新型3-五元碳環螺環氧化吲哚類化合物的合成及其抗人白血病細胞活性
陳智勇, 田民義, 楊 俊, 劉歡歡, 林 冰, 潘玉杰, 劉雄利
(貴州大學 貴州省中藥民族藥創制工程中心,貴州 貴陽 550025)
·研究論文·
新型3-五元碳環螺環氧化吲哚類化合物的合成及其抗人白血病細胞活性
陳智勇, 田民義, 楊 俊, 劉歡歡, 林 冰*, 潘玉杰, 劉雄利
(貴州大學 貴州省中藥民族藥創制工程中心,貴州 貴陽 550025)
以氧化吲哚與鄰芳基二甲醛為原料,經Knoevenagel縮合(或Michael,環化反應),制得7個3-五元碳環螺環氧化吲哚(4a~4c,產率67%~86%,d/r值4 ∶1~10 ∶1)和4d~4g; 4d~4g與哌啶(或四氫吡咯)和多聚甲醛經胺甲基化反應,合成了4個3-五元碳環螺環氧化吲哚(5d~5g),產率55%~67%,d/r值10 ∶1~>20 ∶1,其結構經1H NMR,13C NMR和HR-MS(ESI-TOF)表征。采用MTT法研究了4a~4c和5d~5g對人白血病細胞(K562)的體外抗腫瘤活性。結果表明:4b, 5d和5f對K562抑制活性較好,IC50分別為29.3 μmol·L-1, 27.4 μmol·L-1和34.2 μmol·L-1,與陽性對照藥順鉑(26.8 μmol·L-1)相當。
氧化吲哚; 3-五元碳環螺環氧化吲哚類化合物; Knoevenagel縮合反應; 胺甲基化反應; 合成; 抗腫瘤活性
3-五元碳環螺環氧化吲哚骨架廣泛存在于天然產物和合成藥物分子中,吸引了諸多化學工作者和醫藥研發團隊的廣泛關注。如天然產物marcfortine A, B; paraherquamide A~E; notoamide A~N和sclerotiamide等具有抗炎、抗癌、抗微生物等多種藥理作用[1-4]。目前,已有文獻[5-11]報道了其合成方法。
本課題組主要從事3-季碳氧化吲哚骨架化合物的設計、合成及其生物活性的研究[12-14]。本文在課題組前期工作的基礎上,以氧化吲哚(1a~1g)與鄰芳基二甲醛(2, 2′)為原料,經Knoevenagel縮合(或Michael,環化反應),制得7個3-五元碳環螺環氧化吲哚(4a~4c,產率67%~86%,d/r值4 ∶1~10 ∶1)和4d~4g; 4d~4g與哌啶[3或四氫吡咯(3′)]和多聚甲醛經胺甲基化反應,合成了4個3-五元碳環螺環氧化吲哚(5d~5g, Scheme 1),產率55%~67%,d/r值10 ∶1~>20 ∶1,其結構經1H NMR,13C NMR和HR-MS(ESI-TOF)表征。采用MTT法研究了4a~4c和5d~5g對人白血病細胞(K562)的體外抗腫瘤活性。
1 實驗部分
1.1 儀器與試劑
WRS-1B型數字熔點儀;Bruker-400 MHz型核磁共振儀(CD3Cl或DMSO-d6為溶劑,TMS為內標); MicroTMQ-TOF型高分辨質譜儀。
所用試劑均為分析純。
1.2 合成
(1) 4a~4c的合成(以4a為例)
攪拌下,在反應管中依次加入N-甲基-5-氟氧化吲哚(1a)148.5 mg(0.90 mmol), 2 40.2 mg(0.3 mmol), 10 mol%3和MeOH 6.0 mL,回流反應16 h。經硅膠柱層析[洗脫劑:A=V(石油醚):V(乙酸乙酯)=4 ∶1~2 ∶1]純化得白色固體3-五元碳環螺環氧化吲哚(4a)115.1 mg。
用類似的方法合成白色固體4b和4c。
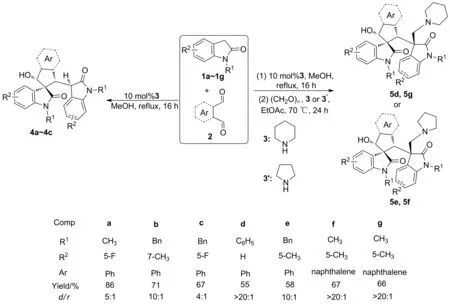
Scheme 1
4a: m.p.243.5~244.1 ℃;1H NMRδ(major+minor): 2.55(s, 3H, major), 2.69(s, 3H, major), 2.78(s, 0.8H, minor), 3.14(s, 0.8H, minor), 4.43~4.50(m, 1.3H, major+minor), 4.56~4.60(m, 1.3H, major+minor), 5.33~5.37(m, 1.3H, major+minor), 5.69~5.73(m, 1.3H, major+minor), 6.23(dd,J=8.2 Hz, 1.8 Hz, 1H, major), 6.59~6.62(m, 2.6H, major+minor), 6.81~6.86(m, 0.6H, minor), 6.93~7.08(m, 3.3H, major+minor), 7.36~7.56(m, 4H, major+minor), 