兩個(gè)基于2,2′-聯(lián)吡啶-5,5′-二羧酸配體構(gòu)筑的堿土金屬配合物的合成、晶體結(jié)構(gòu)及其光致發(fā)光性質(zhì)
區(qū)泳聰鐘均星 宋縈怡 朱嵐嵐 吳建中
(廣州市能源轉(zhuǎn)化與儲(chǔ)能材料重點(diǎn)實(shí)驗(yàn)室,華南師范大學(xué)化學(xué)與環(huán)境學(xué)院,廣州 510006)
?
兩個(gè)基于2,2′-聯(lián)吡啶-5,5′-二羧酸配體構(gòu)筑的堿土金屬配合物的合成、晶體結(jié)構(gòu)及其光致發(fā)光性質(zhì)
區(qū)泳聰*鐘均星宋縈怡朱嵐嵐吳建中
(廣州市能源轉(zhuǎn)化與儲(chǔ)能材料重點(diǎn)實(shí)驗(yàn)室,華南師范大學(xué)化學(xué)與環(huán)境學(xué)院,廣州510006)
摘要:從2,2′-聯(lián)吡啶-5,5′-二羧酸配體出發(fā),利用溶劑熱的方法,合成了兩種新穎的堿土金屬配位聚合物[Ca(bpdc)(DMF)2(H2O)2]n(1)和[Mg(bpdc)(H2O)3]n(2),并且對(duì)其進(jìn)行了元素分析、紅外光譜、熱重分析和光致發(fā)光性質(zhì)表征。單晶結(jié)構(gòu)分析表明,配合物1和2都是一維鏈結(jié)構(gòu),并且通過分子間的氫鍵和π-π堆積作用連接成三維的超分子結(jié)構(gòu)。有趣的是,固態(tài)熒光光譜顯示兩種結(jié)構(gòu)的熒光出現(xiàn)了明顯的不同,聯(lián)系結(jié)構(gòu)分析推斷可能是有機(jī)配體的不同配位構(gòu)象導(dǎo)致的。
關(guān)鍵詞:堿土金屬配合物;溶劑熱合成;構(gòu)象;光致發(fā)光
國(guó)家自然科學(xué)基金(No.21401058)和廣東普通高校青年創(chuàng)新人才項(xiàng)目(No.2014KQNCX058)資助。*通信聯(lián)系人。E-mail:ouyongcong@m.scnu.edu.cn;會(huì)員登記號(hào):S06N6724M1506。
Metal coordination polymers (MCPs) have been widely researched in recent years not only due to their novel and intriguing topological structures, but also because of various potential applications in many aspects, for example, as porous, magnetic, luminescent and catalytic materials[1-4]. Among them, transition metal and lanthanide metal complexes have been extensively studied, but there are relatively less reported for the alkaline-earth metal complexes[5-7]. As the characteristics of alkaline-earth are intriguing for their electronicstructures, wide emission peaks and large Stokes shift, the syntheses of alkaline-earth complexes would be pregnant.
2,2′-Bipyridine-5,5′-dicarboxylic acid (H2bpdc), as a multifunctional bridging ligand, has been used to construct various MCPs[8-15]. According to our previous studies[16-17], the different coordination modes might lead to the different properties. It′s interesting that H2bpdc can be fixed in the structure in two modes: the N-atoms are in trans- or cis-position. To our best knowledge, the researches on the different photoluminescent properties based on the different conformation of H2bpdc are rare[9,12,18]. Herein, two novel coordination complexes, [Ca(bpdc)(DMF)2(H2O)2]n(1) and [Mg(bpdc) (H2O)3]n(2), are constructed via solvothermal conditions and characterized by elemental analysis, IR spectra, thermogravimetric analysis and photoluminescent measurement. To be noticed that the solid-state fluorescent emissions of 1 and 2 are different in related to the different conformation modes of the bpdc2 -ligand.
