Netrin-1通過下調(diào)氧糖剝奪誘導(dǎo)的人神經(jīng)母細(xì)胞瘤自噬促進(jìn)細(xì)胞存活
闕嘉麗, 李晨光, 劉可嘉, 余 劍
?
Netrin-1通過下調(diào)氧糖剝奪誘導(dǎo)的人神經(jīng)母細(xì)胞瘤自噬促進(jìn)細(xì)胞存活
闕嘉麗, 李晨光, 劉可嘉, 余 劍
目的 通過建立體外人神經(jīng)母細(xì)胞瘤(SH-SY5Y)氧糖剝奪(oxygen and glucose deprication,OGD)模型模擬腦梗死后神經(jīng)元缺血缺氧環(huán)境(OGD),探索神經(jīng)導(dǎo)向因子Netrin-1是否影響OGD后細(xì)胞的生存,以及自噬在其中的可能作用。方法 體外培養(yǎng)SH-SY5Y細(xì)胞,檢測(cè)Netrin-1對(duì)細(xì)胞活性及自噬的影響。使用自噬誘導(dǎo)劑雷帕霉素、自噬抑制劑3-甲基腺嘌呤干預(yù)自噬水平,同時(shí)構(gòu)建過表達(dá)Netrin-1受體UNC5H2-HA基因的HEK293T細(xì)胞;CCK-8試劑盒檢測(cè)細(xì)胞活性,免疫印跡檢測(cè)自噬相關(guān)標(biāo)志物(Beclin 1、LC3、p62)的表達(dá)及免疫熒光檢測(cè)LC3斑點(diǎn)的形成。結(jié)果 與OGD對(duì)照組相比,Netrin-1及3-甲基腺嘌呤的干預(yù)均顯著提高細(xì)胞活性,伴有LC3-Ⅱ斑點(diǎn)形成減少;相反,雷帕霉素增加LC3-Ⅱ斑點(diǎn)的同時(shí)抑制細(xì)胞活性,并減弱了Netrin-1提高細(xì)胞活性的作用。過表達(dá)UNC5H2-HA細(xì)胞較對(duì)照質(zhì)粒組相比,LC3-Ⅱ表達(dá)增加;同時(shí)進(jìn)行Netrin-1干預(yù)后僅過表達(dá)組出現(xiàn)LC3-Ⅱ表達(dá)下降,p62表達(dá)上升。結(jié)論 Netrin-1可能通過受體UNC5H2下調(diào)OGD損傷的人神經(jīng)母細(xì)胞瘤自噬而提高細(xì)胞活性。
Netrin-1; 氧糖剝奪; 自噬; UNC5H2
腦卒中是導(dǎo)致我國(guó)成人殘疾和死亡的第一原因[1]。腦梗死后神經(jīng)元自噬過程被激活,研究發(fā)現(xiàn)降低自噬活性能夠降低神經(jīng)元死亡數(shù)量,減小梗死面積并促進(jìn)神經(jīng)功能恢復(fù)[2,3],但其調(diào)節(jié)因素尚未完全闡明。作為一種神經(jīng)導(dǎo)向因子,Netrin-1不僅在發(fā)育中神經(jīng)系統(tǒng)調(diào)節(jié)細(xì)胞遷移黏附[4],而且在成年后與依賴性受體UNC5H2結(jié)合后,顯著地減少腦卒中后細(xì)胞凋亡,并改善運(yùn)動(dòng)功能恢復(fù)[5~7],提示其在腦保護(hù)作用上具有治療潛能。
進(jìn)一步地研究顯示Netrin-1在減少缺血導(dǎo)致心肌細(xì)胞死亡的同時(shí)也顯著地降低了自噬活性[8],但在腦梗死后尚缺乏兩者的研究。
本實(shí)驗(yàn)利用穩(wěn)定表達(dá)Netrin-1及其受體UNC5H2的人神經(jīng)母細(xì)胞瘤(SH-SY5Y)建立模擬腦梗死環(huán)境的體外氧糖剝奪細(xì)胞模型[9,10],同時(shí)構(gòu)建UNC5H2過表達(dá)的HEK 293T細(xì)胞系,旨在探討Netrin-1對(duì)缺氧損傷后自噬及細(xì)胞活性的調(diào)節(jié)作用,以及其受體UNC5H2對(duì)細(xì)胞自噬的影響,為Netrin-1的腦保護(hù)作用探尋新的機(jī)制。
1 材料與方法
