乙型肝炎病毒剪接特異性蛋白HBSP與TGFβ1誘導蛋白1相互作用促進TGFβ1誘導的肝癌細胞上皮間質轉化
陳婉南,黃俊高,梁菲菲,閆小利,軒丹丹,林 旭
?
乙型肝炎病毒剪接特異性蛋白HBSP與TGFβ1誘導蛋白1相互作用促進TGFβ1誘導的肝癌細胞上皮間質轉化
陳婉南,黃俊高,梁菲菲,閆小利,軒丹丹,林 旭
目的 探討乙型肝炎病毒剪接特異性蛋白(Hepatitis B spliced protein, HBSP)與轉化生長因子1誘導蛋白1(transforming growth factor-β1-induced transcript 1,TGFβ1I1)相互作用對TGFβ1誘導的肝癌細胞上皮間質轉化(epithelial-mesenchymal transition,EMT)的影響。方法 構建HBSP慢病毒表達載體,利用293T細胞包裝慢病毒顆粒,感染Huh7肝癌細胞株。以5 ng/mLTGFβ1分別誘導穩定表達HBSP的慢病毒細胞株及其對照細胞株,觀察細胞形態的變化,并提取細胞蛋白,Westernblot檢測上皮間質轉化標志物E-鈣黏素(E-cadherin,E-cad)、緊密連接蛋白(Claudin-1)、β-鏈蛋白(β-catenin)、N-鈣黏素(N-cadherin,N-cad)的變化。進而以TGFβ1I1特異性siRNA轉染上述細胞,Westernblot觀察以上指標變化情況。最后以侵襲小室實驗和劃痕實驗分別檢測TGFβ1誘導的細胞侵襲與遷移能力的變化。結果 篩選獲得穩定表達HBSP的慢病毒細胞株Huh7-HBSP-flag-HIV及其對照細胞株Huh7-flag-HIV。以TGFβ1誘導后在顯微鏡下觀察到細胞形態由緊密的上皮形態變為松散的間質形態;特異性抗體檢測表明上皮標志物E-cad、Claudin-1、β-catenin表達量下降,而間質標志物N-cad表達上升。侵襲小室實驗和劃痕實驗表明TGFβ1誘導的HBSP表達株侵襲及遷移能力增強。而轉染TGFβ1I1特異性siRNA可逆轉以上現象。結論 HBSP與TGFβ1I1相互作用可促進TGFβ1誘導的肝癌細胞上皮間質轉化并增強其侵襲遷移能力,提示HBSP在HBV相關性肝細胞肝癌發生發展中具有重要的致病意義。
乙型肝炎病毒;RNA剪接;轉化生長因子β1誘導蛋白1;上皮間質轉化;侵襲
乙型肝炎病毒剪接特異蛋白HBSP由HBV前基因組RNA (pregenomic RNA, pgRNA)在2 447 nt-489 nt發生RNA剪接產生的長度為2.2 kb的剪接變異體編碼產生,已有研究表明該蛋白在HBV感染不同病程均可檢出,與病毒的持續性感染及致病性相關[1-4]。HBSP定位于細胞漿[5],為了深入闡明HBSP在HBV導致肝癌發生發展的過程中具體的致病機制,本實驗室在前期工作中采用細胞質酵母雙雜交(CytoTrapTMtwo-hybrid system,Stratagene)篩選的方法,發現HBSP可以和轉化生長因子1誘導蛋白1相互作用[6]。已有研究表明,TGFβ1I1可以促進鼠和人的上皮細胞發生上皮間質轉化,對腫瘤的發生發展有著重要意義[7]。本文旨在通過研究HBSP與TGFβ1I1相互作用對肝癌細胞株上皮間質轉化以及侵襲性的影響,更好地闡明HBV導致肝癌發生發展的機制。
1 材料和方法
1.1 材料
1.1.1 質粒與細胞 Huh7肝癌細胞株由本實驗室保存。人胚腎293T細胞株、慢病毒表達載體pCDH-EF1-MCS-T2A-Puro與包裝質粒pMDL、pVSVG、pREV由廈門大學吳喬教授惠贈。
1.1.2 主要試劑 高保真Taq DNA、轉染試劑Lipofectamine 3000聚合酶購自Invitrogen公司,限制性內切酶和T4 DNA連接酶購自NEB公司,質粒提取試劑盒購自Qiagen公司。DMEM高糖培養基、胰酶、Puromycin抗生素購自Gibco公司,胎牛血清購自PAN公司,磷酸鈣轉染試劑盒、anti-TGFβ1I1抗體和Boyden Transwell侵襲小室購自BD公司。TGFβ1購自Sigma-Aldrich公司,TGFβ1I1特異性siRNA混合物及無關序列對照NC-siRNA、兔二抗購自Santa Cruz公司。RIPA裂解液、BCA蛋白定量試劑盒購自碧云天生物技術公司,PVDF膜購自Millipore公司,CDP-STAR化學發光檢測底物購自Roche公司。anti-E-cadherin,anti-N-cadherin,anti-β-catenin,anti-Claudin-1抗體、anti-Flag抗體購自Cell Signaling Technology公司,anti-β-actin抗體購自康為世紀,anti-alpha Smooth Actin抗體、anti-Fibronectin抗體購自Abcam公司,鼠二抗購自Calbiochem公司。
1.2 方法
1.2.1 慢病毒載體pCDH-HBSP的構建 以pSos-HBSP為模板[6],PCR擴增獲得C末端帶有FLAG標簽的HBSP基因,插入慢病毒表達載體pCDH-EF1-MCS-T2A-Puro,獲得重組載體pCDH-HBSP-FLAG。空載體對照pCDH-FLAG為將8個氨基酸大小的FLAG編碼基因插入上述慢病毒表達載體獲得。