1 Experiment
1.1 Materials and physical measurements
The chemicals and solvents were commercially available and used without further purification. C, H, and N microanalyses were carried out with Elementar Vario-EL CHN elemental analyzer. FT-IR spectra were recorded on Nicolet FT-IR-170SX spectrophotometer in KBr tablets in the range of 4 000~400 cm-1. X-ray powder diffraction (XPRD) intensities for polycrystalline samples of complex 1 and 2 were measured at 293 K on Bruker D8 Advance Diffrato-meter (Cu Kα,λ=0.154 056 nm) by scanning over the range of 5°~50°(3°~50°for 2) with step of 0.2°·s-1. Simulated XPRD patterns were generated with Mercury. Thermogravimetric analyses were performed on Perkin-Elmer TGA 7. Luminescent spectra for solid state samples were recorded on Hitachi F-2500 at room temperature with a xenon arc lamp as the light source.
1.2 Synthesis of [Ca(bpdc)(DMF)2(H2O)2]n(1)
A mixture of H2bpdc (0.1 mmol, 24 mg), CaCl2(0.1 mmol, 120 mg) and succinic acid (0.1 mmol, 120 mg) in DMF (4 mL) was sonicated 5 min and then heated in a stainless steel reactor with Teflon liner (23 mL)at 120℃for 72 h and cooled to ambient temperature at a rate of ca. 5℃·h-1to give colorless sheet crystals (Yield based on H2bpdc: 35 mg, 71.6%). Elemental analysis calcd. for C18H24O8N4Ca(%): C 46.54, H 5.21, N 12.06; Found(%): C 46.35, H, 5.29, N 11.96.
1.3 Synthesis of [Mg(bpdc)(H2O)3]n(2)
A mixture of H2bpdc (0.1 mmol, 23 mg), MgCl2(0.1 mmol, 90 mg) and two drops triethylamine in DMF (3 mL) and H2O (2 mL) was sonicated 5 min and then heated in a stainless steel reactor with Teflon liner (23 mL) at 120℃for 72 h and cooled to ambient temperature at a rate of ca. 5℃·h-1to give colorless column crystals (Yield based on H2bpdc: 27 mg, 89.4%). Elemental analysis calcd. for C12H12O7N2Mg(%): C 44.96, H 3.77, N 8.74; Found(%): C 45.10, H, 3.79, N 8.81.
1.4 X-ray crystallography
Diffraction data for compound 1 was collected on Rigaku R-AXIS SPIDER Image Plate diffractometer with graphite-monochromated Mo Kα radiation (λ= 0.071 073 nm) at 150(2) K. Diffraction data for compound 2 was recorded on Bruker smart ApexⅡCCD area detector diffractometer (Mo Kα radiation,λ= 0.071 073 nm) at 296(2) K. The intensities were integrated using SAINT+. Corrections for Lorentz and polarization effects were applied. Absorption corrections were applied by using the multi-scan program SADABS[19]. The structures were solved by direct method, and all non-hydrogen atoms were refined anisotropically by least-squares method on F2using the SHELXTL program[20]. The organic hydrogen atoms were generated geometrically; the aqua hydrogen atoms were located from difference maps and refined with isotropic temperature factors. Crystal data as well as details of data collection and refinements for all compounds are summarized in Table 1. Table 2 and Table 3 give the data of selected bond lengths and angles and the hydrogen bond parameters for complexes 1 and 2 respectively.
CCDC: 1444695, 1; 1444696, 2.
