal.. New full-color-emitting phosphor, Eu2+-doped Na2-xAl2-xSixO4(0≤x≤1), obtained using phase transitions for solid-state white lighting [J].J.Mater.Chem., 2012, 22(12):5374-5381.
[22] WU C Q, ZHANG J C, FENG P F,etal.. Blue photoluminescence and long lasting phosphorescence properties of a novel chloride phosphate phosphor∶Sr5(PO4)3Cl∶Eu2+[J].J.Lumin., 2014, 147:229-234.
[23] YU J J, GONG W T, XIAO Z G,etal.. Spectral structure of barium-phosphate-silicate phosphor Ba10(PO4)4(SiO4)2∶EuM+[J].J.Lumin., 2012, 132(11):2957-2960.
[24] KOTTAISAMY M, JAGANNATHAN R, JEYAGOPAL P,etal.. Eu2+luminescence inM5(PO4)3Xapatites, whereMis Ca2+, Sr2+and Ba2+, andXis F-, Cl-, Br-and OH-[J].J.Phys. D:Appl.Phys., 1994, 27(10):2210-2215.
[25] N?TZOLD D, WULFF H, HERZOG G. Structural and optical properties of the system (Ca, Sr, Eu)5(PO4)3Cl [J].Phys.Stat.Sol.(b), 1995, 191(1):21-30.
[26] RAMESH R, JAGANNATHAN R. Optical properties of Ce3+in self-assembled strontium chloro(hydroxy)apatite nanocrystals [J].J.Phys.Chem. B, 2000, 104(35):8351-8360.
[27] SONG Y H, YOU H P, YANG M,etal.. Facile synthesis and luminescence of Sr5(PO4)3Cl∶Eu2+nanorod bundlesviaa hydrothermal route [J].Inorg.Chem., 2010, 49(4):1674-1678.
[28] AL-KATTAN A, DUFOUR P, DEXPERT-GHYS J,etal.. Preparation and physicochemical characteristics of luminescent apatite-based colloids [J].J.Phys.Chem. C, 2010, 114(7):2918-2924.
[29] TAO Z X, HUANG Y L, SEO H J. Blue luminescence and structural properties of Ce3+-activated phosphosilicate apatite Sr5(PO4)2(SiO4) [J].DaltonTrans., 2012, 42(6):2121-2129.
[30] KIM D, PARK D, OH N,etal.. Luminescent properties of rare earth fully activated apatites, LiRE9(SiO4)6O2(RE=Ce, Eu, and Tb): site selective crystal field effect [J].Inorg.Chem., 2015, 54(4):1325-1336.
[31] XIA Z G, MOLOKEEV M S, IM W B,etal.. Crystal structure and photoluminescence evolution of La5(Si2+xB1-x)(O13-xNx)∶Ce3+solid solution phosphors [J].J.Phys.Chem. C, 2015, 119(17):9488-9495.
[32] BOYER L, PIRIOU B, CARPENA J,etal.. Study of sites occupation and chemical environment of Eu3+in phosphate-silicates oxyapatites by luminescence [J].J.AlloysCompd., 2000, 311(2):143-152.
[33] BACHMANN V, RONDA C, OECKLER O,etal.. Color point tuning for (Sr, Ca, Ba)Si2O2N2∶Eu2+for white light LEDs [J].Chem.Mater., 2009, 21(2):316-325.
[34] LU S Z, ZHANG J S. Study on UV excitation properties of Eu3+-doped rare-earth phosphates [J].J.Lumin., 2007, 122-123:500-502.
[35] NAGPURE I M, PITALE S S, COETSEE E,etal.. Lattice site dependent cathodoluminescence behavior and surface chemical changes in a Sr5(PO4)3F host [J].Phys. B:Condens.Matter, 2012, 407(10):1505-1508.
[36] BOUZIDI C, FERHI M, ELHOUICHET H,etal.. Spectroscopic properties of rare-earth (Eu3+, Sm3+) doped BaWO4powders [J].J.Lumin., 2015, 161:448-455.
[37] MA P C, SONG Y H, SHENG Y,etal.. Single-component and white light-emitting phosphor BaAl2Si2O8∶Dy3+, Eu3+synthesis, luminescence, energy transfer, and tunable color [J].Opt.Mater., 2016, 60:196-203.
[38] ZHANG Z W, LIU L, SONG S T,etal.. A novel red-emitting phosphor Ca9Bi(PO4)7∶Eu3+for near ultraviolet white light-emitting diodes [J].Curr.Appl.Phys., 2015, 15(3):248-252.

程志遠(1989-),男,山東聊城人,碩士研究生,2010年于山東大學獲得學士學位,主要從事白光LED熒光粉的研究。
E-mail: 381136875@qq.com

于晶杰(1974-),女,遼寧大連人,博士,教授級高級工程師,2013年于大連理工大學獲得博士學位,主要從事半導體照明發光材料以及光源器件的研究。
E-mail: yujingjie@dlpu.edu.cn

張彥杰(1981-),男,山西祁縣人,博士,副研究員,2008年于中國科學院蘭州化學物理研究所獲得博士學位,主要從事白光LED用新型高效發光材料的研究。
E-mail: zhang_yj@dlpu.edu.cn
2016-12-07;
2017-01-21
遼寧省自然科學基金(20170540074); 大連工業大學博士啟動基金 (61020726)資助項目 Supported by Natural Science Foundation of Liaoning Province (20170540074); Start-up Funding for Doctoral Researchers of Dalian Polytechnic University (61020726)
單一基質雙光色Ba10-x(PO4)4(SiO4)2∶xEu2+熒光粉的兩步法制備與光譜調控
程志遠, 張彥杰*, 于晶杰*, 李德勝, 曹冠英, 趙作人
(大連工業大學 光子學研究所, 遼寧 大連 116034)
采用兩步法成功合成了單一基質雙光色Ba10-x(PO4)4(SiO4)2∶xEu2+熒光粉,研究了稀土離子占據不同的晶格格位對熒光粉光譜特性的影響。結果表明:兩步法合成的熒光粉發射光譜由414 nm的藍光波帶和504 nm綠光波帶兩種光色組成,而傳統的高溫固相法制備的熒光粉只有504 nm處的綠光發射。熒光粉發光性能與Eu2+離子在磷灰石晶體結構中占據的晶格位置關系十分密切。兩步法熒光粉雙光色的形成主要是由于在第一步氧化氣氛合成過程中Eu3+離子取代了基質結構中的BaⅠ和BaⅡ兩個格位的Ba2+離子;在第二步還原過程結束后,Eu2+離子仍然占據著兩種格位,從而形成了兩種具有不同配位環境的發光中心。此外,雙發射峰的相對強度能夠通過Eu2+離子對BaⅠ格位的取代率而調節,進而實現光譜的調變。
熒光粉; 單基質; 光致發光; Ba10(PO4)4(SiO4)2
1000-7032(2017)07-0874-08
O611.65 Document code: A
10.3788/fgxb20173807.0874
*Corresponding Authors, E-mail: yujingjie@dlpu.edu.cn; zhang_yj@dlpu.edu.cn

