三個喹啉氧基乙酰胺鑭系(Eu、Gd、Er)配合物的合成、結(jié)構(gòu)及Eu配合物的熒光性質(zhì)
毛盼東 陳亮 吳偉娜 賈磊 王元
(河南理工大學(xué)物理化學(xué)學(xué)院,焦作454000)
三個喹啉氧基乙酰胺鑭系(Eu、Gd、Er)配合物的合成、結(jié)構(gòu)及Eu配合物的熒光性質(zhì)
毛盼東陳亮*吳偉娜*賈磊王元
(河南理工大學(xué)物理化學(xué)學(xué)院,焦作454000)
合成并通過單晶衍射表征了3個稀土配合物L(fēng)n(L)(NO3)3(H2O)(L=N-苯基-2-(5-氯-8-喹啉氧基)乙酰胺,Ln=Eu(1),Gd(2),Er (3)),結(jié)構(gòu)與擁有相同有機配體的Pr,Nd和Sm配合物同構(gòu)。在每個配合物中,十配位的稀土離子采取扭曲的雙帽四方反棱柱配位構(gòu)型,分別與來自1個配體L的2個氧原子和1個氮原子,3個雙齒配位硝酸根和1個水分子配位。配合物1能夠發(fā)射Eu離子特征熒光,熒光壽命為437 μs。
酰胺配體;喹啉;稀土配合物;熒光;晶體結(jié)構(gòu)
Euand Tbcomplexes have been paid much attention due to their good luminescent properties, such as the large stokes shifts,narrow emission profiles,long lifetimes and so on[1-2].Generally,direct excitation of Euor Tbion is not efficient because of its inherently small absorption cross section[2].In particular,an organic chromophore,which serves as an antenna or sensitizer,absorbing the excitation light and transferring the energy from its lowest triplet state energy level(T)to the resonance level of Euand Tbions,is highly desired[1-8].
In fact,our previous work has proved that the Smcomplex with N-phenyl-2-(5-chloro-quinolin-8-yloxl)acetamide exhibit the characteristic emission ofthe Smion[9].As a continuation of our research,we report here the structures of the ligand′s another three Lncomplexes(Ln=Eu,Gd and Er),together with the fluorescence property of the Eu complex.
1 Experimental
1.1Materials and measurements
Solvents and starting materials for synthesis were purchasedcommerciallyandusedasreceived. Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.Fluorescent emission spectra were determined on an Edinburgh FLS980 spectrophotometer.
1.2Preparations of complexes 1~3
The Ln(Ln=Eu,1;Gd,2;Er,3)complexes have been synthesized according to the literature method[9].
1:Colorless blocks.Anal.Calcd.for C17H14ClN5O12Eu(%):C,30.58;H,2.11;N,10.49.Found(%):C, 30.62;H,2.00;N,10.55.FT IR(cm-1):ν(O-H)3 410, ν(C=O)1 661,ν(C=N)1 575,ν(Ar-O-C)1 170,ν1(NO3) 1 502,ν4(NO3)1 293,ρ(O-H)882.
2:Colorless blocks.Anal.Calcd.for C17H14ClN5O12Gd(%):C,30.34;H,2.10;N,10.41.Found(%):C, 30.23;H,2.21;N,10.49.FT IR(cm-1):ν(O-H)3 400, ν(C=O)1 663,ν(C=N)1 570,ν(Ar-O-C)1 169,ν1(NO3) 1 506,ν4(NO3)1 290,ρ(O-H)876.
3:Colorless blocks.Anal.Calcd.for C17H14ClN5O12Er(%):C,29.89;H,2.07;N,10.25.Found(%):C, 30.12;H,1.94;N,10.33.FT IR(cm-1):ν(O-H)3 404, ν(C=O)1 661,ν(C=N)1 575,ν(Ar-O-C)1 169,ν1(NO3) 1 502,ν4(NO3)1 287,ρ(O-H)878.