7.70~7.76(m, 1.6H, major+minor);13C NMRδ(major+minor): 25.7, 26.0, 26.8, 43.5, 50.2, 66.8, 79.1, 79.8, 108.0, 108.1, 108.2, 108.3, 113.1, 113.3, 114.3, 114.6, 114.7, 114.9, 115.0, 115.3, 123.5, 126.1, 126.5, 127.2, 127.6, 127.7, 128.7, 138.4, 140.6, 140.9, 143.1, 156.2, 157.0, 174.6, 178.1; HR-MS(ESI-TOF)m/z: Calcd for C26H20N2O3F2Na{[M+Na]+}469.134 0, found 469.134 2。
4b: m.p.223.8~224.2 ℃;1H NMRδ(major+minor): 1.92(s, 3.3H, major+minor), 1.98(s, 3.3H, major+minor), 4.59(s, 2.2H, major+minor), 4.69(d,J=16.0 Hz, 1H, major), 4.94(d,J=16.0 Hz, 1H, major), 5.45(d,J=8.1 Hz, 1H, major), 5.76~5.81(m, 1.1H, major+minor), 6.41(d,J=8.4 Hz, 1.1H, major+minor), 6.67(t,J=8.4 Hz, 1.1H, major+minor), 6.87~6.96(m, 5.5H, major+minor), 7.04~7.11(m, 3.3H, major+minor), 7.18~7.25(m, 6.6H, major+minor), 7.36(s, 3.3H, major+minor), 7.66~7.69(m, 0.3H, minor), 7.72~7.76(m, 1.3H, major+minor);13C NMRδ: 18.4, 52.0, 65.3, 80.3, 118.4, 123.3, 125.5, 125.9, 126.2, 126.6, 127.3, 129.0, 132.0, 132.3, 138.3, 138.6, 138.8, 141.5, 143.9, 176.0, 179.9;HR-MS(ESI-TOF)m/z: Calcd for C40H34N2O3Na{[M+Na]+}613.246 7, found 613.246 9。
4c: m.p.188.4~188.9 ℃;1H NMRδ(major+minor): 3.93~4.03(m, 1.2H, major+minor), 4.14~4.25(m, 3.6H, major+minor), 4.43(d,J=16.0 Hz, 1.2H, major+minor), 4.71(d,J=16.4 Hz, 1.2H, major+minor), 4.93(d,J=16.1 Hz, 1.2H, major+minor), 5.07(d,J=16.2 Hz , 1.2H, major+minor), 6.02~6.14(m, 3.1H, major+minor), 6.55~6.57(m, 2H, major), 6.66~6.70(m, 1.2H, major+minor), 6.79~6.83(m, 1.2H, major+minor), 6.93~6.96(m, 1.2H, major+minor), 7.08~7.18(m, 5.7H, major+minor), 7.27~7.35(m, 6.2H, major+minor), 7.44~7.46(m, 2.4H, major+minor), 7.56(s, 1.2H, major+minor), 7.66~7.73(m, 2H, major), 8.04(s, 1H, major);13C NMRδ(major+minor): 11.3, 14.0, 14.4, 19.1, 22.9, 23.7, 28.8, 30.5, 43.1, 48.3, 50.7, 65.5, 82.9, 124.6, 127.0, 127.7, 127.9, 128.9, 129.1, 132.0, 135.9, 136.6, 145.2, 176.0, 177.7; HR-MS(ESI-TOF)m/z: Calcd for C38H28N2O3F2Na{[M+Na]+}621.196 6, found 621.197 1。