1.2.2 慢病毒包裝與感染、篩選穩定表達HBSP的肝癌細胞株 使用以下質粒混合物、按照試劑盒說明書以磷酸鈣轉染法轉染293T細胞:pCDH-HBSP-FLAG或pCDH-FLAG 2 μg、pMDL 1 μg、pVSVG 0.6 μg、pREV 0.4 μg, 48 h后收集含有病毒的細胞培養上清液,離心過濾后均勻滴加至Huh7肝癌細胞中,以1 μg/mL Puromycin抗生素篩選培養2周,取106個細胞接種于60 mm培養皿。次日以RIPA裂解液提取細胞總蛋白,以anti-FLAG抗體(1∶2 000稀釋)檢測目的蛋白HBSP的表達。其余細胞凍存至-80 ℃備用。
1.2.3 TGFβ1處理穩定表達HBSP的肝癌細胞株
將經過鑒定穩定表達HBSP的Huh7細胞株于加藥處理前一天接種5×105個細胞于35 mm板。次日用PBS清洗后換成不含血清的DMEM培養液,加入TGFβ1(終濃度為5 ng/mL)作用24 h后觀察細胞形態的變化。
1.2.4 Westernblot檢測TGFβ1處理后EMT相關標志物 同上以5 ng/mL的TGFβ1處理HBSP表達細胞株,24 h后提取細胞總蛋白,以EMT標志物E-cad,N-cad,β-catenin,Claudin-1抗體(均為1∶1 000稀釋)檢測、Quantity One軟件(Biorad)分析計算蛋白條帶的灰度值。以各組蛋白條帶的灰度值相對于內參β-actin條帶的灰度值進行標準化。實驗重復5次取平均值作圖分析。
1.2.5 TGFβ1I1特異性siRNA轉染,Westernblot檢測EMT指標 同上以5 ng/mL的TGFβ1處理HBSP表達細胞株,24 h后采用Lipofectamine 3000轉染TGFβ1I1特異性siRNA混合物,使用無關序列NC-siRNA作為對照。轉染方法參照試劑說明書,轉染后24 h提取細胞總蛋白,同上檢測EMT相關標志物。實驗重復5次取平均值作圖分析。
1.2.6 侵襲小室實驗 HBSP表達細胞株以5 ng/mL的TGFβ1處理24 h后,用TGFβ1I1特異性siRNA混合物以及對照NC-siRNA轉染細胞24 h,以無血清培養液吹打混勻消化后的細胞,分別計數5 ×104個細胞接種到Boyden Transwell小室內,36 h,后用 0.1 %結晶紫染液染色,在倒置顯微鏡下隨機取5個視野計數細胞取平均值并分別拍照,實驗重復5次。
1.2.7 劃痕實驗 同上獲得TGFβ1處理并使用TGFβ1I1特異性siRNA混合物轉染的HBSP表達細胞株,接種于6孔板次日用滅菌槍頭在培養皿底部垂直劃出一道直線,在倒置顯微鏡下隨機取5個視野拍照并測量劃痕寬度并取平均值,繼續無血清培養48 h后,拍照測量劃痕寬度取平均值,實驗重復5次。
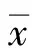
2 結 果
2.1 慢病毒感染Huh7細胞,篩選獲得穩定表達HBSP的肝癌細胞株 PCR擴增HBSP基因,經XbaI和BamH I雙酶切位點克隆入慢病毒表達載體pCDH-EF1-MCS-T2A-Puro,重組載體經酶切(圖1A)及測序證實插入的HBSP DNA片段正確無誤,命名為pCDH-HBSP-FLAG。包裝載體pMDL、pVSVG、pREV,以及pCDH-HBSP-FLAG或空載體對照pCDH-FLAG共轉染293T細胞;獲得慢病毒液,感染并以Puromycin篩選Huh7細胞,以anti-FLAG抗體檢驗目的蛋白的表達(圖1B),獲得的穩定表達HBSP的細胞株命名為Huh7-HBSP-flag-HIV,對照細胞株命名為Huh7-flag-HIV。
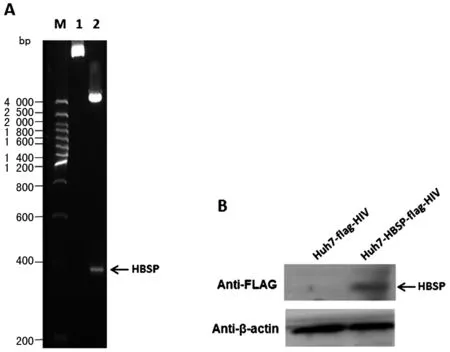
A.慢病毒重組載體雙酶切鑒定 B.1. 未經酶切的重組載體;2. XbaI和BamH I雙酶切重組載體 B.Westernblot檢測Huh7細胞株HBSP蛋白的表達A.Restriction enzyme analysis of recombinant lentiviral expression vector 1.uncut recombinant plasmids; 2. recombinant plasmids digested with XbaI and BamH I B.Westernblot detection of HBSP expression in the established Huh7 cell lines圖1 慢病毒表達載體構建及Westernblot檢測HBSP蛋白表達的Huh7慢病毒細胞株Fig.1 Lentiviral vector construction and HBSP protein detection in the established Huh7 cell lines
