基于cDNA—SCoT差異顯示的甘蔗應答水分脅迫基因表達譜分析
吳凱朝 黃誠梅 鄧智年 魏源文 曹輝慶 徐林 蔣勝理 吳建明 楊麗濤 李楊瑞
Abstract:【Objective】The present study was conducted to study the expression of differential genes in sugarcane response to water stress and its molecular mechanism in order to provide scientific basis for developing genetic improvement of sugarcane drought resistant integrity. 【Method】Sugarcane cultivar GT11 was used as material to design three treatments viz. control, drought and rewatering. The +1st leaves of sugarcane for three treatments were used for extracting total RNA to construct their equal quantity mixed solutions, and the genetic expressing profiles of cultivar GT11 at elongation stage under water stress were constructed by using cDNA-SCoT differential display. The transcript derived fragments(TDFs) were screened and isolated to sequencing, followed by the BLAST homology searching in NCBI database to deduce gene function based on homological genes. 【Result】The 180 TDFs were screened by using 42 primers of SCoT marker, of which 100 TDFs were of higher similarity with the accessed genes in NCBI database. According to homologous gene function, these 100 TDFs could be classified into 14 types, which included 11 metabolism related genes, 12 energy metabolism related genes, 5 transport pathway related genes, 6 communication and signal transduction related genes, 3 cell cycle and DNA processing related genes, 3 transcript regulated factor related genes, 17 protein synthesis related genes, 1 protein processing related genes, 7 associated-functional protein related genes, 3 interaction with the environment related genes, 5 transposons, toxicity and plasmid protein related genes, 1 cell component biological synthesis related genes, 4 defense related genes, and 22 un-functional protein related genes. 【Conclusion】The regulating pathways of sugarcane response to water stress are of multiformity, complexity, coordination and networked; and the obtained water stress-related TDFs by cDNA-SCoT differential display could provide important information to further explore molecular mechanism sugarcane response to water regulation pathway.
Key words: sugarcane; cDNA-SCoT; water stress; rewatering; gene expressing profile
CLC number: S566 Document code: A Article:2095-1191(2016)12-1999-10
摘要:【目的】研究甘蔗應答水分脅迫差異基因表達,進一步探究甘蔗應答水分脅迫的分子機制,為開展甘蔗抗旱綜合性遺傳改良研究提供科學依據。【方法】選用GT11為材料,分別構建對照、干旱、復水3個處理總RNA混合池,使用cDNA-SCoT差異顯示構建GT11伸長期應答水分脅迫的基因表達譜,篩選、分離TDFs進行測序,在NCBI數據庫進行BLAST同源性檢索,根據同源基因推測基因功能。【結果】通過42條SCoT引物篩選得到180個TDFs,有100個TDFs與NCBI數據庫中已錄入的基因具有較高的相似性,根據同源基因功能可分為14類:新陳代謝相關基因(11個)、能量代謝相關基因(12個)、運輸途徑相關基因(5個)、通信及信號轉導相關基因(6個)、細胞循環及DNA加工相關基因(3個)、轉錄調控因子相關基因(3個)、蛋白質合成相關基因(17個)、蛋白質加工相關基因(1個)、結合功能蛋白相關基因(7個)、環境互作相關基因(3個)、轉座子、毒性及質粒蛋白相關基因(5個)、細胞成分生物合成相關基因(1個)、防御相關基因(4個)和未知功能蛋白基因(22個)。【結論】甘蔗應答水分脅迫調節途徑具有多樣性、復雜性、協調性和網絡性;應用cDNA-SCoT差異顯示篩選獲得水分脅迫相關TDFs,可為進一步研究甘蔗應答水分調控途徑的分子機理提供重要信息。
關鍵詞: 甘蔗;cDNA-SCoT;水分脅迫;復水;基因表達譜
0 Introduction
【Research significance】Over 80% of sugarcane in China are planted in dry sloping fields without irriga-
ting conditions, and yearly seasonal drought has become the main constraint factor on sugarcane production and sugar industry safety in China. At present, the high-yield and high-sucrose new sugarcane varieties with strong drought resistance and wide adap-
tability that bred by traditional breeding methods cant thoroughly solve sustaining threaten of drought and bring great loss in sugarcane production. Whereas, the comprehensive improvement of sugarcane drought resistance through molecular genetics maybe become one of alternative pathway to screen new drought resistant sugarcane varieties. Therefore, to explain the molecular mechanism of sugarcane response to water stress would be of important guiding significance on the genetic improvement of drought resistant integrity of sugarcane varieties.【Research progress】Star codon targeted polymorphism(SCoT) marker is a genome amplification technique by using single primer. It is characterized by fine repeatability, simple steps and low cost, and has been applied in genetic diversity researches of many crops viz. rice(Collard and Mackill,2009), longan(Chen et al., 2010), sugarcane(Chen et al., 2011), peanut(Xiong et al.,2011), Citrus sinensis(Jiang et al.,2011), Citrus(Han et al., 2011), potato(Gorji et al., 2011), grape(Guo et al., 2012), pineapple(Chen et al., 2012) and mango(Luo et al., 2011, 2012), and so on. cDNA-SCoT differential display is a new method for mRNA differential display analysis that bases on SCoT marker system and combines with RT-PCR. The cDNA-SCoT differential display is firstly applied in sugarcane by Wu et al.(2010) for analy-