1.3X-ray crystallography
The X-ray diffraction measurement for complexes 1~3 were performed on a Bruker SMART APEX II CCDdiffractometerequippedwithagraphite monochromatized Mo Kα radiation(λ=0.071 073 nm) by using φ-ω scan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[10].The structures were solved by direct methods and refined by fullmatrixleast-square on F2using the SHELXTL-97 program[11].All nonhydrogen atoms were refined anisotropically.The H atoms for water molecules are located from difference Fourier map and refined with restraints in bond length and thermal parameters.All the other H atoms were positioned geometrically and refined using a riding model.Details of the crystal parameters,data collection and refinements for complexes 1~3 are summarized in Table 1.
CCDC:1419225,1;1419226,2;1419227,3.
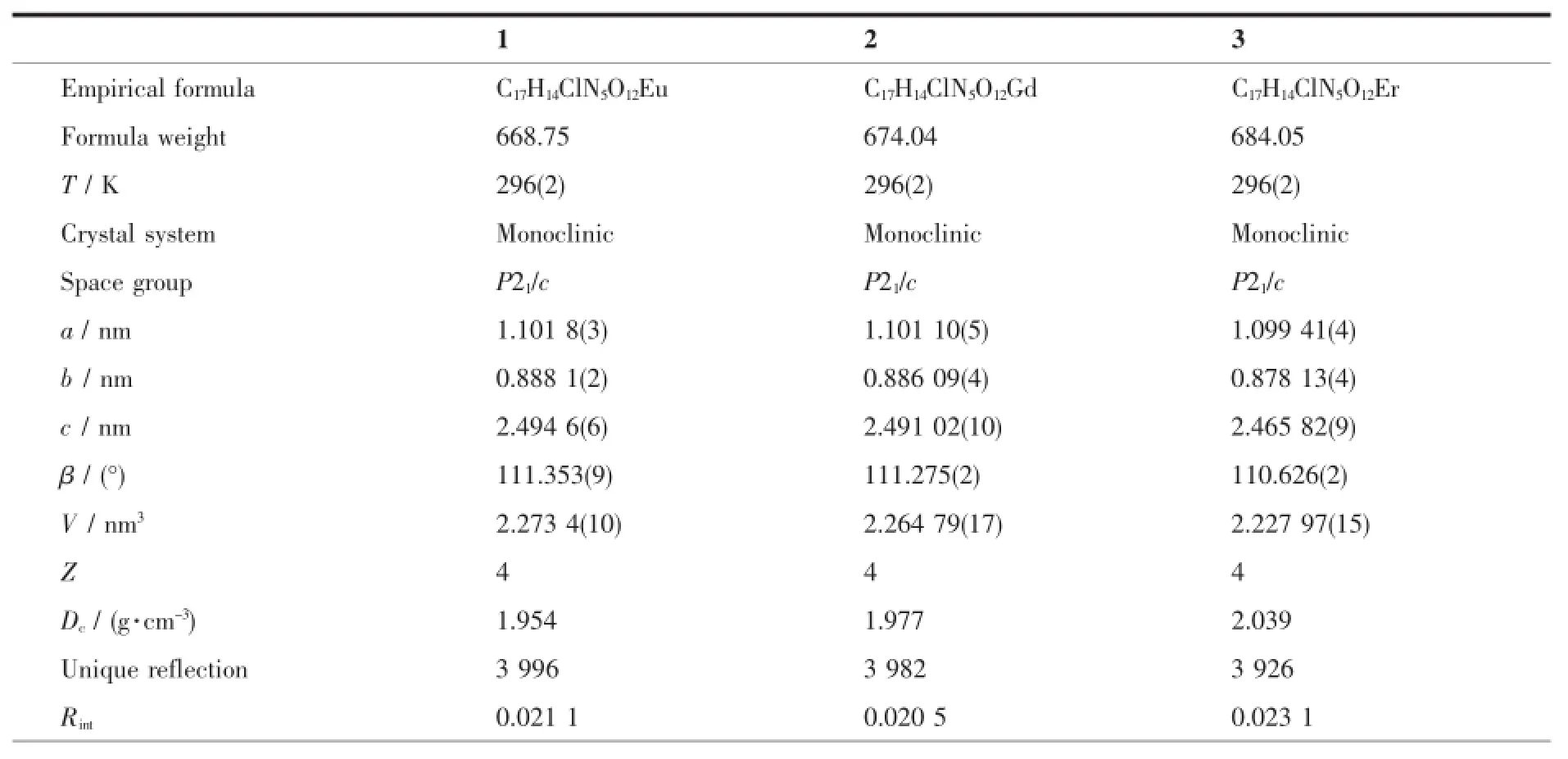
Table 1Selected crystallographic data for complexes 1~3
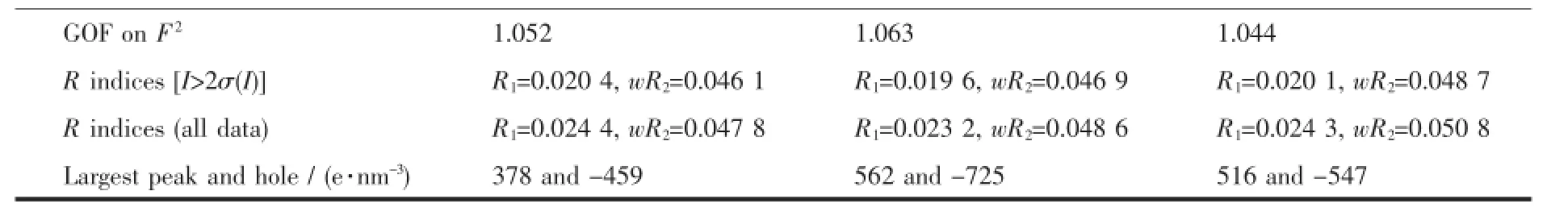
Continued Table 1
2Results and discussion

Table 2Selected bond lengths(nm)and bond angles(°)in complexes 1~3
2.1Crystal structures of the complexes
Selected bond distances,hydrogen bonds information for complexes 1~3 are summarized in Table 2 and 3,respectively.As shown in Fig.1a~c,complexes 1~3,namely Ln(L)(NO3)3(H2O)(Ln=Eu(1),Gd(2),Er (3))are isostructural and crystallize in the monoclinic, space group P21/c,same as the reported Pr,Nd and Sm complexes with same ligand[9].The Lnion ineach complex is surrounded by one tridentate L with NO2donor set,three bidentate nitrate anions and one water molecule,thus giving bicapped square antiprism coordinationgeometry.TheLn-O/Nbondlength decreases gradually with the increase of the atomic number of Lnions,which is consistent with the effect of lanthanide contraction.Similarly,in the crystal of each complex,intermolecular O-H…O (O12-H12A…O4iand O12-H12B…O8ii)and N-H…O(N2-H2A…O3iii)hydrogen bonds link the complexes into a 2D supramolecular network(Symmetry code:i-x+1,y+1/2,-z+1/2;ii-x+1,y-1/2,-z+1/2;iii-x+1, -y,-z).