1.1 細(xì)胞培養(yǎng)及建立過表達(dá)體系 人神經(jīng)母細(xì)胞瘤(SH-SY5Y)、HEK 293T細(xì)胞購(gòu)自American Type Culture Collection(ATCC)公司。細(xì)胞接種于含10%胎牛血清的DMEM培養(yǎng)基(Gibco)中,置于37 ℃、5%CO2的細(xì)胞培養(yǎng)箱中培養(yǎng)。每2~3 d更換培養(yǎng)液一次,生長(zhǎng)達(dá)80%融合時(shí)傳代,取第2-7代細(xì)胞分組進(jìn)行實(shí)驗(yàn)。HEK 293T細(xì)胞按104/ml的密度種植于六孔板及培養(yǎng)皿中,12 h貼壁后,按Lipofectamine 3000(Life Technologies)說明書步驟瞬轉(zhuǎn)UNC5H2-HA-tag質(zhì)粒及對(duì)照質(zhì)粒(吉?jiǎng)P生物),使用HA抗體(1∶1000,Abcam)進(jìn)行免疫熒光法測(cè)定轉(zhuǎn)染效率,觀察計(jì)數(shù)100個(gè)細(xì)胞并計(jì)算帶綠色熒光的細(xì)胞的比例。
1.2 OGD模型建立 該模型用于模擬體內(nèi)腦梗死環(huán)境并進(jìn)行自噬和細(xì)胞活性檢測(cè)[11]。SH-SY5Y按105/ml正常接種于96孔板后,吸凈原有培養(yǎng)基,更換成DMEM無糖培養(yǎng)基(Gibco),置于含有氧氣指示劑(日本三菱)的缺氧罐內(nèi),使用真空抽氣泵抽空罐內(nèi)空氣后,通入由95%N2及5%CO2組成的混合氣體,反復(fù)抽吸3次構(gòu)建缺氧環(huán)境,氧氣指示劑持續(xù)顯示粉紅色表明缺氧過程成功,最后將缺氧罐放置在37 ℃恒溫箱中2 h后置于正常氧糖環(huán)境中。
1.3 CCK-8試劑盒檢測(cè)細(xì)胞活性 SH-SY5Y接種于96孔板中,接種密度為105/孔,設(shè)6個(gè)復(fù)孔,培養(yǎng)24 h后細(xì)胞貼壁,分別加入以下藥物:對(duì)照組(不加藥);Netrin-1組(50 ng/ml);自噬誘導(dǎo)劑雷帕霉素組(Rapamycin,RAPA,50 nmol/L);Netrin-1+RAPA組;自噬抑制劑3-甲基腺嘌呤(3-Methyladenine,3-MA,1mmol/L);Netrin-1+3-MA組。藥物預(yù)處理2 h后置于OGD模型中,2 h后每孔加入10 μl CCK-8(日本同仁),37 ℃孵育1.5 h后用酶標(biāo)儀在450 mm波長(zhǎng)下進(jìn)行比色,計(jì)算相對(duì)吸光度,吸光度越高,細(xì)胞活性越高。
1.4 免疫熒光檢測(cè)自噬標(biāo)記物L(fēng)C3斑點(diǎn)形成 SH-SY5Y經(jīng)100%甲醇固定15 min,0.01 mol PBS搖床洗5 min 3次,加入封閉液封閉1 h后孵育兔抗大鼠LC3一抗(1∶100,CST)檢測(cè)LC3斑點(diǎn)形成,4 ℃冰箱放置過夜,加入delight 555標(biāo)記的山羊抗兔熒光二抗(1∶600,CST)室溫孵育1 h后再漂洗,封片后于共聚焦顯微鏡下拍照。
1.5 Western blot HEK 293T細(xì)胞用細(xì)胞裂解液(博彩生物)提取蛋白,BCA試劑盒(Thermo)進(jìn)行蛋白定量測(cè)定,蛋白樣品(每孔40 μg)進(jìn)行12% SDS-PAGE垂直電泳,恒流轉(zhuǎn)移至PVDF膜上,5%脫脂奶粉室溫封閉1 h,分別加入兔抗人一抗UNC5B、Beclin 1、p62、LC3及GAPDH,4 ℃孵育過夜,次日加入抗兔二抗室溫孵育1 h,加入化學(xué)發(fā)光HRP底物(Millipore)于熒光化學(xué)發(fā)光成像分析系統(tǒng)(Image Quant Las4000 mini)中曝光成像,采用Quantity One 分析條帶灰值。

2 結(jié) 果