zing the internode elongation-related gene segments of sugarcane response to gibberellin-induced, the results showed that this method was of simple operation, better repeatability, high recovery rate of differential segments and so on, which would play important role on gene differential expression. Hereafter, five gene segments of low temperature induced-related genes viz., dehydrin, cysteine-peptidase, polyamine oxidase, phosphate carboxylase, and catalase were amplified from sugarcane by using cDNA-SCoT differential display(Chen et al., 2010). The 22 disease-related gene segments could be amplified from sugarcane by analyzing differential expression induced of ratoon stunting disease through cDNA-SCoT(Chen et al., 2013). The 11 segments of cold resistant-related genes were amplified from Dendrobium candidum under cold stress condition by using differential display(Li et al., 2013). The gene expression change of artificial allopolyploid of Arachis at evolving early stage was analyzed by cDNA-SCoT differential display, and the results showed that 35 segments of related genes could be obtained from Arachis allopolyploid(He et al., 2014). 【Research breakthrough point】The above-mentioned results indicated that cDNA-SCoT differential display is an effective technology for studying plant gene expressing variation. It always takes 10-12 years to breed new drought resistant sugarcane va-
rieties by using traditional breed methods, whereas it only takes 1-2 years by transgenic genetic improvement method. Molecular genetic improvement could shorten new variety breeding period. Although some researching progresses of sugarcane drought resistance have been made by related researchers, while diffe-
rential gene expression of sugarcane response to water stress have not been reported by using cDNA-SCoT technology. 【Solving problems】In the present study, the biological information of differential expressing genes in sugarcane under water stress would be analyzed thoroughly to explain molecular mechanism of sugarcane resistant to drought, in order to provide scientific basis on studying genetic improvement of sugarcane drought resistant integrity and supplying corresponding candidate genes for other crops.
1 Materials and methods
1. 1 Experimental materials
This experiment was conducted in intelligent greenhouse in 2011. Sugarcane cultivar GT11 was used as tested material, and its seed-canes with single bud were treated with 30 min of 52 ℃ hot water for virus-free, followed by pre-germinating at 32 ℃ for 3 days before planting. Five neat germinating seed-canes were planted in each black plastic buckets(35 cm of height and upper diameter, 28 cm of bottom diameter), and 60 buckets in total were conducted on 26 June, 2011. After growth of sugarcane seedlings, only 3 seedlings were contained in each bucket.
1. 2 Experimental design
The experiment designed 3 treatments as the control(CK), drought(D) and re-watering(R) by using completely randomized block arrangement. The sugarcane seedlings of the control were given water normally with 75%-85% of relative water content in soil; the seedlings of drought treatment were stopped to water on 18, September and its leaves were sampled at 9:00 a.m. after 2, 5 and 9 days; the seedlings of re-watering treatment were re-watered on 9th day, and their leaves were sampled on 6, 12 and 24 hours after re-watering. The +1st leaves of 3-5 plants were sampled from each treatment, followed by cutting the leaf segment of 50-60 cm in length from hypertrophy zone to wipe off midrib. All samples were treated with liquid nitrogen freezing and saved at -80 ℃.