2.2 HBSP 促進TGFβ1誘導的肝癌細胞上皮間質轉化 以5 ng/mL的TGFβ1誘導Huh7-HBSP-flag-HIV與對照細胞株Huh7-flag-HIV24 h,鏡下可見細胞形態發生變化:處理前,對照組Huh7-flag-HIV細胞呈鋪路石樣,細胞之間連接緊密,呈現典型的上皮細胞形態(圖2A),而Huh7-HBSP-flag-HIV 細胞(圖2B)為多邊形,部分為梭形;經過5 ng/mL TGFβ1處理24 h,Huh7-flag-HIV 細胞間隙變寬,細胞間連接變得松散,多呈間質細胞形態(圖2C),而Huh7-HBSP-flag-HIV細胞比對照細胞連接更松散,細胞變長呈梭形,幾乎全為間質細胞形態(圖2D)。提示HBSP促進TGFβ1誘導的肝癌細胞發生上皮間質轉化。
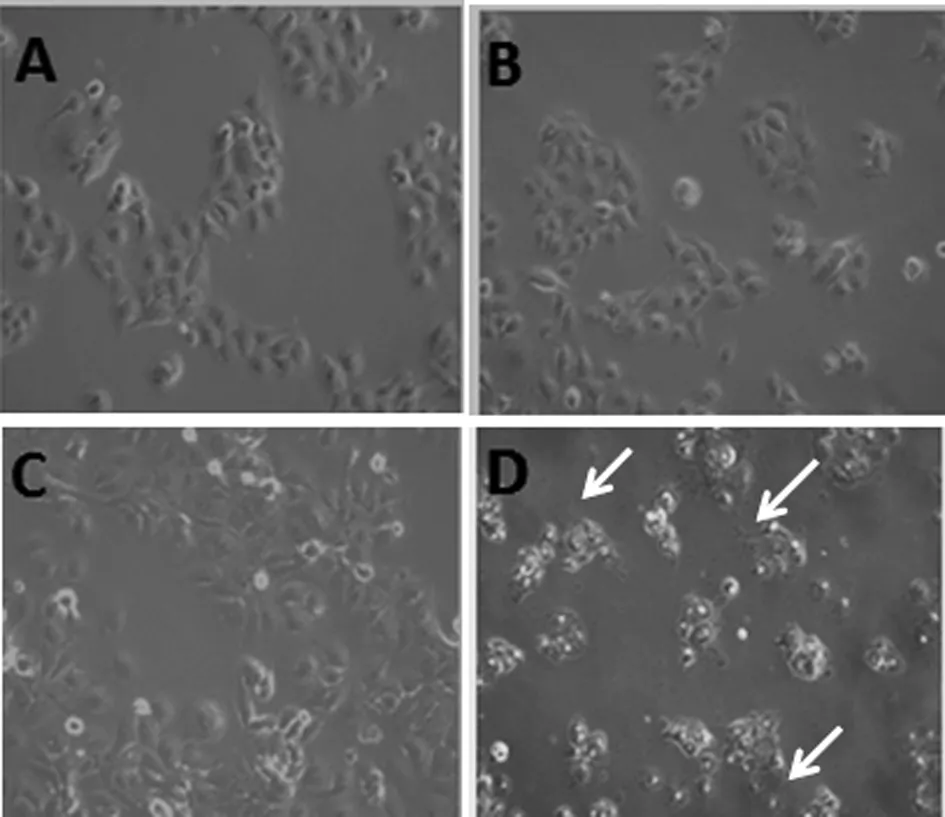
A:Huh7-flag-HIV細胞; B:Huh7-HBSP-flag-HIV細胞; C:5 ng/mL TGFβ1處理Huh7-flag-HIV細胞24 h后;D:5 ng/mL TGFβ1處理Huh7-HBSP-flag-HIV細胞24 h后A:Huh7-flag-HIVcells; B:Huh7-HBSP-flag-HIV cells; C:Huh7-flag-HIV cells treated with 5 ng/mL TGFβ1 for 24 hours; D:Huh7-HBSP-flag-HIV cells treated with 5 ng/mL TGFβ1 for 24 hours.圖2 TGFβ1處理前后 Huh7-HBSP-flag-HIV 及對照組 Huh7-flag-HIV 細胞株形態學變化(10×)Fig.2 Huh7-HBSP-flag-HIV and Huh7-flag-HIV cells images before and after TGFβ1 treatment (10×)
2.3 HBSP促進TGFβ1誘導的肝癌細胞上皮間
質轉化相關指標變化 以5 ng/mL TGFβ1誘導細胞24 h后,提取細胞總蛋白,用特異性抗體檢測EMT相關指標表達水平,結果顯示,Huh7-HBSP-flag-HIV與Huh7-flag-HIV對照細胞株相比,上皮樣標志物E-cadherin、Claudin-1、β-catenin表達量都出現明顯下降(n=5,P<0.01),而間質樣標志物N-cadherin表達量出現明顯升高(n=5,P<0.01)(圖3)。HBSP在肝癌細胞株內過表達可下調TGFβ1誘導的肝癌細胞上皮樣標志物表達水平,并上調間質樣標志物表達水平,進一步證明HBSP可促進TGFβ1誘導的肝癌細胞發生上皮間質轉化。
2.4 下調TGFβ1I1可逆轉TGFβ1誘導的HBSP表達細胞株上皮間質轉化 以5 ng/mL TGFβ1誘導Huh7-HBSP-flag-HIV以及Huh7-flag-HIV對照株,并分別轉染TGFβ1I1 siRNA混合物與無關對照NC-siRNA,觀察TGFβ1I1表達水平被特異性siRNA下調后(圖4A)EMT相關指標的變化情況。結果顯示:轉染無關對照NC-siRNA組,Huh7-HBSP-flag-HIV細胞與Huh7-flag-HIV對照細胞株相比,上皮細胞相關指標E-cadherin、Claudin-1、β-catenin表達量都下降,而間質細胞標志物N-cadherin表達量增高(圖4A,第三泳道與第一泳道對比,n=5,P<0.01);而TGFβ1I1 siRNA 混合物轉染Huh7-HBSP-flag-HIV細胞,其上皮樣標志物E-cadherin、Claudin-1、β-catenin表達量逆轉上調,間質樣標志物N-cadherin表達下降(圖4A,第四泳道與第三泳道對比,n=5,P<0.01),說明下調TGFβ1I1可逆轉TGFβ1誘導的HBSP表達細胞株上皮間質轉化,提示HBSP借由與TGFβ1I1的相互作用來影響TGFβ1誘導的肝癌細胞上皮間質轉化。
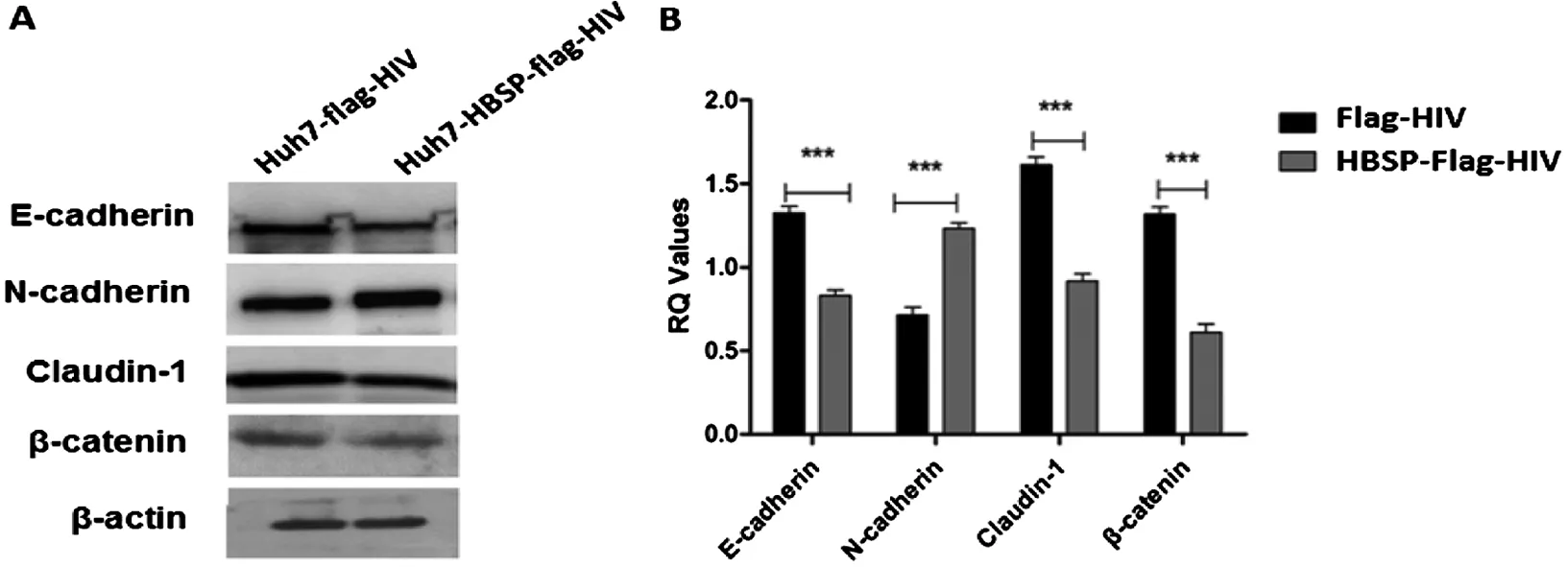
A:Westernblot檢測Huh7 細胞株EMT標志物 B:蛋白條帶灰度值比較A:Westernblot analysis of EMT markers; B:Relative intensities of plotted proteins.*** n=5, P<0.0001圖3 TGFβ1誘導的肝癌細胞上皮間質轉化相關指標變化Fig.3 TGFβ1-induced EMT markers detection in Huh7 cell lines
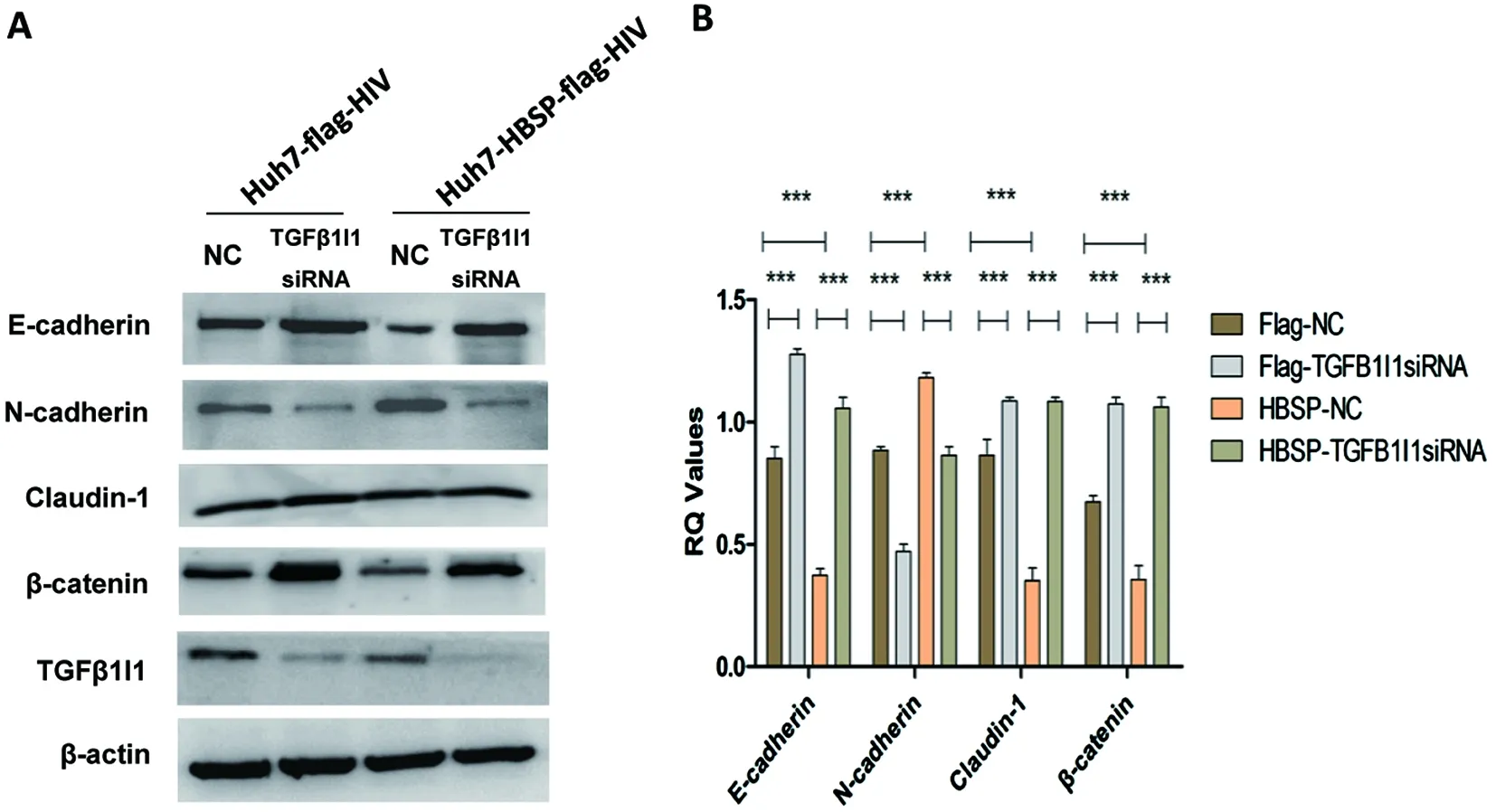