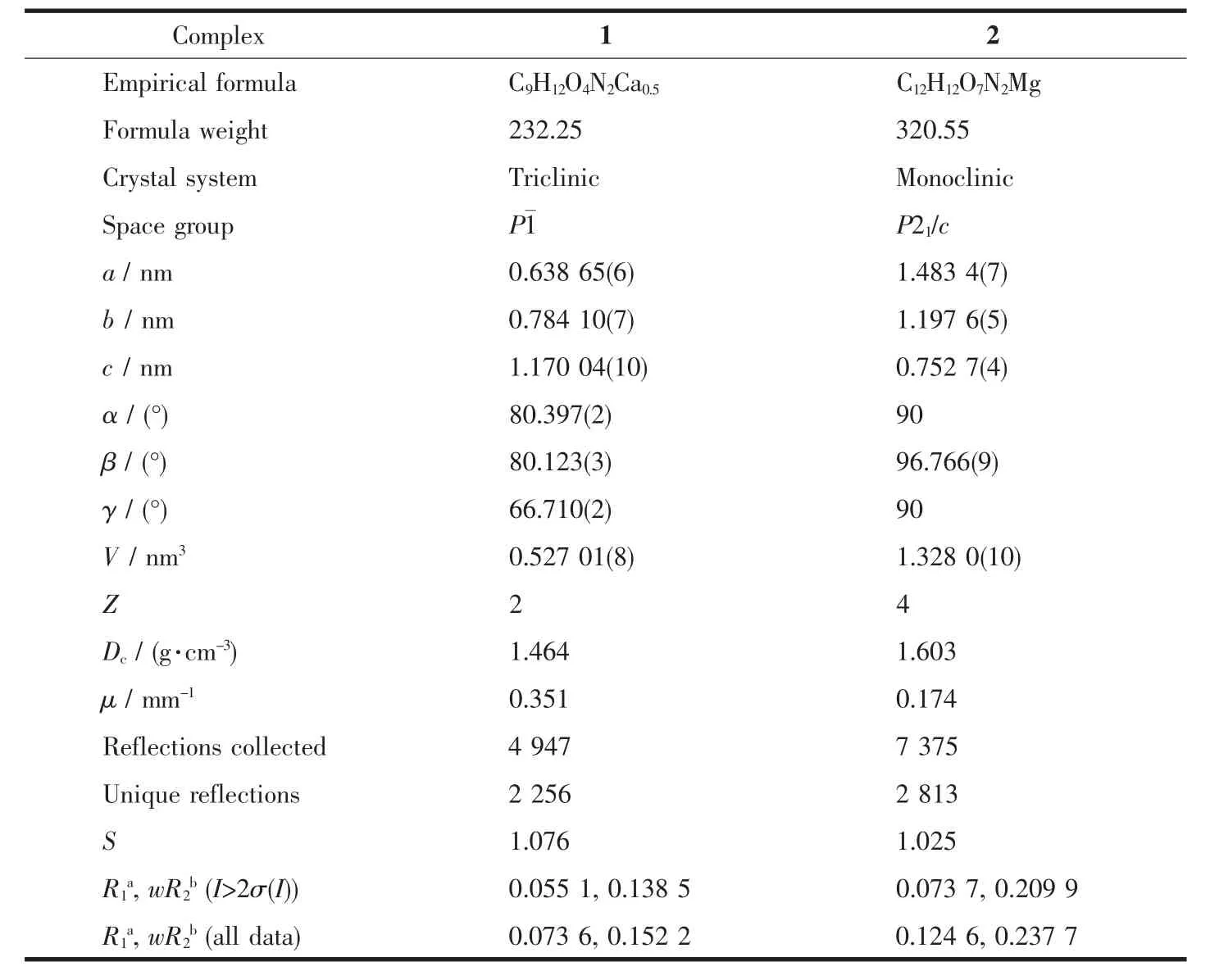
Table1 Crystal data and structure refinements for 1 and 2
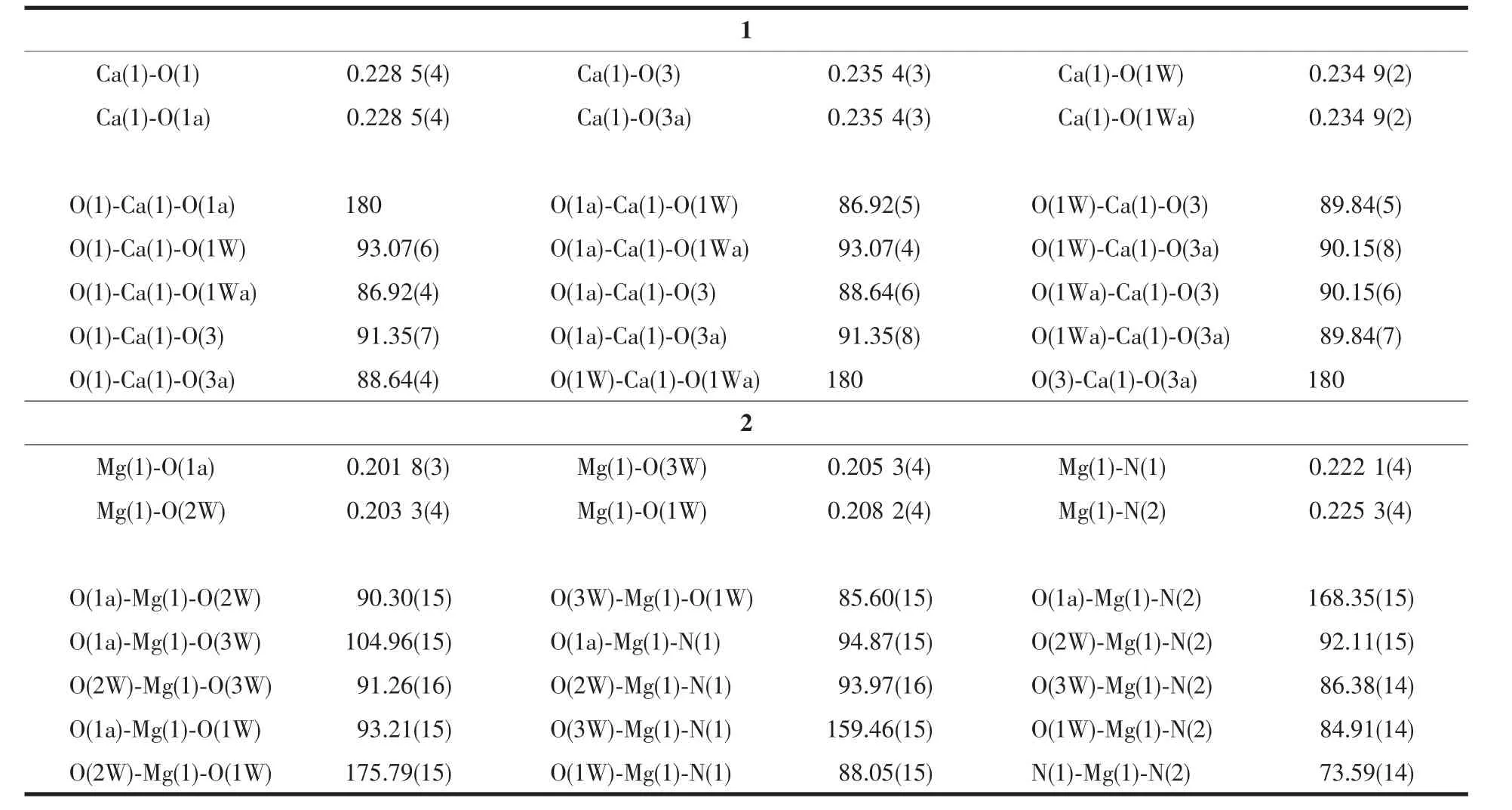
Table2 Selected bond lengths (nm) and angles (°) for 1 and 2

Table3 Hydrogen bond parameters for 1 and 2
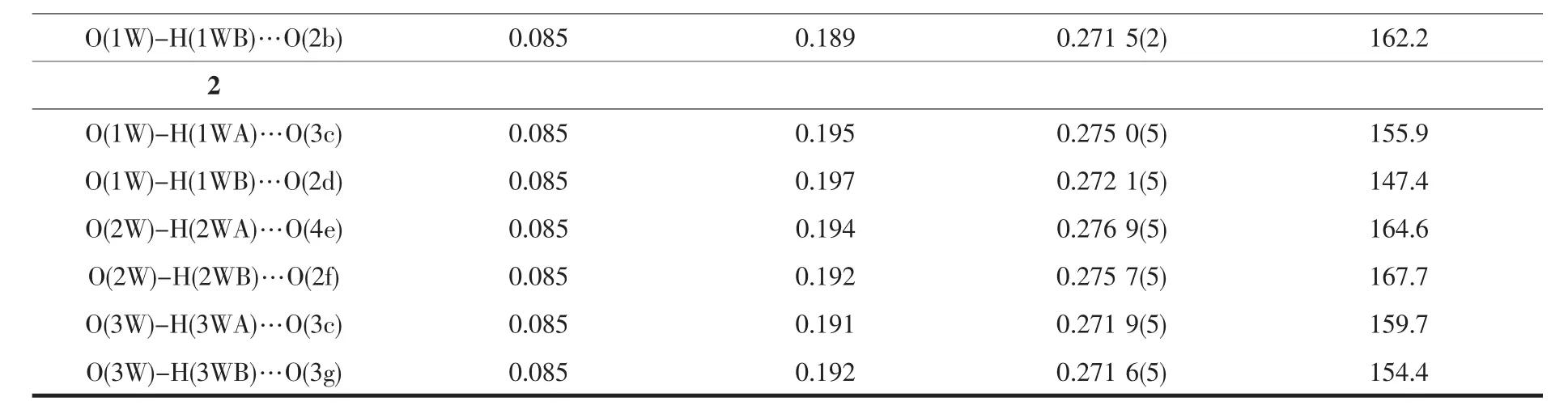
Continued Table 3
2 Results and discussion
2.1 Crystal structures
Single crystal structure shows that complex 1 crysyallizes in P1 space group and contains half calcium ion, half bpdc2-ligand, one DMF molecule and one water molecule in asymmetric unit (Fig.1a). Ca2+ion is surrounded with six oxygen atoms from two carboxylate groups of bpdc2 -ligands, two