The relative water content of soil was determined by using drying weighing method. The relative water content of soil for drought treatment was 52.0%, 40.5% and 28.2% after stopping water for 2, 5 and 9 days, respectively, which reached moderate drought and serve drought(Zhu et al., 2010). After re-watering, the relative water content of soil reached about 80.0% for a suitable range of sugarcane growth at elongation stage.
1. 3 Total RNA extraction, RNA mixed solution construction and cDNA first strand synthesis
Total RNA was extracted from leaf samples by referenced method of Wu et al.(2012). The purification and concentration of RNA were detected by UV Spectrophotometer, and equal amount of total RNA of each treatment were mixed to construct sample mix-ture of the control, drought and re-watering treatment. The cDNA first strand synthesis was conducted by using AMV First Strand cDNA Kit of Fermentas Company. 1. 4 Amplification of SCoT-PCR and its products detection
The amplification system of SCoT-PCR was conducted according to method of Wu et al.(2013). The 20.0 μL reaction system was as follows: 10× Recation Buffer 2.0 μL, 2.5 mmol/L dNTPs Mixture 0.4 μL, 10 μmol/L SCoT Primer 2.0 μL, 5 U/μL BioReady rTaq 0.4 μL, 80 ng/μL cDNA template 1.0 μL, ddH2O 14.2 μL. The amplified reaction program was designed as follow: predegeneration for 5 min at 94 ℃; 35 cycles of degeneration for 30 s at 94 ℃, annealing for 45 s at 50 ℃, and extending for 60 s at 72 ℃; at last, extending for 10 min at 72 ℃. The 1.0 μL of amplified product was performed electrophoresis at 7.0% non-denaturing polyacrylamid gel, and gel was stained and colorated with silver staining-NaOH method.
1. 5 Recovery,verification,cloning,sequencing and analysis of TDFs
The differential bands were cut and put into 1.5 mL centrifuge tube, followed by supplying 50.0 μL TE solution into tube for standing 24 hours at normal temperature and placed in waterbath at 65 ℃ for 1 hour. After cooling, the tubes were centrifuged at 12000×g for 10 min. The supernatant of TDFs were used as template to perform PCR amplification with corresponding primers, the amplified reaction system and program were the same with SCoT-PCR method above-mentioned. The 1.0 μL PCR product was detec-
ted at 2% agarose gel; then the obtained TDFs was re-amplified by using 200.0 μL of PCR system and purified with Biospin Gel Extraction Kit(BioFlux Company). The reverse Northern blotting of purified TDFs was performed by using DIG-High Detection Kit instruction, followed by positive TDFs cloning using pMDTM18-T Vector Cloning Kit(TaKaRa) and competence strain Trans 5α. The cloned plasmids were sequenced by Sinogenomax Company. The TDFs sequences were searched for Blast homology in NCBI database(http://blast.ncbi.nlm.nih.gov/Blast.cgi) and deduced gene function based on homologous genes. The functional category of TDFs were performed in The MIPS Functional Catalogue Database(http://www.helmholtz-muenchen.de/).
2 Results and analysis
2. 1 RNA extraction and reverse-transcription
The clear and completing bands amplified were found in 28S and 18S of 3 treatments, whereas dispersive bands were found in 5S; the 28S band was thicker than that of 18S and no trailing, which indicated that the RNA extracted presented fine completeness without degradation and DNA contamination(Fig.1). The OD260/OD230 and OD260/OD230 of RNA samples was 2.05-2.27 and 1.92-2.00, which showed the high purification of RNA samples that could meet the demand of cDNA first strand synthesis. The dispersive bands of first strand cDNA were well-distributed, indicating that reverse transcription reacted very well to get better quality bands to meet the demand of cDNA-SCoT differential display analysis(Fig.2).
2. 2 Gene expressing profile construction of cDNA- SCoT in leaves of sugarcane, screening and functional category of TDFs under water stress
By using 42 SCoT primers to conduct PCR amplification of cDNA first strand plate of three treatments, about 1900 bands could be obtained and ranged from 100 to 2000 bp, and each primer could get 21-87 bands amplified, indicating that cDNA-SCoT had abundant diversity and isolating effectiveness in differ-
ential expressing genes. The amplified results of primer SP2, SP10, SP22, SP23, SP27 were shown in Fig.3. Through recovery of differential bands with fine repeating effects, 316 TDFs could be obtained and 180 TDFs with blue blots were gotten by verification of reverse northern blotting. The verified results of partial TDFs for different treatments were present in following Fig.4.