A:Westernblot檢測 EMT標志物 B:蛋白條帶灰度值比較A:Western blot detection of EMT markers; B:Relative intensities of plotted proteins.*** n=5, P<0.0001.圖4 siRNA下調TGFβ1I1可逆轉TGFβ1誘導的HBSP表達細胞株上皮間質轉化Fig.4 TGFβ1I1 knockdown by siRNA could reverse epithelial-mesenchymal transition in HBSP expressed Huh7 hepatoma cells
2.5 HBSP與TGFβ1I1的相互作用促進TGFβ1誘導的肝癌細胞侵襲與遷移能力 Huh7-HBSP-flag-HIV以及Huh7-flag-HIV對照株分別以5 ng/mL TGFβ1處理24 h后分別轉染TGFβ1I1 siRNA混合物與NC-siRNA,作用24 h后接種于侵襲小室,36 h后觀察到:轉染無關對照NC-siRNA,Huh7-HBSP-flag-HIV細胞與Huh7-flag-HIV細胞株相比(見圖5A,第三列與第一列相比),細胞數明顯增加(94±3 vs. 29±3,P<0.05),說明HBSP能夠促進TGFβ1誘導的肝癌細胞侵襲能力。Huh7-HBSP-flag-HIV細胞株轉染TGFβ1I1特異性siRNA后(見圖5A,第四列與第三列相比)細胞數明顯下降(29±3 vs. 9±2,P<0.05)。
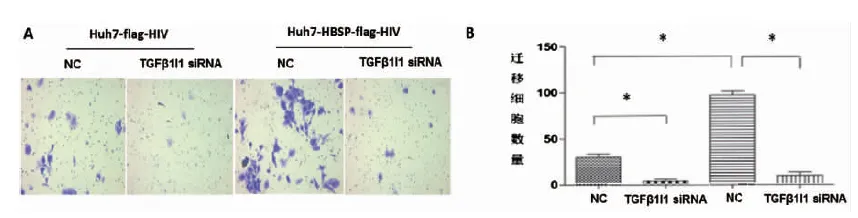
A:鏡下觀察遷移穿過transwell小室的細胞 B:遷移細胞數量比較A:Images of Huh7 invaded through membrane into the bottom of transwell chamber; B:Comparison of invaded cells quantities.* n=5, P<0.05.圖5 HBSP與TGFβ1I1相互作用促進TGFβ1誘導的肝癌細胞侵襲能力Fig.5 HBSP-TGFβ1I1 interaction promotes invasion of TGFβ1-induced Huh7 hepatoma cells
同樣地,對5 ng/mL TGFβ1處理Huh7-HBSP-flag-HIV以及Huh7-flag-HIV對照株轉染TGFβ1I1 siRNA混合物與NC-siRNA 24 h后,進行劃痕實驗,48 h后觀察細胞修復情況。結果與侵襲實驗類似,轉染無關對照NC-siRNA,Huh7-HBSP-flag-HIV細胞與Huh7-flag-HIV細胞株相比,細胞遷移能力明顯加強(見圖6A,第三列與第一列相比),說明HBSP促進TGFβ1誘導的肝癌細胞遷移能力。當TGFβ1I1被siRNA下調后,Huh7-HBSP-flag-HIV細胞株遷移能力下降(見圖6A,第四列與第三列相比)。上述結果提示HBSP與TGFβ1I1的相互作用促進TGFβ1誘導的肝癌細胞侵襲與遷移能力。
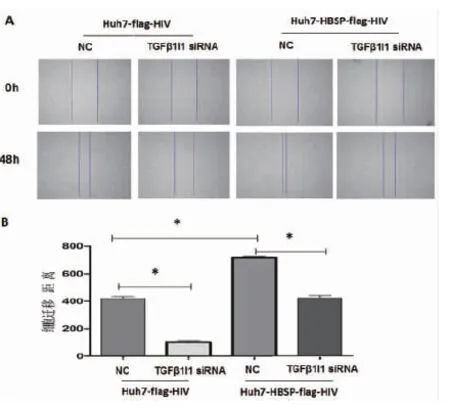
A:鏡下觀察劃痕實驗前后的細胞 B:劃痕修復寬度比較A:Images of Huh7 with wound healing assay captured at 0 h and 48 h; B:Quantify the total distance of cells moved from the edge of the scratch toward the center.* n=5,P<0.05.圖6 HBSP與TGFβ1I1相互作用促進TGFβ1誘導的肝癌細胞遷移能力Fig.6 HBSP-TGFβ1I1 interaction promotes migration of TGFβ1-induced Huh7 hepatoma cells
3 討 論
乙型肝炎病毒是一種在其復制周期中存在逆轉錄過程的DNA病毒,由前基因組RNA剪接所產生的剪接變異體可編碼產生一些剪接變異體特異性新蛋白。HBSP是HBV感染者中常見的一種剪接蛋白[8],已有研究認為,HBSP可誘導肝細胞凋亡,加重肝炎病情,還與肝纖維化有關[2];并通過誘導特異性細胞毒性T淋巴細胞(cytotoxic lymphocyte,CTL)活性,導致肝臟損傷[3]。我們先前的研究也顯示HBSP與組織蛋白酶B(cathepsin B, CTSB)相互作用,通過PI3K/Akt通路及MAPK通路增強肝癌細胞株的侵襲能力[4]; HBSP與纖維蛋白原(fibrinogen gamma chain,FGG)相互作用,抑制其交聯,能抑制血小板凝集、活化,造成凝血障礙[5]; HBSP與微粒體環氧化物酶(microsomal epoxidehydrolase, mEH)相互作用,通過促進后者活性,導致苯丙芘(Benzo(a)pyrene, B[a]P)的致癌產物增加,協同促進肝癌細胞生長[9]。為了更好地了解定位于細胞漿內的HBSP與肝細胞蛋白相互作用的情況,本實驗室在前期工作中采用細胞質酵母雙雜交研究與HBSP相互作用的蛋白,篩選出轉化生長因子1誘導蛋白1,并利用哺乳動物細胞雙雜交、免疫共沉淀等方法證實了HBSP可與其相互作用[6]。