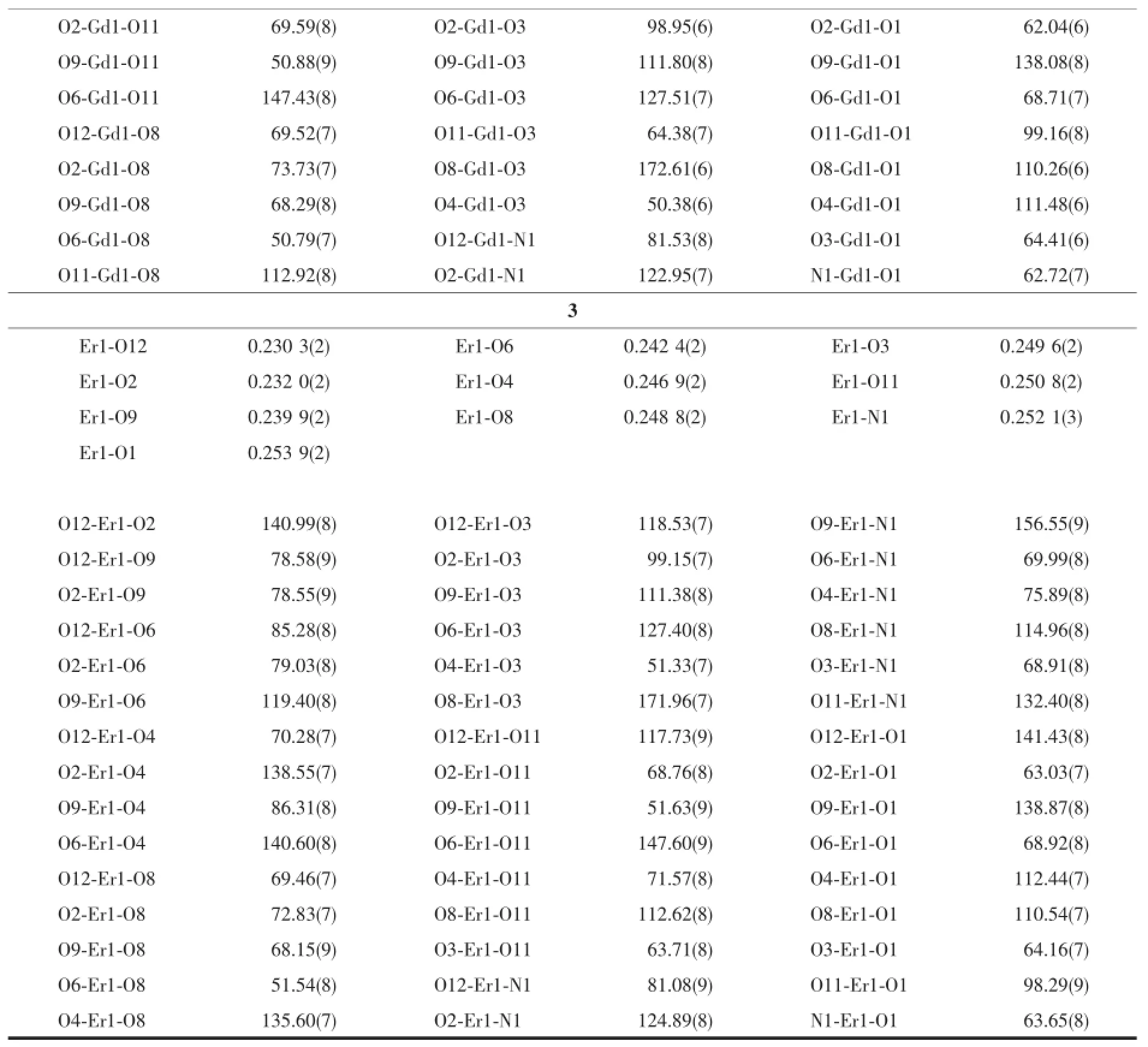
Continued Table 2

Table 3 Hydrogen bond information in complexes 1~3
2.2IR spectra
The spectral regions for all the complexes are more or less similar due to the similarity in coordination modes of the ligand with the metal centre. The free ligand L exhibit three absorption bands at 1 682,1 598 and 1 241 cm-1,assigned to ν(C=O), ν(C=N)and ν(C-O-C),respectively[5-9].However,in the complexes,three absorption bands assigned to ν(C= O),ν(C=N)and ν(C-O-C)in free ligand shift evidently to lower frequency,indicating that the oxygen atoms of the carbonyl group,quinoline nitrogen atoms and ethereal oxygen atoms take part in coordination to the central Lnion.Additionally,for all complexes,the characteristic absorptions of bidentate coordinated nitrate anions and coordinative water molecule could be observed[9].It is in accordance with the result of the crystal structure study.