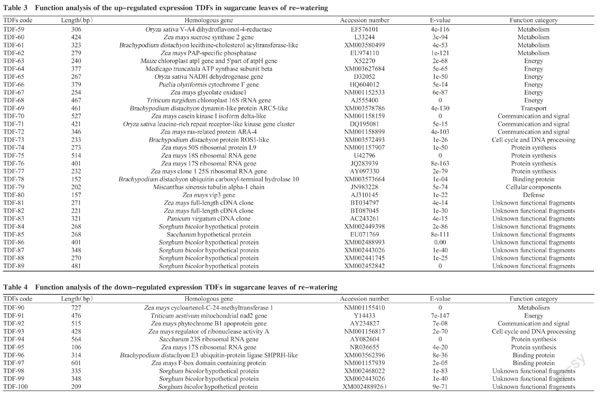
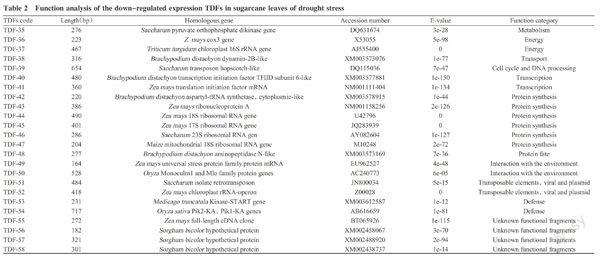
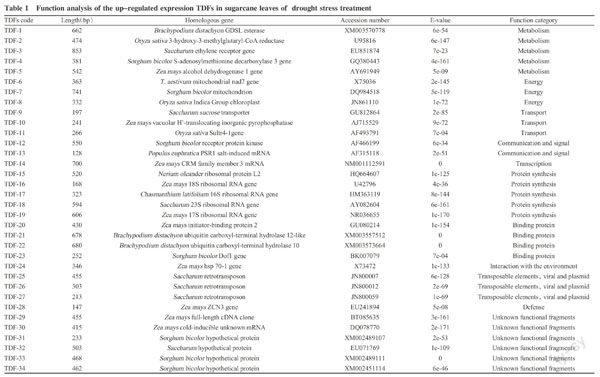
The positive TDFs verified were cloned and sequenced, followed by sequence searching and alignment by Blastx in NCBI database, and function determination of homologous genes. The results showed that 100 TDFs had higher similarity with the accessed genes in NCBI database. These TDFs could be classified into 14 types based on their functions, including metabolism related genes(11), energy metabolism related genes(12), transport pathway related genes(5), communication and signal transduction related genes(6), cell cycle and DNA processing related genes(3), transcript regulated factor related genes(3), protein synthesis related genes(17), protein processing related gene(1), associated-functional protein related genes(7), environmental interaction related genes(3), transposon, toxicity and plasmid protein related genes(5), cell component biological synthesis related gene(1), defense related genes(4) and unknown functional protein related genes(22)(Fig.5). Of 100 TDFs, 34 and 31 up-regulated expressing TDFs were found in drought and re-watering treatment, respectively, as well as 24 and 11 down-regulated expressing TDFs in them.
2. 3 Induced expressing genes analysis of sugarcane response to water stress
2. 3. 1 Up-regulated genes analysis in sugarcane leaves of drought treatment Under drought stress, 34 TDFs found to be presented up-regulated expression(Table 1). Of which, 5 TDFs involved in metabolism, 3 involved in energy metabolism, 3 involved in transportation pathway, 2 involved in communication and signal transduction, 4 involved in binding protein, 1 involved in transcriptional regulation, 5 involved in protein synthesis, 1 involved in interaction with the environment, 3 involved in transposon, toxicity and plasmid protein, and 1 involved in defense. These results indicated that sugarcane could activate response reaction from many aspects under drought stress, and their related genes expression presented as mRNA response diversity; the drought defense mechanism of sugarcane is a complete network by polygenes coordinating and regulating. According to the functions of homologous genes predicted, GDSL type lipase/esterlysis gene, 3-hydroxy-3-methyl-glutaryl CoA reductase gene, ethylene receptor gene,S-ademetionine decarboxylase gene, vacuole H+-pyrophosphatase gene, zinc finger protein gene and heat shock protein gene played an important regulating effects on plant stress resistance, their up-regulated expression showed that sugarcane positively responded to drought stress, and actively defended and regulated to keep its normal growth.