(2) 5d~5g的合成(以5d為例)
攪拌下,在反應管中依次加入N-苯基氧化吲哚(1d) 188.2 mg(0.90 mmol), 2 40.2 mg(0.3 mmol), 10 mol%3和MeOH 6.0 mL,回流反應16 h。經硅膠柱層析[洗脫劑:V(正己烷) ∶V(乙酸乙酯)=4 ∶1~2 ∶1]純化得4d。加入3 51 mg(0.6 mmol)的EtOAc(6.0 mL)溶液和多聚甲醛36 mg(1.2 mmol),于70 ℃(浴溫)反應24 h。經硅膠柱層析(洗脫劑:A=3 ∶1~2 ∶1)純化得白色固體5d 104.2 mg。
用類似的方法合成白色固體5e, 5f和淡黃色油狀液體5g。
5d: m.p.133.6~134.4 ℃;1H NMRδ: 1.05~1.15(m, 6H), 1.77(d,J=8.1 Hz, 1H), 1.87(m, 2H), 2.25(m, 2H), 3.06~3.16(m, 2H), 4.14(s, 1H), 5.51(d,J=8.2 Hz, 1H), 5.96~5.98(m, 1H), 6.20(d,J=8.4 Hz, 1H), 6.67~6.75(m, 4H), 7.06~7.10(m, 2H), 7.30~7.40(m, 12H), 7.49~7.55(m, 5H);13C NMRδ: 24.0, 26.7, 29.8, 55.1, 56.6, 57.4, 65.1, 80.6, 108.9, 109.5, 121.7, 122.4, 122.6, 122.9, 124.6, 127.4, 127.7, 128.0, 128.3, 128.7, 129.0, 129.1, 129.3, 129.4, 130.3, 130.5, 134.6, 135.2, 140.6, 144.0, 145.6, 146.4, 174.8, 178.4; HR-MS(ESI-TOF)m/z: Calcd for C42H37N3O3Na{[M+Na]+}654.273 3, found 654.273 3。
5b: m.p.215.0~216.1 ℃;1H NMRδ(major+minor): 1.47~1.52(m, 5.5H, major+minor), 2.09~2.13(m, 2.2H, major+minor), 2.16(s, 3.3H, major+minor), 2.37(s, 3.3H, major+minor), 2.42~2.44(m, 2.2H, major+minor), 3.10~3.17(m, 1.1H, major+minor), 3.31~3.46(m, 1.1H, major+minor), 3.63(m, 1H, major), 4.56(s, 1.1H, major+minor), 4.93(d,J=16.1 Hz, 1.1H, major+minor), 5.04~5.13(m, 1.2H, major+minor), 5.49(d,J=12.4 Hz, 1H, major), 5.92~5.96(m, 1.1H, major+minor), 6.08~6.17(m, 1.1H, major+minor), 6.21~6.23(m, 0.3H, minor), 6.34(s, 1H, major), 6.42(m, 0.1H, minor), 6.56~6.59(m, 0.1H, minor), 6.66~6.71(m, 0.1H, minor), 6.79~6.84(m, 2.5H, major+minor), 7.10~7.25(m, 14.5H, major+minor), 7.32~7.64(m, 5.5H, major+minor), 8.23(s, 1.1H, major+minor);13C NMRδ(major+minor): 21.2, 21.3, 21.4, 24.0, 43.0, 43.2, 52.2, 55.2, 56.4, 64.3, 67.5, 81.7, 107.7, 108.8, 123.2, 124.1, 125.7, 126.7, 127.0, 127.1, 127.4, 127.7, 128.3, 128.7, 129.4, 129.5, 130.1, 130.8, 135.7, 136.2, 137.8, 141.4, 141.7, 142.9, 177.5, 178.2; HR-MS(ESI-TOF)m/z: Calcd for C45H43N3O3Na{[M+Na]+}696.320 2, found 696.320 7。
