低氧環境對臍帶間充質干細胞相關細胞因子表達的影響
張沖,徐敏,毛成剛,錫洪敏,聶娜娜,高宏,李自普
低氧環境對臍帶間充質干細胞相關細胞因子表達的影響
張沖,徐敏,毛成剛,錫洪敏,聶娜娜,高宏,李自普
目的 觀察低氧環境對人臍帶間充質干細胞(hUCMSCs)分泌血管內皮生長因子(VEGF)、肝細胞生長因子(HGF)、堿性成纖維細胞生長因子(bFGF)、基質衍生因子-1(SDF-1)、干細胞因子(SCF)、胰島素樣生長因子(IGF-1)和粒細胞集落刺激因子(G-CSF)表達的影響。
方法 將第 3 代 hUCMSCs 分別置于 20% O2(常氧組)和 5% O2(低氧組)環境中培養,于 8、24、32、48、56、72 h ELISA 法檢測培養前及培養后上清液中 HGF、IGF-1、SDF-1、VEGF、bFGF、SCF、G-CSF 的濃度。
結果 低氧組培養上清液中 HGF、SDF-1、SCF、G-CSF 的濃度于培養后 72 h 明顯高于常氧組(P < 0.05);VEGF 的濃度于培養后 56 h 明顯高于常氧組(P < 0.05);bFGF 濃度于培養后 32 h 明顯高于常氧組(P < 0.05);IGF-1 濃度在培養后較常氧組無明顯變化(P > 0.05)。
結論 臍帶間充質干細胞培養后可持續表達細胞因子VEGF、HGF、IGF、bFGF、SCF、SDF-1 和 G-CSF,且 5%低氧培養環境可促進 VEGF、HGF、bFGF、SCF、SDF-1 和G-CSF 的表達,但對 IGF-1 表達無影響。
間質干細胞; 細胞低氧; 干細胞因子
www.cmbp.net.cn 中國醫藥生物技術, 2016, 11(5):437-440
人臍帶間充質干細胞(human umbilical cord mesenchymal stem cells,hUCMSCs)存在于臍帶沃頓膠和血管周圍組織,較其他間充質干細胞(mesenchymal stem cells,MSCs)具有更強的增殖分化能力、HLA-I 表達和神經誘導分化能力,且免疫功能低,無異體排斥反應。同時還具有來源廣泛,取材方便,便于保存及運輸,對供體無影響、無倫理爭議等優點,因此具有更為廣闊的應用前景。MSCs 可分泌血管內皮生長因子(vascular endothelial growth factor,VEGF)、基質衍生因子-1(stromal cell derived factor-1,SDF-1)等多種細胞因子,參與細胞遷移募集,促進血管生成,改善臟器功能。然而,目前大多體外細胞培養在常氧環境下進行,與體內生理或病理狀態下的低氧狀態相差甚遠。本研究通過觀察常氧和低氧環境下hUCMSCs 多種細胞因子分泌的變化,旨在探討低氧環境對 hUCMSCs 細胞因子分泌能力的影響。
1 材料與方法
1.1 材料