2. 3. 2 Down-regulated genes analysis in sugarcane leaves of drought treatment Under drought stress, 24 TDFs in sugarcane leaves presented up-regulated expression, and they involved to metabolism, energy meta-bolism, transportation pathway, cell cycle and DNA processing, transcription, protein synthesis, protein processing, interaction with the environment, transposon, toxicity and plasmid protein, and defense(Table 2). The sugarcane could defend the drought and keep normal growth through down-regulated response of these TDFs in leaves. The TFDs related to protein synthesis and modification presented higher frequency for 6 times, indicating that synthesis of many proteins might be hindered under drought stress, which corresponded to the results that lower protein content was found in sugarcane leaves under drought stress at previous researches(Wu et al.,2015). The tillering genes in leaves of sugarcane presented down-regulated expression to inhibit tillering emergence, reduce matter energy loss, and elude drought environment temporarily, which might be response to drought avoiding. The down-regulated expression of pyruvate phosphate dikinase, mitochondrial cytochrome oxidase I(mtCOI) and chloroplast gene and so on might be a defensive response for keeping normal growth through regulating energy loss reduction by respiratory meta-
bolism. The down-regulated expression of disease-related genes meant the sugarcane disease resistance weakening after suffering from drought.
2. 3. 3 Up-regulated genes analysis of re-watering treatment It found that, among all the up-regulated expression TDFs obtained from leaves of sugarcane given by re-watering after drought, both the metabolism-related genes and protein synthesis genes appeared four times; the energy metabolism-related genes appeared six times, the communication and signal-related genes appeared three times; the transport-related gene, cell cycle and DNA processing-related gene, cellular component-related gene, and defense-related gene only appeared one time(Table 3). These results indicated that, sugarcane accepted many aspects signal to fully initiate vigorous metabolic activities, synthesize substances and accelerate their transpotation for plant growth after re-watering. The vigorous metabolic activity was the basis for sugarcane growth. Sugarcane activated many aspects of metabolic regulation after re-watering to ensure its normal growth recovery. Especially, tubulin was the major component of plant microtubules and involves in cell division, its up-re-
gulated expression after re-watering in sugarcane leaves might be a marker for recovering rapid growth of sugarcane.
2. 3. 4 Down-regulated genes analysis in leaves of re-watering treatment The results shown in Table 4 indicated that, many related genes presented down-regulated expressing in leaves of sugarcane after re-wagering, which included cyclic steroid methyltransferase gene, NADH dehydrogenase gene, phytochrome mutant B1 gene, ribosomal RNA gene, RNase inhibitor, biotin ligase E3 gene and F-box protein gene. Of which, the phytochrome mutant B1 gene was able to transmit stress signal to cell and start related signal transduction factors of defense mechanism. Both biotin ligase E3 and F-box protein genes were related to protein regulation and had plant stress response related genes. So, the down-regulated genes of re-watering treatment were likely to be the up-regulated genes in sugarcane under drought condition, which would be further explored.