5c: m.p.218.1~219.36 ℃;1H NMRδ: 1.54~1.57(m, 4H), 1.85(d,J=12.0 Hz, 1H), 2.04(s, 3H), 2.34~2.37(m, 5H), 2.48~2.51(m, 5H), 2.68(s, 3H), 3.43~3.54(m, 2H), 4.80(s, 1H), 5.52(d,J=12.4 Hz, 1H), 6.13~6.17(m, 2H), 6.27(d,J=8.2 Hz, 1H), 6.85(d,J=8.4 Hz, 2H), 7.21(s, 1H), 7.46~7.50(m, 2H), 7.76(s, 1H), 7.80~7.84(m, 1H), 7.97~7.99(m, 1H), 8.60(s, 1H);13C NMRδ: 21.1, 21.4, 24.2, 25.3, 25.9, 51.4, 54.9, 56.5, 63.8, 67.3, 81.02, 106.5, 107.5, 121.6, 124.1, 125.7, 125.8, 126.0, 127.6, 127.7, 127.9, 128.1, 129.1, 129.3, 129.7, 130.2, 130.9, 133.0, 133.5, 135.8, 141.7, 141.8, 142.6, 177.1, 177.8; HR-MS(ESI-TOF)m/z: Calcd for C37H37N3O3Na{[M+Na]+}594.273 3, found 594.273 5。
5d:1H NMRδ: 1.32~1.36(m, 4H), 2.03(s, 3H), 2.35(m, 7H), 2.50~2.55(m, 5H), 2.69(s, 3H), 3.01(d,J=16.4 Hz, 1H), 3.38(d,J=12.2 Hz, 1H), 4.82(s, 1H), 5.54(s, 1H), 6.14(d,J=8.1 Hz, 1H), 6.25(d,J=8.3 Hz, 1H), 6.85(d,J=8.2 Hz, 2H), 7.30(s, 1H), 7.46~7.51(m, 2H), 7.77~7.83(m, 2H), 7.98(d,J=8.7 Hz, 1H), 8.59(s, 1H);13C NMRδ: 11.4, 21.0, 21.4, 24.0, 25.2, 25.9, 26.5, 46.1, 50.6, 54.8, 56.8, 64.9, 67.3, 81.0, 106.4, 107.5, 121.6, 124.1, 125.7, 125.8, 126.0, 127.5, 128.0, 129.1, 129.2, 129.3, 130.0, 130.8, 133.0, 133.5, 136.1, 141.6, 142.0, 142.7, 177.2, 177.8; HR-MS(ESI-TOF)m/z: Calcd for C38H39N3O3Na{[M+Na]+}608.288 9, found 608.289 1。
1.3 體外抗人白血病細胞活性測試
采用MTT法[15-16]測試了4a~4c和5d~5g對人白血病細胞(K562)的體外抗腫瘤活性,以順鉑為陽性對照藥。
K562用RPMI-1640培養基(含10%胎牛血清,100 U·mL-1青霉素和100 U·mL-1鏈霉素)培養。細胞以每孔5 000個的濃度加至96孔中,于37 ℃在含5%CO2潮濕空氣的培養箱中培養24 h。依次將新配4a~4c和5d~5g的二甲基亞砜溶液以濃度梯度加入到各孔中,使孔中化合物的終濃度為6 μmol·L-1, 12 μmol·L-1, 25 μmol·L-1, 50 μmol·L-1和100 μmol·L-1。靜置48 h,每孔加入5 mg·mL-1MTT的磷酸鹽緩沖液10 μL,于37 ℃培養4 h。離心5 min除去未轉化的MTT,每孔加入二甲基亞砜150 μL。用酶標儀在波長490 nm處測定吸光度A490。半抑制濃度(IC50)由IBM-SPSS(19.0)軟件分析得到。
2 結果與討論
2. 1 合成
通過底物擴展,我們發現該反應的活性較高。第一步發生Knoevenagel縮合(Michael或環化反應)生成4, 16 h內基本反應完全(TLC檢測)。TLC顯示反應有少量副產物產生,但NMR和MS分析沒有發現有效的結構信息,因此可能為分解產物或聚合物。第二步胺甲基化反應生成5的反應活性也較高,以乙酸乙酯為溶劑,幾乎沒有羥基化副產物生成。
2.2 抗人白血病細胞活性