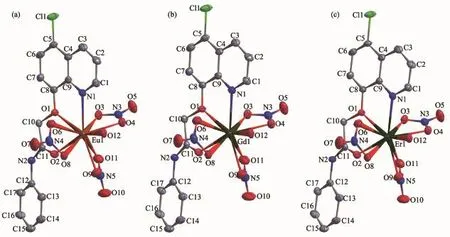
Fig.1Molecular structures of complexes 1(a),2(b)and 3(c)shown with 30%probability displacement ellipsoids
2.3Fluorescence spectra
As shown in Fig.2,emission transitions from5D0to7FJ(J=1,2,3)of Euion are clearly observed in the spectrum of Eucomplex 1(excited at 338 nm)[4-8]. It is found that the intensity of5D0→7F2transition is higher than that of5D0→7F1transition,and the intensity ratio is equal to 5.39,showing that the linelike emission spectrum results from intra-4f transitions of predominantly electric dipole character.Thus,Eulies in a non-centrosymmetric ligand field[5-8],which is in agreement with the result of the X-ray crystal structure analysis.By contrast,the emission intensity of the literature complex[Eu(L1)2(NO3)2]NO3(L1=N-(phenyl)-2-(quinolin-8-yloxy)acetamide)[8](excited at 332 nm)is much less than that of complex 1.In addition,complex 1 exhibits almost none of the free ligandemissionband,indicatinganefficiently intermolecular energy transfer from the ligand L to the Euion[12-13].The luminescence decay of complexes 1 and[Eu(L1)2(NO3)2]NO3were measured in solid state at room temperature(Fig.3).The lifetime of complex 1 and[Eu(L1)2(NO3)2]NO3is fitted to be 437 and 325 μs, respectively,which is moderate compared with some reported Euβ-diketonate complexes[12].
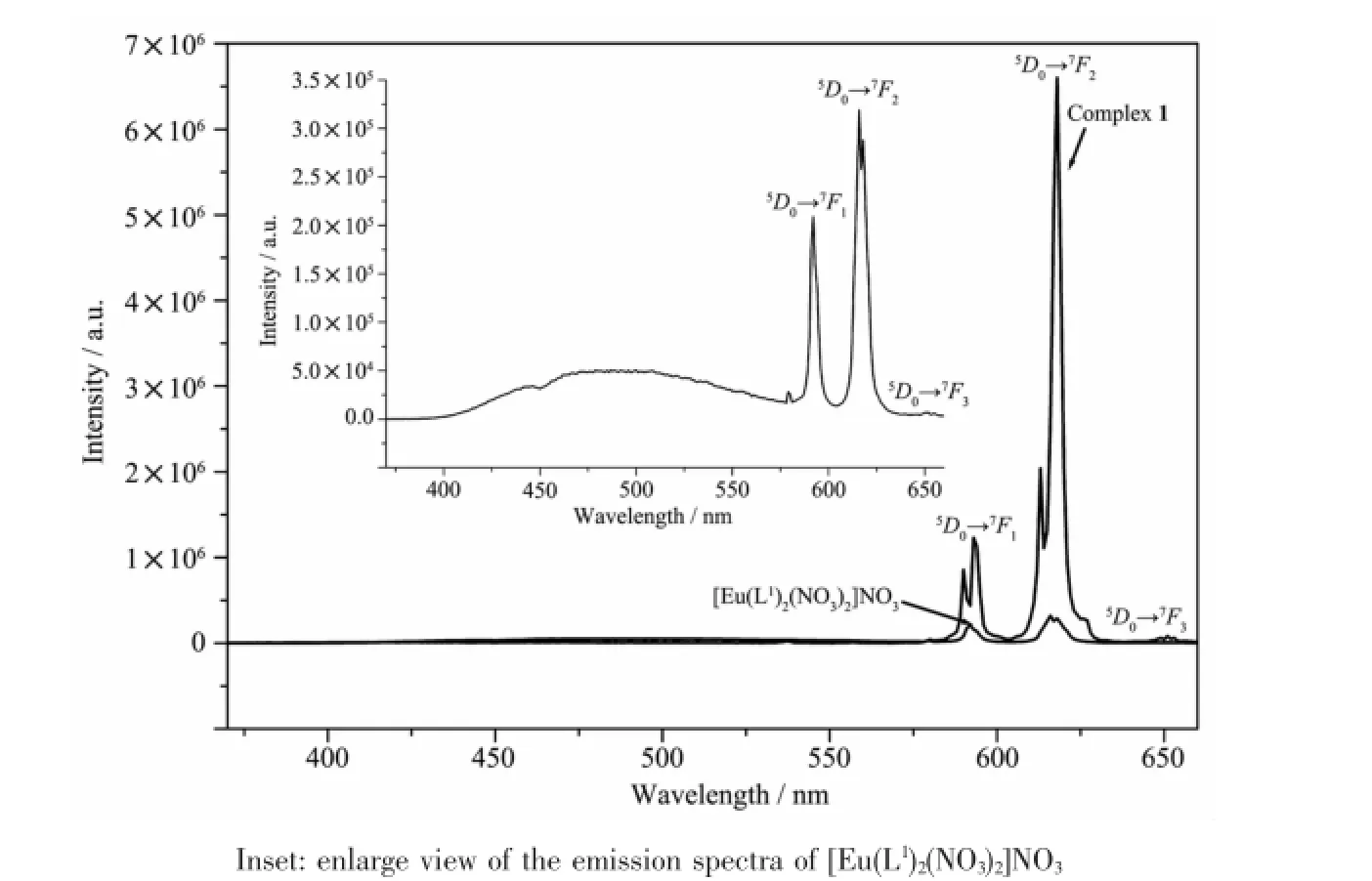