人臍血間充質干細胞由青島大學醫學院附屬醫院干細胞中心提供,產婦及家屬同意用于科學研究。實驗用間充質干細胞均經過微生物檢測及流式細胞儀檢測,細胞表型符合間充質干細胞的表型特征:CD34(-),CD45(-),CD90(+),CD105(+),HLA-DR(-)。微生物檢測:支原體檢測陰性,乙型肝炎病毒陰性,丙型肝炎病毒陰性,梅毒螺旋體陰性,巨細胞病毒陰性,HIV 陰性,需氧菌培養陰性,真菌培養陰性。
1.2 方法
將第 3 代的 hUCMSCs 按照 1 × 104個/cm2接種 6 孔培養板,每孔加入培養基 2 ml(DMEM培養基 + 10% FBS),并將其分為 2 組,正常氧濃度組(20% O2、5% CO2、75% N2,簡稱常氧組);低氧濃度組(5% O2、5% CO2、90% N2,簡稱低氧組)。將常氧組各培養板放入 20% O2濃度的培養箱中,低氧組各培養板放入 5% O2濃度的三氣培養箱中培養,分別于培養后第 8、24、32、48、56、72 小時取相應培養孔的上清液,離心。應用 ELISA法檢測上清液中各細胞因子的濃度。
1.3 統計學處理
2 結果
兩組 hUCMSCs 在不同培養時間 VEGF、SDF-1、肝細胞生長因子(hepatocyte growth factor,HGF)、堿性成纖維細胞生長因子(basic fibroblast growth factor,bFGF)、胰島素樣生長因子-1(insulin-like growth factor-1,IGF-1)、干細胞因子(stem cell factor,SCF)、粒細胞集落刺激因子(granulocyte colony-stimulating factor,G-CSF)的表達水平見表 l。與常氧組比較,低氧組中 HGF、G-CSF 濃度于 72 h 開始明顯高于常氧組,VEGF濃度于 56 h 開始明顯高于常氧組,bFGF 濃度于32 h 開始明顯高于常氧組,且差異均有統計學意義(P < 0.05)。SDF-1 濃度于培養開始 8 h 較常氧組明顯減低(P < 0.05),后隨培養時間延長逐漸升高,并于 72 h 明顯高于常氧組(P < 0.05)。SCF 濃度于 8、24、32 h 明顯低于常氧組(P 均 < 0.01),后隨時間延長逐漸升高,并于 72 h 明顯高于常氧組(P < 0.05)。IGF-1 濃度則較常氧組無明顯變化(P > 0.05)。
3 討論
目前,基于間充質干細胞的替代治療及組織工程等方面已經做了大量實驗研究和臨床試驗。間充質干細胞的旁分泌作用越來越受到研究者的重視。Ye 等[1]報道間充質干細胞可以分泌多種細胞因子及調節肽等,至少包括 34 種蛋白質。大量研究證實這些因子參與細胞的存活凋亡、營養代謝、增殖分化、遷移歸巢,有利于促進血管的生成、組織修復、改善心臟功能[2-6]。Tang 等[7]將 MSCs 注入大鼠心肌梗死邊緣區,2 周后發現 VEGF、bFGF、SDF-1 表達明顯升高,且提高了左室收縮功能,而Bax 表達明顯下降。從而提示 MSCs 可以通過旁分泌作用抑制心肌細胞凋亡,促進血管生成,改善心功能。隨著研究的深入,人們發現 MSCs 的旁分泌與受損部位的低氧環境密切相關。