2.1 CCK8檢測(cè)細(xì)胞活性 各組經(jīng)OGD處理后,與OGD對(duì)照組(0.63±0.03)相比,Netrin-1組(0.77±0.04)、3-MA組(0.76±0.03)以及3-MA+Netrin-1組(0.77±0.04)明顯增強(qiáng)SH-SY5Y活性(P均<0.05);而RAPA組(0.63±0.01)對(duì)細(xì)胞活性影響不明顯;與Netrin-1組相比,RAPA+Netrin-1組(0.65±0.02)的細(xì)胞活性下降(P<0.05)(見圖1),表明50 nmol/L RAPA能夠有效減弱OGD后Netrin-1對(duì)細(xì)胞活性的增強(qiáng)作用,而3-MA對(duì)此無明顯影響。
2.2 免疫熒光檢測(cè)LC3斑點(diǎn)形成 微管相關(guān)蛋白LC3是自噬標(biāo)志物。自噬小體形成時(shí),胞漿型LC3即LC3-I與磷脂酰乙醇胺共價(jià)結(jié)合,形成結(jié)合在自噬小體膜上的斑點(diǎn)狀LC3-Ⅱ(見圖2)。OGD時(shí)SH-SY5Y胞質(zhì)中可見大量LC3斑點(diǎn)形成,RAPA作用后斑點(diǎn)明顯增加,但Netrin-1組及3-MA組、Netrin-1+3-MA組則出現(xiàn)斑點(diǎn)數(shù)目明顯減少,提示Netrin-1減輕SH-SY5Y自噬活性。另外,Netrin-1+RAPA組的斑點(diǎn)數(shù)較Netrin-1組增加。
2.3 Western blot 過表達(dá)UNC5H2-HA組較對(duì)照質(zhì)粒組LC3-Ⅱ/GAPDH比值增高(P<0.05);對(duì)照質(zhì)粒組中進(jìn)行Netrin-1干預(yù)對(duì)自噬標(biāo)志物無明顯變化,而在UNC5H2-HA組中Netrin-1干預(yù)使LC3-Ⅱ相對(duì)比值下降,及Beclin 1相對(duì)下降,p62相對(duì)升高(P<0.05),提示細(xì)胞自噬活性降低(見圖3)。
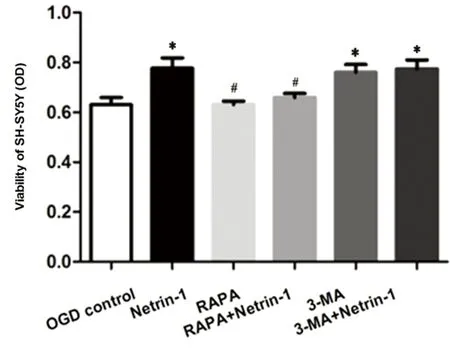
與對(duì)照組相比*P<0.05;與Netrin-1組相比#P<0.05
圖1 CCK8試劑盒檢測(cè)細(xì)胞活性

A:對(duì)照組;B:RAPA組;C:3-MA組;D:Netrin-1組;E:RAPA+Netrin-1組;F:3-MA+Netrin-1組 紅色熒光為L(zhǎng)C3-Ⅱ蛋白,藍(lán)色熒光為細(xì)胞核,1000×,標(biāo)尺=5μm
圖2 LC3免疫熒光染色

A:蛋白泳道從左到右:vehicle組,vehicle組+Netrin-1,UNC5H2-HA組,UNC5H2-HA組+Netrin-1;B:Beclin 1蛋白的相對(duì)比值變化;C:p62蛋白的相對(duì)比值變化;D:LC3-Ⅱ蛋白的相對(duì)比值變化與vehicle對(duì)照組相比*P<0.05; 與UNC5H2-HA組相比#P<0.05
圖3 過表達(dá)UNC5H2-HA及添加Netrin-1后的自噬標(biāo)志物表達(dá)改變
3 討 論
本實(shí)驗(yàn)通過對(duì)SH-SY5Y細(xì)胞活性和自噬相關(guān)標(biāo)志物L(fēng)C3的檢測(cè),結(jié)果發(fā)現(xiàn),OGD后SH-SY5Y自噬增強(qiáng),細(xì)胞活性降低,而給予Netrin-1預(yù)處理后,自噬明顯減弱并伴細(xì)胞活性增強(qiáng),這種增強(qiáng)作用可被自噬誘導(dǎo)劑RAPA所減弱。另外,過表達(dá)UNC5H2的HEK 293T細(xì)胞也出現(xiàn)自噬增強(qiáng),并且Netrin-1僅減弱該過表達(dá)系的自噬活性,提示Netrin-1在體外對(duì)OGD后的SH-SY5Y生存具有保護(hù)作用,且該保護(hù)作用可能通過UNC5H2介導(dǎo)降低自噬而實(shí)現(xiàn)。