Fig.2 Fluorescence emission spectra of complex 1(excited at 338 nm)and[Eu(L1)2(NO3)2]NO3(excited at 332 nm)in solid state at room temperature
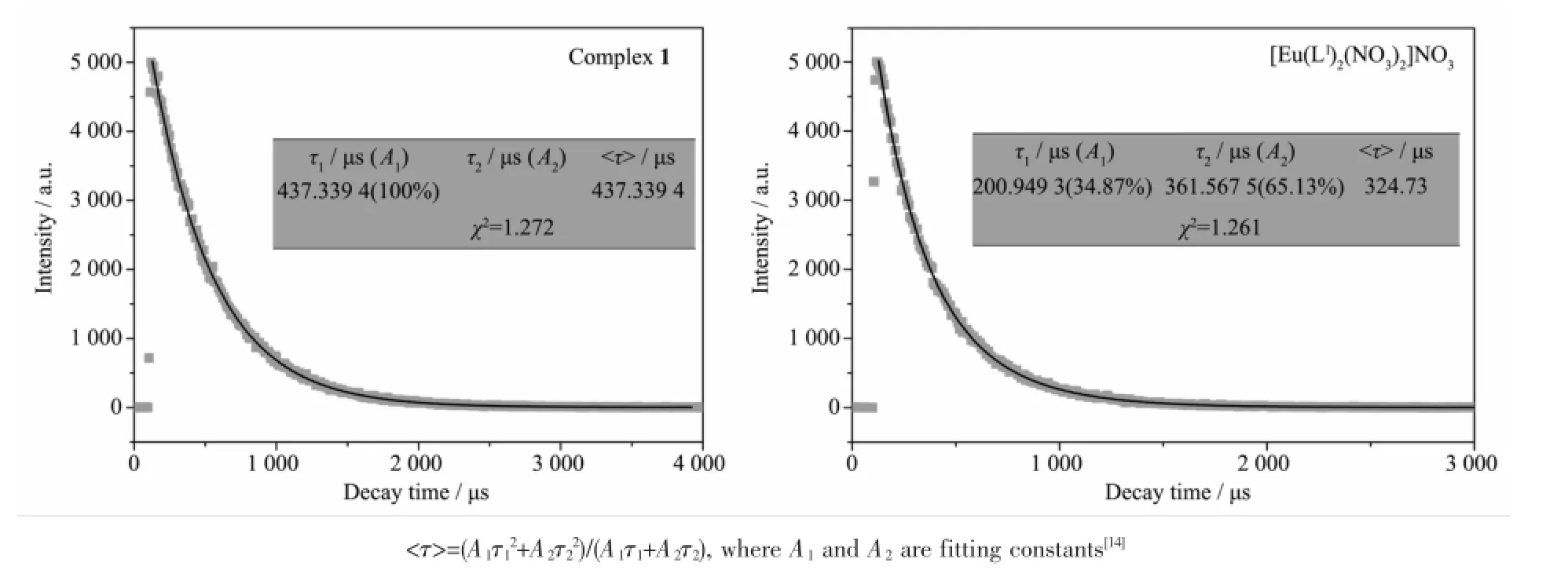
Fig.3 Fluorescence decay dots and fitted curves of complex 1 and[Eu(L1)2(NO3)2]NO3
To fully understand the energy transfer processes between the Euion and the organic ligand L, phosphorescence spectrum of the Gd complex 2 was recorded.At 77 K in a solution of methanol-ethanol (1∶1,V/V),complex 2 display only one weak phosphorescent emission band at 473 nm,which can be assumed to be the 0-0 transition[4-8].The triplet state energy level T of the ligand L,which was calculated from the shortest wavelength phosphorescence band of the corresponding Gdcomplex 2[4-8],is 21 142 cm-1, while that of L1is 22 831 cm-1(438 nm)as reported[8]. The energy level T of the ligand L is above the lowest excited resonance level5D1of Eu(19 020 cm-1), indicating that the absorbed energy could be transferred from ligand to the Eu ion.Furthermore,it is demonstratedthattheintramoleculartransfer efficiency depends mainly on two energy transfer processes:one is from the lowest triplet level of ligand to the resonance level of Lnby resonant exchange interaction;the other is just an inverse energy transfer by the thermal de-excitation mechanism.Both energy transfer rate constants