轉化生長因子β1誘導蛋白1受TGFβ1誘導[10],因其又受H2O2誘導,也稱為Hic-5(hydrogen peroxide-inducible clone-5)[11]。TGFβ1I1對TGFβ1存在正反饋調節機制,即TGFβ1I1具有維持局部組織TGFβ1不斷自分泌的作用[12]。TGFβ1I1可促進細胞發生上皮間質轉化[13],且這類由TGFβ1誘導的EMT可促進細胞間基質的降解和提高細胞的侵襲轉移能力[14],有研究表明,TGFβ1誘導的EMT與肝細胞性肝癌發生發展密切相關[15-16]。EMT是腫瘤轉移的重要機制之一,主要特征是上皮細胞極性消失,轉換成運動更為靈活、易于侵襲的間質細胞,最直觀的表現就是其細胞形態常常發生改變[17]。本研究觀察到乙型肝炎病毒剪接特異性蛋白HBSP在肝癌細胞株Huh7中過表達可促進TGFβ1誘導的EMT現象,經過5 ng/mL TGFβ1刺激24 h后,表達HBSP的慢病毒細胞株大部分發生明顯形態改變,表現為細胞之間連接變得松散,細胞間隙變寬,細胞變長呈梭形,即上皮源性的肝細胞呈現出典型的間質細胞形態。為深入探討其機制,我們進一步利用特異性抗體檢測同樣處理條件下EMT的一些標志分子,可見相應細胞上皮標志物E-cad、Claudin-1、β-catenin表達量下降,而間質標志物N-cad表達量升高,且該現象可隨TGFβ1I1被特異性siRNA下調而逆轉。提示HBSP過表達Huh7細胞株受TGFβ1誘導發生EMT,可能與HBSP-TGFβ1I蛋白相互作用有關。E-cad是一種跨膜類型的糖蛋白,在胞漿內通過α、β連接素(α、β-catenin)間接的與肌動蛋白骨架連接,對于穩定細胞骨架和保持上皮細胞間連接起重要作用,E-cad水平的下降往往同時伴隨有細胞間連接分子Claudin-1和β-catenin的下調[18]。N-cad是間質細胞的主要標志物,在很多腫瘤細胞發生中都出現N-cad的上調[19]。
EMT發生往往導致腫瘤細胞黏附能力下降,遷移運動能力增加,使得腫瘤細胞更易于離開原有位置,發生原位浸潤或者隨血行、淋巴等途徑轉移[17]。為了深入揭示HBSP-TGFβ1I1相互作用導致EMT如何影響肝癌細胞惡性生物學行為,我們進一步做了侵襲小室實驗,發現在TGFβ1誘導下,HBSP過表達組與對照組相比,侵襲能力顯著增強,而當TGFβ1I1被特異性siRNA下調,侵襲能力得以減弱,說明HBSP能通過與TGFβ1I1相互作用加強Huh7細胞的侵襲能力。劃痕實驗也同樣說明了HBSP蛋白與TGFβ1I1相互作用加強了Huh7細胞的遷移能力。
綜上所述,我們認為乙型肝炎病毒編碼的剪接特異性蛋白HBSP可借由與TGFβ1I1的相互作用,促進肝癌細胞發生EMT,繼而促進腫瘤侵襲轉移。本研究所獲得的結果有助于闡明HBV病毒成分導致肝癌轉移的具體機制,并為抑制HBV相關肝癌的侵襲轉移提供新思路。
[1] Wu HL, Chen PJ, Tu SJ, et al. Characterization and genetic analysis of alternatively spliced transcripts of hepatitis B virus in infected human liver tissues and transfected HepG2 cells[J]. J Virol, 1991, 65(4):1680-1686.
[2] Soussan P, Tuveri R, Nalpas B, et al. The expression of hepatitis B spliced protein (HBSP) encoded by a spliced hepatitis B virus RNA is associated with viral replication and liver fibrosis[J]. J Hepatol, 2003, 38(3):343-348. DOI:10.1016/S0168-8278(02)00422-1
[3] Mancini-Bourgine M, Bayard F, Soussan P, et al. Hepatitis B virus splice-generated protein induces T-cell responses in HLA-transgenic mice and hepatitis B virus-infected patients[J]. J Virol, 2007, 81(10):4963-4972. DOI:10.1128/JVI.02619-06
[4] Chen WN, Chen JY, Jiao BY, et al. Interaction of hepatitis B spliced protein (HBSP) with cathepsin B promotes hepatoma cell migration and invasion[J]. J Virol, 2012, 86(24):13533-13541. DOI:10.1128/JVI.02095-12