water moleclues and two acyl groups of DMF molecules. The bpdc2-ligands connect two Ca atoms as bridges to form an infinite chain, and the nitrogen atoms of ligand were fixed in the lattice in trans-position (Fig.1b). Since only one oxygen atom of the carboxylate group coordinated with Ca2+ion, the free oxygen atom can generate two hydrogen bonds with two hydrogen atoms in two water molecules (Table 3) to expand into two dimensional layer (Fig.1c). Then 2D layers are connected into 3D supramolecular structure via the bpdc2-ligands (Fig.1d).
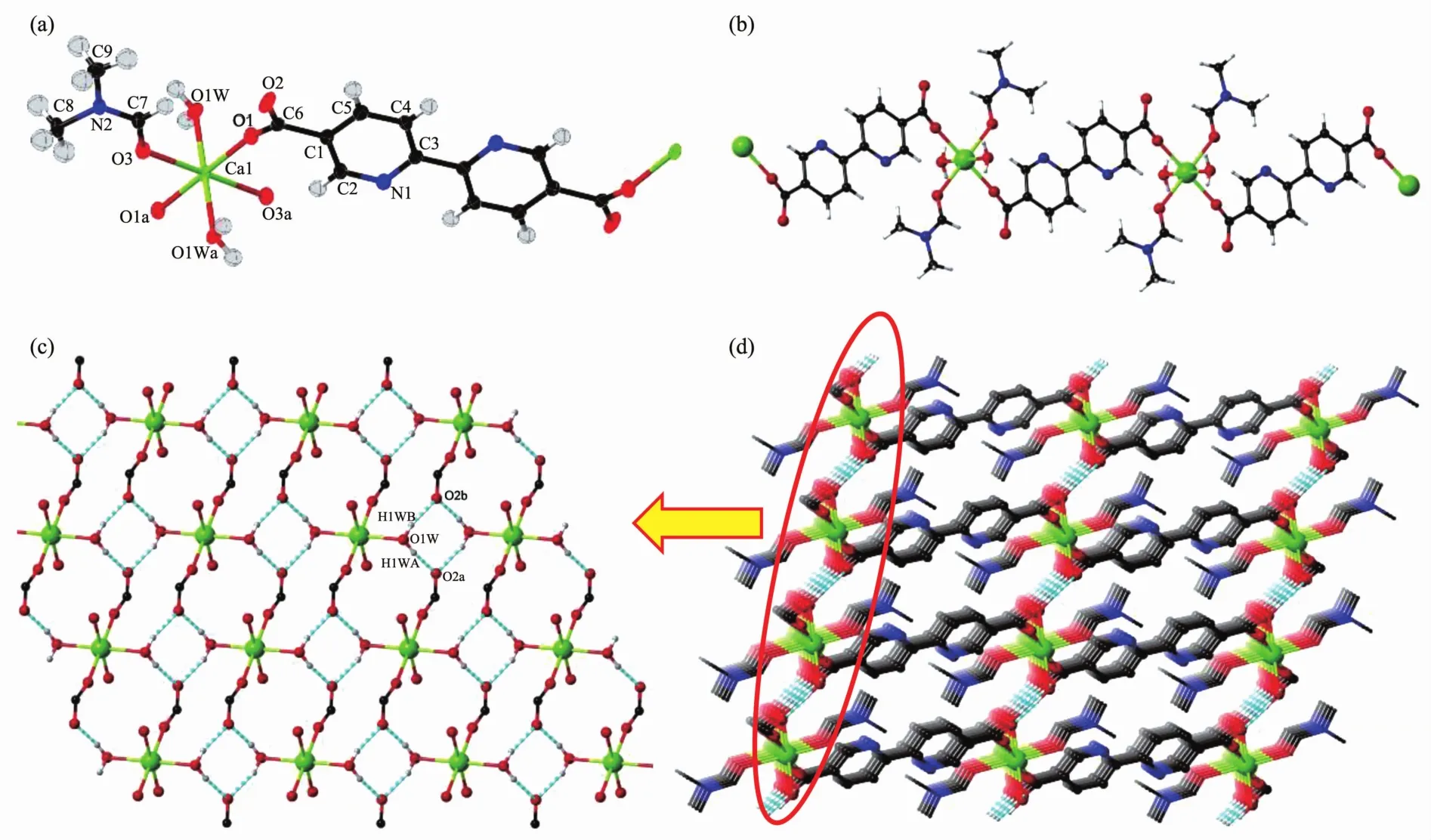