自噬過程與細(xì)胞死亡密切相關(guān),并受mTOR、PI3K等多種因素調(diào)控,適量的自噬有助于適應(yīng)外界刺激促進(jìn)細(xì)胞存活,而過量的自噬則有可能加速細(xì)胞的死亡[12,13]。腦梗死后早期病灶周圍神經(jīng)元自噬即顯著增強(qiáng),但其作用尚存爭(zhēng)議。一般認(rèn)為這種自噬激活是一種損傷機(jī)制[14]。注射自噬抑制劑[10,15]或敲除自噬相關(guān)基因Beclin 1[16]后均可減少腦梗死面積并改善神經(jīng)功能;但也有實(shí)驗(yàn)認(rèn)為自噬激活具有保護(hù)作用,在側(cè)腦室注入自噬抑制劑預(yù)處理后腦梗死面積反而增大[17],這可能與所使用的動(dòng)物模型、藥物劑量和觀測(cè)時(shí)間點(diǎn)等不同相關(guān)[15,17]。本實(shí)驗(yàn)采用的神經(jīng)源性細(xì)胞SH-SY5Y與原代神經(jīng)元自噬活性相當(dāng),采用的50 nmol/L的RAPA和1 mmol/L的3-MA在保留有效調(diào)控自噬的同時(shí)可盡量避免對(duì)細(xì)胞造成損傷[18]。在該條件下通過觀察OGD后自噬對(duì)SH-SY5Y的影響,發(fā)現(xiàn)OGD后自噬增強(qiáng)不利于該細(xì)胞的生存,而Netrin-1對(duì)其生存的促進(jìn)作用則是通過減弱這種自噬來實(shí)現(xiàn)的,表現(xiàn)為L(zhǎng)C3的減低和p62的增高。
已經(jīng)發(fā)現(xiàn)過表達(dá)Netrin-1能夠減少腦缺血導(dǎo)致的神經(jīng)元死亡并改善神經(jīng)功能恢復(fù)[5~7],同時(shí),Netrin-1與其依賴性受體UNC5H2結(jié)合后能抑制細(xì)胞凋亡,促進(jìn)細(xì)胞存活[19]。為進(jìn)一步了解是否UNC5H2也介導(dǎo)Netrin-1對(duì)自噬的調(diào)節(jié)作用,我們構(gòu)建了過表達(dá)UNC5H2的HEK 293T細(xì)胞系,結(jié)果發(fā)現(xiàn)過表達(dá)UNC5H2明顯提高細(xì)胞的自噬活性,而同時(shí)給予Netrin-1干預(yù)后可減低這種自噬活性,提示Netrin-1/UNC5H2可能是自噬調(diào)節(jié)中的重要因素。
有研究發(fā)現(xiàn)UNC5在線蟲神經(jīng)元中與自噬相關(guān)基因UNC-51及UNC-14密切相關(guān),后兩者可協(xié)同定位UNC-5的亞細(xì)胞結(jié)構(gòu),并引導(dǎo)神經(jīng)元生長(zhǎng)[20]。另外,Netrin-1/UNC5H2介導(dǎo)的細(xì)胞存活的下游分子DAPK[21]也可能通過激活Beclin 1而啟動(dòng)自噬途徑[22],但其機(jī)制仍有待進(jìn)一步闡明。本實(shí)驗(yàn)沒有檢測(cè)Netrin-1的其他受體,如DCC、neoginin等,尚不能除外這些受體也在Netrin-1對(duì)自噬的調(diào)控起作用,這有待于今后深入研究。
[1]中華醫(yī)學(xué)會(huì)神經(jīng)病學(xué)分會(huì),中華醫(yī)學(xué)會(huì)神經(jīng)病學(xué)分會(huì)腦血管病學(xué)組.中國(guó)急性缺血性腦卒中診治指南2014[J].中華神經(jīng)科雜志,2015,48(4):246-257.
[2]Tian F,Deguchi K,Yamashita T,et al.In vivo imaging of autophagy in a mouse stroke model[J].Autophagy,2010,6(8):1107-1114.