機體正常和受損組織均處于低氧環境,MSCs移植后也處于低氧微環境中,因此研究低氧條件對MSCs 旁分泌作用的影響具有重要意義。國內外學者均已提出低氧可以作為誘導因素,刺激 MSCs的分泌,如 VEGF、HGF、SDF-1 等,但也有少數研究者得出不同結論。Wairiuko 等[8]提出體外低氧應激情況下,能激活 MSCs 的分泌活性,顯著促進 VEGF 和 bFGF 的釋放。Rosova 等[9]對 MSCs進行低氧預處理后發現培養基中 HGF 釋放明顯增加。Liu 等[10]于體外 3% O2濃度下培養BMSCs,發現 24、36、48 h 其分泌的 SDF-1 及其表面受體 CXCR4 均較常氧環境明顯升高,但Jing 等[11]的研究結果則與其相反。L?nne 等[12]于2.5% 的 O2環境下培養臍帶間充質干細胞 3 d,結果發現 VEGF、IGF、SCF 明顯增加,但 bFGF 較對照組無明顯變化。本實驗將臍帶間充質干細胞分別置于 20% O2、5% O2環境下進行培養,于 8、24、32、48、56、72 h 動態檢測培養基上清液中各生長因子的濃度,發現臍帶間充質干細胞可持續表達 HGF、SDF-1、SCF、G-CSF、VEGF、bFGF、IGF-1 多種細胞因子。低氧組 HGF、SDF-1、SCF、G-CSF、VEGF、bFGF 濃度隨培養時間逐漸升高,并明顯高于常氧組,提示低氧促進臍帶間充質干細胞 VEGF、bFGF、HGF、SDF-1、SCF、G-CSF 的分泌。但 IGF 濃度則無明顯變化,考慮此結果與既往文獻報道有所差異,原因可能為:① IGF-1 對低氧環境的敏感性低,此次低氧預處理的氧濃度未能達到其有效刺激濃度。②培養時間較短,培養液中游離的 IGF-1 太少,仍需進一步研究。此外本實驗還發現低氧環境下,細胞培養早期 VEGF、HGF、SDF-1、SCF、G-CSF 濃度較常氧環境低,后隨時間推移逐漸升高,并分別于不同時間點高于常氧組,提示臍帶間充質干細胞對低氧刺激需要一定的適應及反應時間。
表 1 低氧組和常氧組 hUCMSCs 細胞因子的表達()Table 1 The concentrations between hypoxia group and normoxia group ()
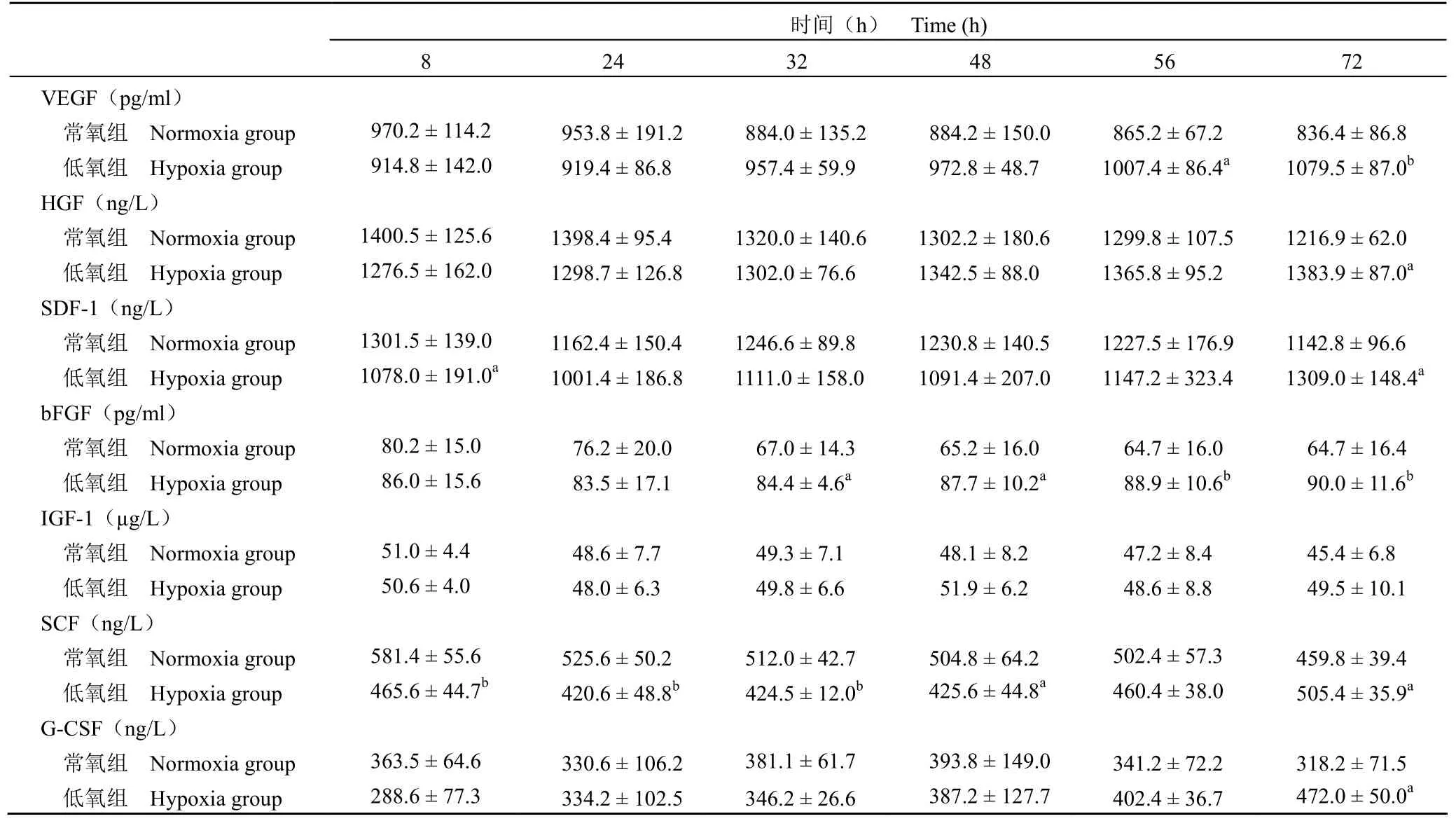
表 1 低氧組和常氧組 hUCMSCs 細胞因子的表達()Table 1 The concentrations between hypoxia group and normoxia group ()
注:與常氧組相比,aP < 0.05,bP < 0.01。Note: Compared with normoxia group,aP < 0.05,bP < 0.01.