H atoms with carbon atoms are omitted for clear in (d); Symmetry codes: a: x+1, y, z; b: -x+1, -y+1, -z+1Fig.1 (a) Coordination environment of Ca2+and bpdc2-ligand with ellipsoids at the 50% probability level for 1; (b) View of 1D coordination chain; (c) 2D layer connected by hydrogen bonds; (d) 3D supramolecular structure of 1
Complex 2 is also a 1D coordination chain structure composed of one magnesium ion, one bpdc2-ligand and three coordination water molecules in the asymmetric unit (Fig.2a). Mg2+ion is coordinated with two nitrogen atoms and one oxygen atom from a bpdc2-ligand and three water molecules. It′s different from complex 1 that the coordination mode of bpdc2-ligand in 2 is T-shape connection with metal atoms through oxygen atom in one carboxylate group and two nitrogen atoms which were fixed in cis-position. Therefore, 1D z-type chains are constructed and stacking along caxis via π-π interactions (d=0.328 nm) (Fig.2b). To be noticed that the coordinated water molecules O3w are hydrogen bonded with O3 atoms and then forms an interesting infinite hydrogen bonding chain which further connects with the other molecules, O1w and O2w, with hydrogen bonds along c-axis as branches (Fig.2c, Table 3). Due to the hydrogen bonding interactions above, 1D chains could be linked into 3D supramolecular structure (Fig.2d).