[5] Chen JY, Chen WN, Liu LL, et al. Hepatitis B spliced protein (HBSP) generated by a spliced hepatitis B virus RNA participates in abnormality of fibrin formation and functions by binding to fibrinogen γ chain[J]. J Med Virol, 2010, 82(12):2019-2026. DOI:10.1002/jmv.21918
[6] Sciencepaper Online.Interaction of the hepatitis B spliced protein with transforming growth factor beta 1 induced transcript 1[EB/OL].[2015-7-13].http://www.paper.edu.cn/html/releasepaper/201507-118.
[7] Tumbarello DA, Turner CE. Hic-5 contributes to epithelial-mesenchymal transformation through a RhoA/ROCK-dependent pathway[J]. J Cellular Physiol, 2007, 211(3):736-47. DOI:10.1002/jcp.20991
[8] Soussan P, Garreau F, Zylberberg H, et al.Invivoexpression of a new hepatitis B virus protein encoded by a spliced RNA[J]. J Clin Investigat, 2000, 105(1):55-60. DOI:10.1172/JCI8098
[9] Chen JY, Chen WN, Jiao BY, et al. Hepatitis B spliced protein (HBSP) promotes the carcinogenic effects of benzo [alpha] pyrene by interacting with microsomal epoxide hydrolase and enhancing its hydrolysis activity[J]. BMC Cancer, 2014, 14:282. DOI:10.1186/1471-2407-14-282
[10] Shibanuma M, Mashimo J, Mita A, et al. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide[J]. Eur J Biochem, 1993, 217(1):13-19. DOI:10.1111/j.1432-1033.1993.tb18212.x
[11] Shibanuma M, Mashimo J, Kuroki T, et al. Characterization of the TGF beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence[J]. J Biol Chem, 1994, 269(43):26767-26774. DOI:10.1007/s00109-010-0608-3
[12] Dabiri G, Tumbarello DA, Turner CE, et al. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop[J]. J Invest Dermatol, 2008, 128(10):2518-2525. DOI:10.1038/jid.2008.90
[13] Tumbarello DA, Turner CE. Hic-5 contributes to epithelial-mesenchymal transformation through a RhoA/ROCK-dependent pathway[J]. J Cellular Physiol, 2007, 211(3):736-747. DOI:10.1002/jcp.20991
[14] Pignatelli J, Tumbarello DA,Schmidt RP, et al. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition[J]. J Cell Biol, 2012, 197(3):421-437. DOI:10.1083/jcb.201108143