時間(h) Time (h)8 24 32 48 56 72 VEGF(pg/ml)常氧組 Normoxia group 970.2 ± 114.2 953.8 ± 191.2 884.0 ± 135.2 884.2 ± 150.0 865.2 ± 67.2 836.4 ± 86.8低氧組 Hypoxia group 914.8 ± 142.0 919.4 ± 86.8 957.4 ± 59.9 972.8 ± 48.7 1007.4 ± 86.4a1079.5 ± 87.0bHGF(ng/L)常氧組 Normoxia group 1400.5 ± 125.6 1398.4 ± 95.4 1320.0 ± 140.6 1302.2 ± 180.6 1299.8 ± 107.5 1216.9 ± 62.0低氧組 Hypoxia group 1276.5 ± 162.0 1298.7 ± 126.8 1302.0 ± 76.6 1342.5 ± 88.0 1365.8 ± 95.2 1383.9 ± 87.0aSDF-1(ng/L)常氧組 Normoxia group 1301.5 ± 139.0 1162.4 ± 150.4 1246.6 ± 89.8 1230.8 ± 140.5 1227.5 ± 176.9 1142.8 ± 96.6低氧組 Hypoxia group 1078.0 ± 191.0a1001.4 ± 186.8 1111.0 ± 158.0 1091.4 ± 207.0 1147.2 ± 323.4 1309.0 ± 148.4abFGF(pg/ml)常氧組 Normoxia group 80.2 ± 15.0 76.2 ± 20.0 67.0 ± 14.3 65.2 ± 16.0 64.7 ± 16.0 64.7 ± 16.4低氧組 Hypoxia group 86.0 ± 15.6 83.5 ± 17.1 84.4 ± 4.6a87.7 ± 10.2a88.9 ± 10.6b90.0 ± 11.6bIGF-1(μg/L)常氧組 Normoxia group 51.0 ± 4.4 48.6 ± 7.7 49.3 ± 7.1 48.1 ± 8.2 47.2 ± 8.4 45.4 ± 6.8低氧組 Hypoxia group 50.6 ± 4.0 48.0 ± 6.3 49.8 ± 6.6 51.9 ± 6.2 48.6 ± 8.8 49.5 ± 10.1 SCF(ng/L)常氧組 Normoxia group 581.4 ± 55.6 525.6 ± 50.2 512.0 ± 42.7 504.8 ± 64.2 502.4 ± 57.3 459.8 ± 39.4低氧組 Hypoxia group 465.6 ± 44.7b420.6 ± 48.8b424.5 ± 12.0b425.6 ± 44.8a460.4 ± 38.0 505.4 ± 35.9aG-CSF(ng/L)常氧組 Normoxia group 363.5 ± 64.6 330.6 ± 106.2 381.1 ± 61.7 393.8 ± 149.0 341.2 ± 72.2 318.2 ± 71.5低氧組 Hypoxia group 288.6 ± 77.3 334.2 ± 102.5 346.2 ± 26.6 387.2 ± 127.7 402.4 ± 36.7 472.0 ± 50.0a
如上所述,低氧可以通過改變 MSCs 的細胞因子表達,影響其旁分泌作用,但具體機制仍不明確。Martin-Rendon 等[13]將 MSCs 在體外經低氧處理 24 h 后,發現約有 231 種 mRNA 受到缺氧的調節。進一步研究發現主要與低氧誘導因子-1α(HIF-1α)、NF-κB 等有關。郭輝等[14]分別在 21%和 3% O2環境中培養骨髓間充質干細胞 24 h,發現低氧組 HIF-1α 蛋白表達明顯增高。HIF-1α 是細胞適應低氧環境的關鍵蛋白轉錄調節因子,也是影響 MSCs 旁分泌作用的主要原因。HIF-1α 調控的靶基因有 60 多個[15]。VEGF 及 SDF-1 是已經證實的 HIF-1α 的兩大重要靶基因[16-19]。低氧增加間充質干細胞 HIF-1α 的活性,促使 HIF-1α 與VEGF、SDF-1 編碼基因的低氧反應元件結合,增加 VEGF、SDF-1 的分泌。HIF-1α 還可以誘導IGF-1、HGF 等多種細胞因子的表達。NF-κB 是一種存在于真核細胞的轉錄因子。近期的研究數據表明 NF-κB 在誘導因素的作用下可以促進 VEGF、bFGF 等細胞因子的分泌。Crisostomo 等[20]將人間充質干細胞置于含 NF-κB 抑制劑的培養基,并于1% O2環境中培養,24 h 后檢測培養液中 VEGF、bFGF、HGF 及 IGF 的濃度,發現低氧 +NF-κB 抑制劑組中各細胞因子的濃度較對照組明顯減低。此外細胞因子之間的相互作用也影響 MSCs 的分泌。SDF-1 與 CXCR4+ 間充質干細胞結合,可促進 VEGF、bFGF、HGF、IGF-1 等的分泌[10,21]。bFGF、G-CSF 可以促進 HGF 的表達[22]。上述因子通過相互作用,可以形成級聯放大效應,影響細胞的旁分泌作用。