表1為4a~4c和5d~5g對K562的體外抗人白血病細胞活性。由表1可見,4b, 5d和5f對K562的抑制活性較好, IC50分別為29.3 μmol·L-1, 27.4 μmol·L-1和34.2 μmol·L-1,與陽性對照藥順鉑(26.8 μmol·L-1)相當,可作為先導化合物骨架進一步研究。
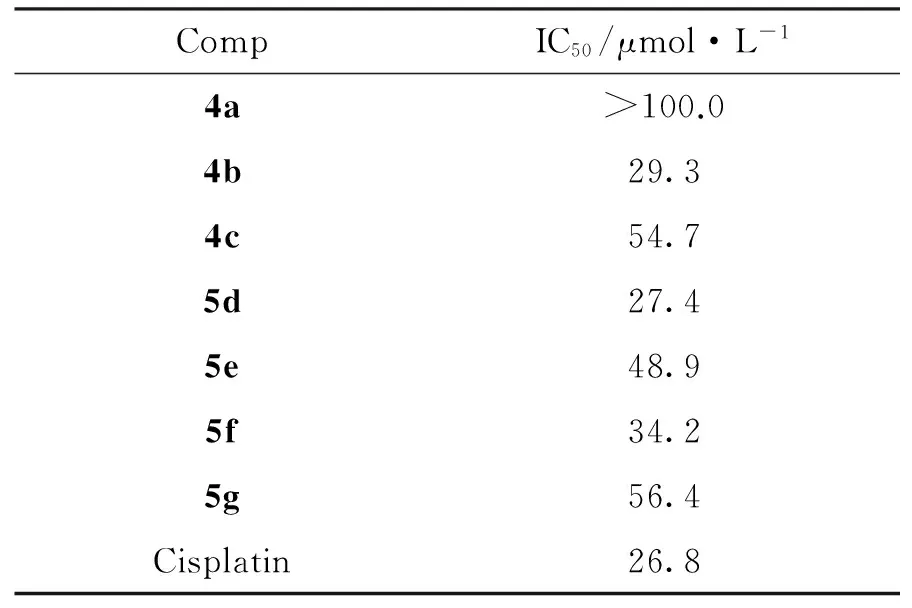
表1 4a~4c和5d~5g的體外抗人白血病細胞活性Table 1 In vitro activities of 4a~4c and 5d~5g against human leukemia cells
合成了7個新型的3-五元碳環螺環氧化吲哚類化合物(4a~4c和5d~5g)。并用MTT法研究了4a~4c和5d~5g對人白血病細胞(K562)的體外抑制活性。結果表明:4b, 5d和5f對K562抑制活性較好,IC50分別為29.3 μmol·L-1, 27.4 μmol·L-1和34.2 μmol·L-1,與陽性對照藥順鉑(26.8 μmol·L-1)相當,可作為先導化合物骨架進一步研究。其他相關藥理活性的研究正在進行中。
[1] Fensome A, Adams W R, Adams A L,etal. Design,synthesis,and SAR of new pyrrole-oxindole progesterone receptor modulators leading to 5-(7-fluoro-3,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-5-yl)-1-methyl-1H-pyrrole-2-carbonitrile(WAY-255348)[J].J Med Chem,2008,51:1861-1873.
[2] Shangary S, Qin D, McEachern D,etal. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition[J].Proc Natl Acad Sci USA,2008,105:3933-3938.
[3] Greshock T J, Grubbs A W, Jiao P,etal. Isolation,structure elucidation,and biomimetic total synthesis of versicolamide-B, and the isolation of antipodal (+)-stephacidin-A and (+)-notoamide-B from aspergillus versicolor NRRL-35600[J].Angew Chem Int Ed,2008,47:3573-3577.
[4] Mugishima T, Tsuda M, Kasai Y,etal. Absolute stereochemistry of citrinadins A and B from marine-derived fungus[J].J Org Chem,2005,70:9430-9435.
[5] Albertshofer K, Tan B, Barbas III C F,etal. Assembly of spirooxindole derivatives containing four consecutive stereocenters via organocatalytic Michael-Henry cascade reactions[J].Org Lett,2012,14:1834-1837.
[6] Sun W, Zhu G, Hong C,etal. An organocatalytic cascade strategy for the enantioselective construction of spirocyclopentane bioxindoles containing three contiguous stereocenters and two spiro quaternary centers[J].Chem Eur J,2012,18:6737-6741.
[7] Tan B, Candeias N R, Barbas III C F,etal. Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst[J].Nat Chem,2011,3:473-477.