[15] Xu Z, Shen MX, Ma DZ, et al. TGF-beta1-promoted epithelial-to-mesenchymal transformation and cell adhesion contribute to TGF-beta1-enhanced cell migration in SMMC-7721 cells [J]. Cell Res, 2003, 13(5):343-350. DOI:10.1038/sj.cr.7290179
[16] Calvisi DF, Pascale RM, Feo F. Epidermal growth factor-like repeats and discoidin I-like domains 3:a multifaceted oncoprotein at the crossroad of MAPK and TGF-beta pathways in human hepatocellular carcinoma[J]. Transl Cancer Res, 2016, 5(2):103-109. DOI:10.21037/tcr.2016.03.09
[17] Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states:acquisition of malignant and stem cell traits[J]. Nat Rev Cancer, 2009, 9(4):265-273. DOI:10.1038/nrc2620
[18] Tepass U, Truong K, Godt D, et al. Cadherins in embryonic and neural morphogenesis[J]. Nat Rev Mol Cell Biol, 2000, 1(2):91-100. DOI:10.1038/35040042
[19] An HT, Yoo S, Ko J.α-Actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of Snail expression and β-catenin stabilization in cervical cancer[J]. Oncogene, 2016, 35(45):5893-5904.DOI:10.1038/onc.2016.117
Lin Xu, Email:linxu@mail.fjmu.edu.cn
Hepatitis B spliced protein triggers TGFβ1-induced epithelial-mesenchymal transition via interaction with transforming growth factor beta-1-induced transcript 1 protein
CHEN Wan-nan, HUANG Jun-gao, LIANG Fei-fei, YAN Xiao-li, XUAN Dan-dan, LIN Xu
(KeyLaboratoryofMinistryofEducationforGastrointestinalCancer,KeyLaboratoryofFujianProvinceforTumorMicrobiology,SchoolofBasicMedicalSciences,FujianMedicalUniversity,Fuzhou350122,China)
To investigate the TGFβ1-induced epithelial-mesenchymal transition (EMT) of Huh7 hepatoma cells caused by interaction of hepatitis B spliced protein (HBSP) with transforming growth factor beta-1-induced transcript 1 protein (TGFβ1I1), coding region of HBSP was cloned into lentiviral expression vector. Huh7 hepatoma cells were infected by recombinant lentivirus packaged in 293T cells. Stable cell lines expressing HBSP or control cells were selected by puromycin. Cells were incubated with 5 ng/mL TGFβ1 for 24 h, and observed under contrast-phase microspcope. Then the whole cell lysates were collected for western blot analysis using specific antibodies against EMT markers including E-cadherin, N-cadherin, Claudin-1 