綜上所述,間充質干細胞可持續分泌一些細胞因子,適度低氧刺激有利于間充質干細胞細胞因子的分泌,但具體機制仍有待進一步研究。
[1] Ye NS, Chen J, Luo GA, et al. Proteomic profiling of rat bone marrow mesenchymal stem cells induced by 5-azacytidine. Stem Cells Dev,2006, 15(5):665-676.
[2] Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res, 2004, 94(4):514-524.
[3] Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res, 2005, 97(7):663-673.
[4] Harada M, Qin Y, Takano H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med, 2005, 11(3):305-311.
[5] Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature, 2004, 428(6983):668-673.
[6] Ma N, Stamm C, Kaminski A, et al. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res, 2005, 66(1):45-54.
[7] Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg,2005, 80(1):229-237.
[8] Wairiuko GM, Crisostomo PR, Wang M, et al. Stem cells improve right ventricular functional recovery after acute pressure overload and ischemia reperfusion injury. J Surg Res, 2007, 141(2):241-246.
[9] Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells, 2008, 26(8):2173-2182.
[10] Liu H, Liu S, Li Y, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One, 2012, 7(4):e34608.
[11] Jing D, Wobus M, Poitz DM, et al. Oxygen tension plays a critical role in the hematopoietic microenvironment in vitro. Haematologica, 2012,97(3):331-339.
[12] L?nne M, Lavrentieva A, Walter JG, et al. Analysis of oxygen-dependent cytokine expression in human mesenchymal stemcells derived from umbilical cord. Cell Tissue Res, 2013, 353(1):117-122.
[13] Martin-Rendon E, Wilmot C, Carr C, et al. Hypoxic preconditioning promotes proliferation of mesenchymal stem cells in vitro and does not alter their effects in the infarcted rat heart in vivo//Annual autumn meeting of the British-society-for-cardiovascular-research. Southampton, England, 2005.