are dependent on the energy difference(ΔET-5D1)[15-16].It has been noted that the well suitable ΔE in the range of 1 000~2 000 cm-1is associated with the strong fluorescence emission of Eucomplex[16].According to this,the strong luminescence of complex 1 should be due to the optimal(2 122 cm-1),thusconfirmingthattheligand L possesses higher energy transfer efficiency to the Euion than the reported ligand L1(3 811 cm-1). Comparing the structures of both ligands,it can be roughly concluded that the high emission intensity of complex 1 may be attributed to the additional electron -accepting chloro-substitute on the quinoline ring in the ligand L.Based on these studies,some new aryl amide type ligands could be synthesized to optimize the fluorescence properties of Eu.
References:
[1]Binnemans K.Coord.Chem.Rev.,2015,295:1-45
[2]Bünzli J C G.Coord.Chem.Rev.,2015,293-294:19-47
[3]Yan Z Z,Hou N,Wang C M.Spectrochim.Acta A,2015, 137:1265-1269
[4]Song X Q,Xing D Y,Lei Y K,et al.Inorg.Chim.Acta, 2013,404:113-122
[5]Wu W N,Tang N,Yan L.Spectrochim.Acta A,2008,71: 1461-1465
[6]Wu W N,Cheng F X,Yan L,et al.J.Coord.Chem.,2008, 61:2207-2215
[7]Wu W N,Tang N,Yan L.J.Fluoresc.,2008,18:101-107
[8]Wu W N,Yuan W B,Tang N,et al.Spectrochim.Acta A, 2006,65:912-918
[9]CAI Hong-Xin(蔡紅新).Thesis for the Master of Henan Polytechnic University(河南理工大學(xué)碩士論文).2014.