3 Discussion
Studying gene functions are always a focused issue by researchers. The gene sequence is firstly obtained to study gene functions for those species with unfinished whole genome sequencing. Sugarcane is one of species without finishing whole genome sequencing, therefore, the gene differential expressing profile analysis is the most important methods for getting drought resistant genes of sugarcane(Zhou, 2010; Rodrigues et al., 2011; Li et al., 2015). At the present study, the function of partial TDFs obtained by cDNA-SCoT differential display was similar with the results of previous researches. The CF0 ATP synthetase gene fragment obtained in our study is an important effecting factor for energy metabolism and photophosphorylation reaction just as CF1 ATPase subunit beta(Zhou, 2010), but their location in cell are different; CF0 ATP synthetase is located at chloroplast membrane, while CF1 ATPase subunit beta is located in chloroplast. The similar regulating pathway of S-adenosylmethionine decarboxylase(SAMDC) gene fragment obtained in our study was found with that of S-adenosylmethionine synthetase(SAMS) gene fragment obtained from sugarcane by GA3-induced using cDNA-SCoT differential display(Wu et al. 2010), which is related to polyamine synthesis. SAMS is one of main enzymes to catalyze S-adenosylmethionine(SAM) synthesis, and SAM is the biological synthesis precursor of ethylene and polyamine(Yue et al.,2008). SAMDC catalyzes SAM decarboxylation to produce aminopropyl that supply substrate for biological synthesis of Spd and Spm. Furthermore, the vacuolar H+-translocating inorganic pyrophosphatase gene and 3-hydroxy-3-methylglutaryl CoA reductase(HMGR) gene got from our study is related to drought resistant matter proline accumulation and terpene synthesis, respectively. These important drought resistant related gene fragments still need to be further identified in their functions to provide scientific basis on genetic improvement of sugarcane integrity of drought resistance.
The functions of TDFs obtained from the present study involved to many related genes viz., metabolism, energy, communication and signal, protein synthesis, cell cycle and DNA processing, binding protein, cellular components, and transposons and so on. This results indicated that sugarcane responding to water stress is a complex trait controlled by multi-genes and collaboratively regulated by multi-pathways, but it is unclear that which pathway or gene plays important role. Through cDNA-SCoT differential display is characterized by simple operation, fine repeatability, and lower cost etc., whereas its less primer limits the possibility to get more genetic information. Therefore, we should get genetic expressing information that thoroughly reflect sugarcane respond to water stress by transcriptome sequencing and differential display analysis in order to reveal its molecular mechanism of drought resistance.
4 Conclusion
In a conclusion, the regulating pathways of sugarcane response to water stress are of multiformity, complexity, coordination and network; and the water stress-related TDFs identified by cDNA-SCoT diffe-
rential display could provide important information to further explore molecular mechanism of sugarcane response to water regulation pathways, and be of important values on enriching drought resistant theory and genetic improvement of sugarcane varietal integrity by using drought genes derived from wilt germplasm resources.
References:
Chen H, He X H, Luo C, Gao M P. 2010. Analysis on the genetic diversity of 24 longan (Dimocarpus longan) accessions by SCoT markers[J]. Acta Horticulturae Sinica, 37(10):1651-1654.
Chen M H, Zhang B Q, Song X P, Chen H, Yang L T, Li Y R, Chen B S. 2013. cDNA-SCoT analysis of differentially expressed genes in sugarcane induced by Leifsonia xyli subsp. xyli[J]. Acta Agronomica Sinica, 39(6):1119-1126.
Chen P H, Chen R K, Xu L P, Wang H B, Chen L P, Lin B, Shi G J, Zhang Z, Gao S J, Guo J L, Pan Y B. 2011. Whole genome amplification of single pollen grains from a sugarcane cultivar and analysis of the genetic relatedness based on SCoT Markers[J]. Chinese Journal of Tropical Crops, 32(11):2069-2075.
Chen X L, Su W Q, Liu Y Q, Ren H, Lu Y Y. 2012. Analysis on genetic diversity of 36 pineapple collections by SCoT markers[J]. Southwest China Journal of Agricultural Sciences, 25(2):625-629.
Chen X L, Li Y R, Yang L T, Wu J M, Luo C, Xiong F Q, Yang L. 2010. cDNA-SCoT differential display of cold resistance related genes in sugarcane under low temperature stress[J]. Biotechnology Bulletin, 20(8): 120-124.
Collard B C Y, Mackill D J. 2009. Start codon targeted(SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants[J]. Plant Molecu-
lar Biology Reporter, 27(1):86-93.
Gorji A M, Poczai P, Polgar Z, Taller J. 2011. Efficiency of arbitrarily amplified dominant markers(SCoT, ISSR and RAPD) for diagnostic fingerprinting in tetraploid potato [J]. American Journal of Potato Research, 88(3): 226-237.
Guo D L, Zhang J Y, Liu C H. 2012. Genetic diversity in some grape varieties revealed by SCoT analyses[J]. Molecular Biology Reports, 39(5): 5307-5313.