[14] Guo H, Zhang YJ, Chen YZ, et al. Expression of vascular endothelial growth factor in bone marrow mesenchymal stem cells under hypoxic conditions. J Clin Rehabil Tissue Eng Res, 2014, 23(18):3627-3632.(in Chinese)郭輝, 張于娟, 陳永珍, 等. 低氧環境下骨髓間充質干細胞中血管內皮生長因子的表達. 中國組織工程研究, 2014, 23(18):3627-3632.
[15] Acarregui MJ, Penisten ST, Goss KL, et al. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am J Respir Cell Mol Biol, 1999, 20(1):14-23.
[16] Busletta C, Novo E, Valfrè Di Bonzo L, et al. Dissection of the biphasic nature of hypoxia-induced motogenic action in bone marrow-derived human mesenchymal stem cells. Stem Cells, 2011,29(6):952-963.
[17] Zou D, Zhang Z, Ye D, et al. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem Cells, 2011, 29(9):1380-1390.
[18] Yun SP, Lee MY, Ryu JM, et al. Role of HIF-1alpha and VEGF in human mesenchymal stem cell proliferation by 17beta-estradiol:involvement of PKC, PI3K/Akt, and MAPKs. Am J Physiol Cell Physiol, 2009, 296(2):C317-C326.
[19] Liu L, Yu Q, Lin J, et al. Hypoxia-inducible factor-1α is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev, 2011, 20(11):1961-1971.
[20] Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol, 2008, 294(3):C675-C682.
[21] Liu X, Duan B, Cheng Z, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell, 2011, 2(10):845-854.
[22] Fujii K, Ishimaru F, Kozuka T, et al. Elevation of serum hepatocyte growth factor during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Br J Haematol, 2004, 124(2):190-194.
Objective To observe the effects of low oxygen on the expression of VEGF, HGF, bFGF, SDF-1, SCF, IGF, G-CSF in human umbilical cord mesenchymal stem cells (hUCMSCs).
Methods The third generation of hUCMSCs was divided into hypoxia group and normoxia group cultured in 5% oxygen and 20% oxygen, respectively. The concentration of HGF, IGF-1, SDF-1, VEGF, bFGF, SCF and G-CSF was detected by ELISA after culturing 8, 24, 32, 48, 56 and 72 h, respectively.
Results The concentration of HGF, SDF-1, SCF and G-CSF in hypoxia group was significantly higher than that of normoxia group after 72 h culture (P < 0.05); The concentration of VEGF and bFGF in hypoxia group was significantly higher than that of normoxia group after 56 h and 32 h culture, respectively (P < 0.05). However, the concentration of IGF-1 in hypoxia group had no significant change as compared to that of normoxia group (P > 0.05).
Conclusions The continuous expression of cytokines in hUCMSCs, such as HGF, IGF-1, SDF-1, VEGF, bFGF, SCF and G-CSF,could be found, and the expression level of the above cytokines except IGF-1 is increased by 5% oxygen.
Author Affiliation: Department of Cardiorenal Pediatrics (ZHANG Chong, XU Min, MAO Cheng-gang, NIE Na-na, LI Zi-pu),Neonatal Department (XI Hong-min), Stem Cell Center (GAO Hong), Affiliated Hospital of Qingdao University, Qingdao 266003,China
www.cmbp.net.cn Chin Med Biotechnol, 2016, 11(5):437-440
Effects of low oxygen on the expression of related cytokines in human umbilical cord mesenchymal stem cells
ZHANG Chong, XU Min, MAO Cheng-gang, XI Hong-min, NIE Na-na, GAO-Hong, LI Zi-pu
Mesenchymal stem cells; Cells hypoxia; Stem cell factor
LI Zi-pu, Email: 13370871121@163.com
10.3969/j.issn.1673-713X.2016.05.009
266003 青島大學附屬醫院心腎免疫兒科(張沖、徐敏、毛成剛、聶娜娜、李自普),新生兒科(錫洪敏),干細胞中心(高宏)
李自普,Email:13370871121@163.com
2016-05-25