and β-catenin. To evaluate the effects of HBSP-TGFβ1I1 interaction on EMT, TGFβ1-induced EMT marker transition, as well as cell invasion and migration were explored after knocking down of TGFβ1I1 by siRNA. Results showed that Huh7 cell lines expressing HBSP (Huh7-HBSP-flag-HIV) and control cell lines (Huh7-flag-HIV) were successfully established. Huh7-HBSP-flag-HIV cells lost their pebble-like shape and tight cell-cell adhesion and transformed into the mesenchymal-like cells in the presence of TGFβ1. Decreased expression level of epithelial marker of E-cadherin, Claudin-1, β-catenin, increased expression level of mesenchymal marker of N-cadherin, and enhanced migration and invasion abilities were observed in Huh7-HBSP-flag-HIV cells as compared to the control cells. Moreover, the changes of EMT markers and metastasis abilities of Huh7-HBSP-flag-HIV cells could be reversed when TGFβ1I1 was knocked down by siRNA. In conclusion, HBSP could promote hepatoma cell migration and invasion by triggering EMT via interaction with TGFβ1I1. Our findings highlight new insights for HBSP-induced HCC progression.
hepatitis B virus; RNA splicing; transforming growth factor beta-1-induced transcript 1 protein; epithelial-mesenchymal transition; metastasis
10.3969/j.issn.1002-2694.2017.04.003
國家自然科學基金青年基金項目 (No. 81201293);福建省衛生廳醫學創新課題(No.2012-CX-14);福建省高校杰出青年科研人才培育計劃(No. JA11104);國家衛生和計劃生育委員會共建科學研究基金-福建省衛生教育聯合攻關計劃(No. WKJ-FJ-29);福建省高等學校新世紀優秀人才支持計劃(No.JA13129);福建省衛生系統中青年骨干人才培養項目(No. 2014-ZQN-ZD-25)聯合資助
林 旭:Email:linxu@mail.fjmu.edu.cn
福建醫科大學基礎醫學院,消化道惡性腫瘤省部共建教育部重點實驗室,福建省腫瘤微生物學重點實驗室,福州 350122
Supported by the National Natural Science Foundation of China (No. 81201293), the Fujian Provincial Medical Innovation Project (No. 2012-CX-14), the Outstanding Young Researchers Training Plan of Fujian Province (No. JA11104), the Joint Research Program of Health and Planning Committee and Education Department of Fujian (No. WKJ-FJ-29), the New Century Talents Supporting Plan of Fujian Education Department (No. JA13129), and the Medical Elite Cultivation Program of Fujian (No. 2014-ZQN-ZD-25)
R373
A
1002-2694(2017)04-0305-07
2016-11-16 編輯:梁小潔