[3]Rami A.Upregulation of Beclin 1 in the ischemic penumbra[J].Autophagy,2008,4(2):227-229.
[4]Ko SY,Dass CR,Nurgali K.Netrin-1 in the developing enteric nervous system and colorectal cancer[J].Trends Mol Med,2012,18(9):544-554.
[5]Lu H,Wang Y,He X,et al.Netrin-1 hyperexpression in mouse brain promotes angiogenesis and long-term neurological recovery after transient focal ischemia[J].Stroke,2012,43(3):838-843.
[6]Sun H,Le T,Chang TT,et al.AAV-mediated netrin-1 overexpression increases peri-infarct blood vessel density and improves motor function recovery after experimental stroke[J].Neurobiol Dis,2011,44(1):73-83.
[7]Lu H,Wang Y,Yuan F,et al.Overexpression of netrin-1 improves neurological outcomes in mice following transient middle cerebral artery occlusion[J].Front Med,2011,5(1):86-93.
[8]Bouhidel JO,Wang P,Siu KL,et al.Netrin-1 improves post-injury cardiac function in vivo via DCC/NO-dependent preservation of mitochondrial integrity,while attenuating autophagy[J].Biochim Biophys Acta,2015,1852(2):277-289.
[9]Goldberg MP,Choi DW.Combined oxygen and glucose deprivation in cortical cell culture:calcium-dependent and calcium-independent mechanisms of neuronal injury[J].J Neurosci,1993,13(8):3510-3524.
[10]Shi R,Weng J,Zhao L,et al.Excessive autophagy contributes to neuron death in cerebral ischemia[J].CNS Neurosci Ther,2012,18(3):250-260.
[11]Luo T,Liu G,Ma H,et al.Inhibition of autophagy via activation of PI3K/Akt pathway contributes to the protection of ginsenoside Rb1 against neuronal death caused by ischemic insults[J].Int J Mol Sci,2014,15(9):15426-15442.
[12]Oral O,Akkoc Y,Bayraktar O,et al.Physiological and pathological significance of the molecular cross-talk between autophagy and apoptosis[J].Histol Histopathol,2016,31(5):479-498.