[10]Sheldrick G M.SADABS,University of G?ttingen,Germany, 1996.
[11]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of G?ttingen, Germany,1997.
[12]Li J,Li H,Yan P,et al.Inorg.Chem.,2012,51:5050-5057
[13]Li L,Zhao X,Xiao N,et al.Inorg.Chim.Acta,2015,426: 107-112
[14]Buddhudu S,Morita M,Murakami S,et al.J.Lumin.,1999, 83-84:199-203
[15]Chai W J,Li W X,Sun X J,et al.J.Lumin.,2011,131:225-230
[16]Sato S,Wada M.Bull.Chem.Soc.Jpn.,1970,43:1955-1962
Three Lanthanide(Eu,Gd,Er)Complexes with Quinolinyloxy Acetamide Ligand: Syntheses,Crystal Structures and Fluorescence Property of Eu Complex
MAO Pan-DongCHEN Liang*WU Wei-Na*JIA LeiWANG Yuan
(Department of Physics and Chemistry,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Three lanthanidecomplexes Ln(L)(NO3)3(H2O)based on L(Ln=Eu(1),Gd(2),Er(3);L=N-phenyl-2-(5-chloro-quinolin-8-yloxy)acetamide)have been synthesized and characterized by elemental analyses,IR spectra and X-ray diffraction analyses.The results reveal that all three complexes are isostructral with those of the Pr,Nd and Sm complexes bearing same ligand.The Lnion in each complex is surrounded by one tridentate L with NO2donor set,three bidentate nitrate anions and one water molecule,thus giving bicapped square antiprism coordination geometry.In addition,complex 1 could exhibit characteristic fluorescent emissions of the Euion in the visible region with the average lifetime being 437 μs.CCDC:1419225,1;1419226,2;1419227,3.
amide type ligand;quinolone;lanthanide complex;fluorescence;crystal structure
O614.33+8;O614.33+9;O614.344
A
1001-4861(2016)05-0336-07
10.11862/CJIC.2016.050
2015-10-25。收修改稿日期:2015-12-22。
國家自然科學(xué)基金(No.21404033,21401046,21001040)和河南省教育廳自然科學(xué)基金(No.12B150011,14B150029)資助項目。
*通信聯(lián)系人。E-mail:cl721@hpu.edu.cn,wuwn08@hpu.edu.cn;會員登記號:S06N6704M1112。
- 無機化學(xué)學(xué)報的其它文章
- 一個基于N′-(3-溴-5-氯-2-羥基苯亞甲基)-3-羥基-4-甲氧基苯甲酰肼的甲基麥芽酚配位的氧釩配合物:合成、晶體結(jié)構(gòu)及其胰島素增強活性
- 由V-型雙咪唑和芳香羧酸根配體共同構(gòu)筑的Zn配位聚合物的合成,結(jié)構(gòu)與性能
- 多次透射反射紅外光譜法靈敏和準(zhǔn)確地測量單晶硅中間隙氧和代位碳的含量
- 基于2-甲基-8-羥基喹啉的鏑單分子磁體的晶體結(jié)構(gòu)及磁性
- 兩個Schiff堿銅配合物的合成、晶體結(jié)構(gòu)、光譜性質(zhì)及取代基效應(yīng)
- 以6-甲基-2,3,5-吡啶三酸為配體的過渡金屬配合物的合成、結(jié)構(gòu)與性質(zhì)