[8] Zhong F, Han X, Wang Y,etal. Highly enantioselective [3+2] annulation of Morita-Baylis-Hillman adducts mediated by L-threonine-derived phosphines:Synthesis of 3-spirocyclopentene-2-oxindoles having two contiguous quaternary centers[J].Angew Chem Int Ed,2011,50:7837-7841.
[9] Tan B, Candeias N R, Barbas III C F,etal. Core-structure-motivated design of a phosphine-catalyzed [3+2] cycloaddition reaction:Enantioselective syntheses of spirocyclopenteneoxindoles[J].J Am Chem Soc,2011,133:4672-4675.
[10] Deng H P, Wei Y, Shi M,etal. Highly regio- and diastereoselective construction of spirocyclopenteneoxindoles through phosphine-catalyzed [3+2] annulation of Morita-Baylis-Hillman carbonates with isatylidene malononitriles[J].Org Lett,2011,13:3348-3351.
[11] Voituriez A, Pinto N, Neel M,etal. An organocatalytic [3+2] cyclisation strategy for the highly enantioselective synthesis of spirooxindoles authors[J].Chem Eur J,2010,16:12541-12544.
[12] 劉雄偉,周根,姚震,等. 異噁唑拼接吡咯螺環氧化吲哚化合物的合成及其抗腫瘤活性[J].合成化學,2016,24(5):389-392.
[13] 彭禮軍,周根,韓朔楠,等. 新型芳姜黃酮拼合吡咯螺環氧化吲哚類化合物的合成及其抗腫瘤活性[J].合成化學,2016,24(8):669-672.
[14] 楊超,楊俊,郭豐敏,等. 新型芝麻酚并吡喃螺環氧化吲哚拼接衍生物的合成[J].合成化學,2015,23(7):599-602.
[15] Mosman T J. Rapid colorimetric assay for eellulair growth and survival:Application and cytotxicity assays[J].Immunol Methods,1983,65:55-63.
[16] Alley M C, Scudiero D A, Monks A,etal. Feasibility of drug screening with panals of human tumor cell lines using a mycroculture tetrazolium assay[J].Cancer Res,1988,48:589-601.
Synthesis of Novel Five-membered Carbocyclic Spirooxindoles and Their Activities Against Human Leukemia Cells
CHEN Zhi-yong, TIAN Min-yi, YANG Jun, LIU Huan-huan,LIN Bing*, PAN Yu-jie, LIU Xiong-li
(Gui zhou Engineering Center for Innovative Traditional Chinese Medicine and Ethnic Medicine,Guizhou University, Guiyang 550025, China)
Seven novel five-membered carbocyclic spirooxindoles(4a~4c and 4d~4g) were prepared by knoevenagel condensation(or Michael, cyclization), using oxindole ando-aryldicarboxaldehyde as the materials. The yields andd/rof 4a~4c were 67%~86% and 4 ∶1~10 ∶1, respectively. Four five-membered carbocyclic spirooxindoles(5d~5g) were synthesized by the aminomethylation reaction of 4d~4g with piperidine(or pyrrdidine) and paraformaldehyde. The yields andd/rof 5d~5g were 55%~67% and 10 ∶1~>20 ∶1, respectively. The structures were characterized by1H NMR,13C NMR and HR-MS(ESI-TOF). Theinvitroantitumor activities against human leukemia cells(K562) were demonstrated by MTT assays. The results showed that 4b, 5d and 5f showed best activities equipotent than the positive control of Cisplatin(26.8 μmol·L-1), with IC50of 29.3 μmol·L-1, 27.4 μmol·L-1and 34.2 μmol·L-1, respectively.
oxindole; five-membered carbocyclic spirooxindole; Knoevenagel condensation; aminomethylation reaction; synthesis; antitumor activity
2016-10-16
國家自然科學基金地區基金資助項目(81560563, 81660576); 貴州省教學改革創新項目(SJJG201423); 研究生創新項目(黔教研合JG字[2016]06); 貴州省中藥現代化科技產業研究開發專項項目(黔科合中藥字【2011】5080號)
陳智勇(1988-),男,漢族,貴州貴陽人,碩士研究生,主要從事天然活性物質的全合成及結構修飾的研究。 E-mail: 253259908@qq.com
林冰,副教授,碩士生導師, E-mail: nlin@gzu.edu.cn
O626.13; O623.7
A
10.15952/j.cnki.cjsc.1005-1511.2017.02.16260