[13]Tovary-Romo LB,Penagos-Puig A,Ramirez-Jarquin JO.Endogenous recovery after brain damage:molecular mechanisms that balance neuronal life/death fate[J].J Neurochem,2016,136(1):13-27.
[14]Wei K,Wang P,Miao CY.A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury[J].CNS Neurosci Ther,2012,18(11):879-886.
[15]Wen YD,Sheng R,Zhang LS,et al.Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways[J].Autophagy,2008,4(6):762-769.
[16]Zheng YQ,Liu JX,Li XZ,et al.RNA interference-mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats[J].Acta Pharmacol Sin,2009,30(7):919-927.
[17]Carloni S,Buonocore G,Balduini W.Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury[J].Neurobiol Dis,2008,32(3):329-339.
[18]李治國(guó),張繼紅,張錦華.mTOR通路對(duì)神經(jīng)母細(xì)胞瘤SH-SY5Y細(xì)胞自噬作用的影響[J].現(xiàn)代腫瘤醫(yī)學(xué),2014,10:2278-2280.
[19]Wu TW,Li WW,Li H.Netrin-1 attenuates ischemic stroke-induced apoptosis[J].Neuroscience,2008,156(3):475-482.
[20]Ogura K,Goshima Y.The autophagy-related kinase UNC-51 and its binding partner UNC-14 regulate the subcellular localization of the Netrin receptor UNC-5 in Caenorhabditis elegans[J].Development,2006,133(17):3441-3450.
[21]Llambi F,Lourenco FC,Gozuacik D,et al.The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase[J].EMBO J,2005,24(6):1192-1201.
[22]Bialik S,Kimchi A.Lethal weapons:DAP-kinase,autophagy and cell death:DAP-kinase regulates autophagy[J].Curr Opin Cell Biol,2010,22(2):199-205.
Netrin-1 protects SH-SY5Y neuroblastoma cells from oxygen-glucose deprivation by attenuating autophagy
QUE Jiali,LI Chenguang,LIU Kejia,et al.
(Department of Neurology,The First Affiliated Hospital of Sun Yat-sen University,Guangzhou 510080,China)
Objective To mimic hypoxic and ischemia environment caused by stroke,in vitro oxygen-glucose deprivation (OGD) model was established.The aim is to explore whether axon guidance factor Netrin-1 protectes SH-SY5Y neuroblastoma cells from OGD by regulating autophagy.Methods SH-SY5Ys were pretreated with Netrin-1 before OGD,apply applied autophagy inducer rapamycin and autophagy inhibitor 3-methyladeni-ne (3-MA) to modulate the autophagy flow,and transient transfected HEK 293T cell with UNC5H2-HA plasmid;cell viability was examined in CCK8 assay,western Western blot detected the autophagy markers (Beclin1,LC3,p62) and immunofluorescence detected punctuate LC3.Results We found that Netrin-1 improved cell viability and decreased the expression of LC3-Ⅱ,a protein related to autophagosome formation,in a manner sensitive to RAPA co-treatment.Additionally,the ability of Netrin-1 to decrease the expression of LC3-Ⅱ was present in UNC5H2-overexpressing HEK 293T cells but not vehivle-transfected cells,indicated the importance of Netrin-1-UNC5H2 interaction to this effect.Conclusion These results indicate that Netrin-1 may improve cell survival by attenuat-ing autophagy via UNC5H2.
Netrin-1; Oxygen-glucose derivation; Autophagy; UNC5H2
1003-2754(2016)06-513-04
2016-02-23;
2016-05-29
國(guó)家自然科學(xué)基金(No.81371276);廣東省自然科學(xué)基金(No.2014A030313065);廣東省重大神經(jīng)疾病診治研究重點(diǎn)實(shí)驗(yàn)室(No.2014B030301035)
(中山大學(xué)附屬第一醫(yī)院神經(jīng)科,衛(wèi)生部國(guó)家臨床重點(diǎn)專科,教育部國(guó)家重點(diǎn)專科,廣東 廣州 510080)
余 劍,E-mail:yujian21cn@163.com
R739.4
A