Han G H, Xiang S Q, Wang W X, Jia Z G, Hong Q B, Liang G L. 2011. Establishment and application of SCoT molecular marker system for Citrus[J]. Acta Horticulturae Sinica, 38(7):1243-1250.
He L Q, Xiong F Q, Tang X M, Jiang J, Han Z Q, Zhong R C, Gao Z K, Li Z, He X H, Tang R H. 2014. Expression variation of genes in early period of Arachis artificial allopolypoidy evolution using cDNA-SCoT technique[J]. Acta Agronomica Sinica, 40(10):1767-1775.
Jiang Q Q, Long G Y, Li W W, Deng Z N. 2011. Identification of genetic variation in Citrus sinensis from hunan based on start codon targeted polymorphism[J]. Agricultural Science & Technology, 12(11):1594-1599.
Li C N, Xie J L, Wang W Z, Liang Q, Li Y J, Dong W B, Liu X Y, Yang L T, Li Y R. 2015. Screening of differentially expressed genes and analysis of plant hormones related genes under water stress in sugarcane[J]. Acta Agronomica Sinica, 41(7):1127-1135.
Li D B, Gao Y H, Si J P. 2013. SCoT differential expression of cold resistance related genes in Dendrobium officinale under low temperature stress[J]. China Journal of Chinese Materia Medica, 38(4):511-515.
Luo C, He X H, Chen H, Ou S J, Gao M P, Brown J S, Tondo C T, Schnell R J. 2011. Genetic diversity of mango cultivars estimated using SCoT and ISSR markers[J]. Biochemical Systematics & Ecology, 39(4):676-684.
Luo C, He X H, Chen H, Hu Y, Ou S J. 2012. Genetic relationship and diversity of Mangifera indica L. rrevealed through SCoT analysis[J]. Genetic Resources and Crop Evolution, 59(7):1505-1515.
Rodrigues F A, DaGraca J P, DeLaia M L, NhaniJr A, Galbiati J A, Ferro M I T, Ferro J A, Zingaretti S M. 2011. Sugarcane genes differentially expressed during water deficit [J]. Biologia Plantarum, 55 (1):43-53.
Wu J M, Li Y R, Wang A Q, Yang L, Yang L T. 2010. Differential expression of genes in gibberellin-induced stalk elongation of sugarcane analyzed with cDNA-SCoT[J]. Acta Agronomica Sininca, 36(11):1883-1890.
Wu K C,Huang C M,Li Y R,Yang L T,Wu J M. 2012. Fast and effective total RNA extraction from different tissues in 3 crops through the Trizol reagent method[J]. Journal of Southern Agriculture, 43(12):1934-1939.
Wu K C, Li Y R, Yang L T, Wu J M. 2013. Establishment, optimization and application of cDNA-SCoT reaction system in sugarcane[J]. Chinese Journal of Tropical Crops, 34(5):892-898.
Wu K C,Huang C M,Deng Z N,Cao H Q,Wei Y W,Xu L,Li Y R,Yang L T. 2015. Effects of drought stress and re-wate-
ring on physiological biochemical characteristics in sugarcane at elongation stage[J]. Journal of Southern Agriculture,46(7):1166-1172.
Xiong F Q, Zhong R C, Han Z Q, Jiang J, He L Q, Zhuang W J, Tang R H. 2011. Star codon targeted polymorphism for evaluation of functional genetic variation and relationships in cultivated peanut (Arachis hypogaea L.) genotypes [J]. Molecular Biology Reports, 38(5):3487-3494.
Yue C W, Xiao J, Ling X, Zeng N. 2008. Effect of low tempe-
rature stress on sweet potato S-adenosyl methionine synthetase gene expression[J]. Journal of Anhui Agricultural Sciences, 9(1):11-14.
Zhou G. 2010. Effect of osmotic stress and ethephon treatment on differential expression of protein in sugarcane[D]. Nanning:Guangxi University.
Zhu L H, Xing Y X, Yang L T. Li Y R, Yang R Z, Mo L X. 2010. Effects of water stress on leaf water and chlorophyll fluorescence parameters of sugarcane seedling[J]. Journal of Anhui Agricultural Sciences, 11(5):17-21.
(責任編輯 韋莉萍)