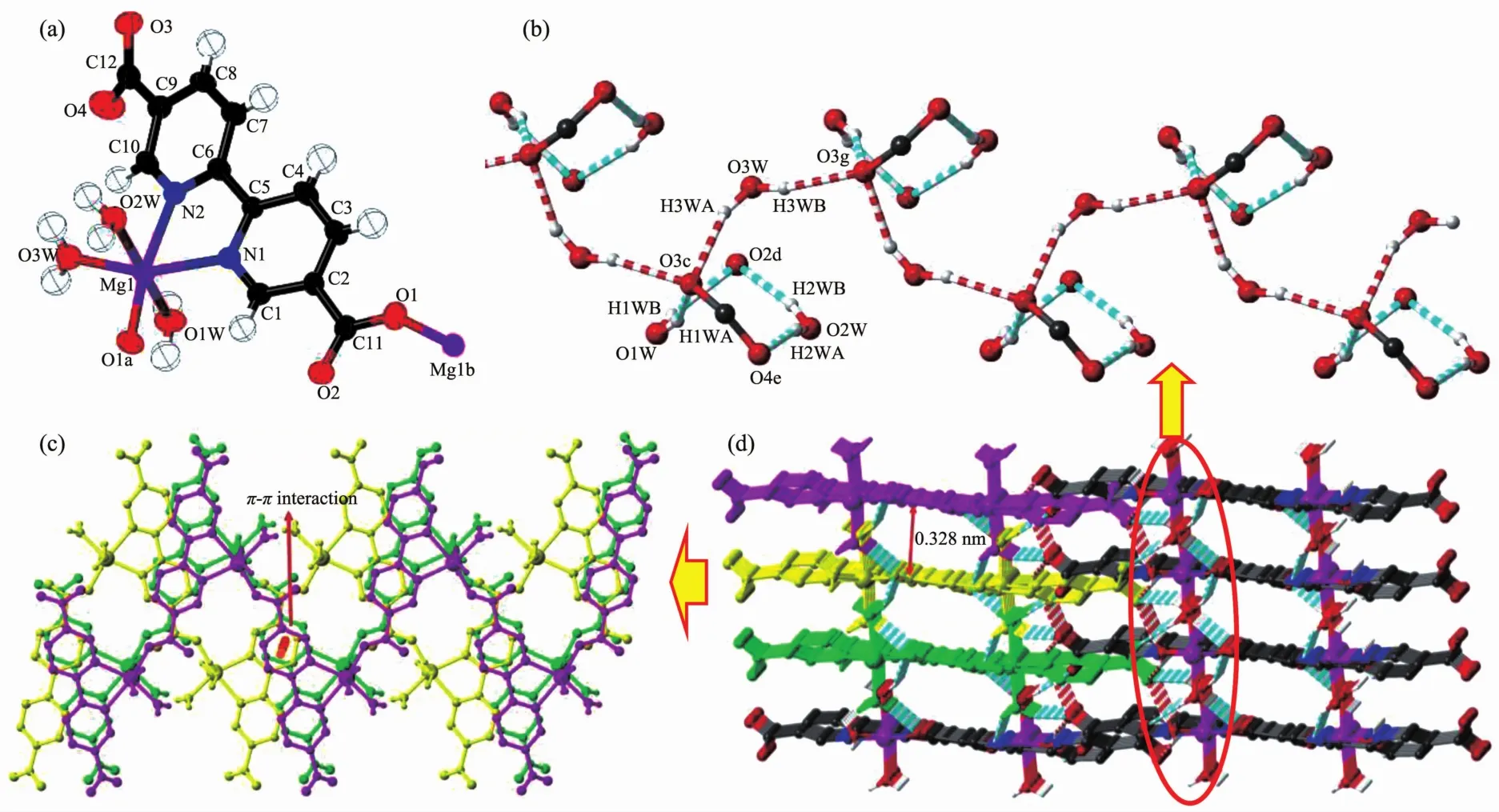
H atoms with carbon atoms are omitted for clear; Symmetry codes: a: -x, y+1/2, -z+1/2; b: -x, y-1/2, -z+1/2; c: -x+1, -y, -z;d: -x, -y, -z; e: -x+1, -y, -z+1; g: -x+1, y+1/2, -z+1/2Fig.2 (a) Coordination environments of Mg2+ion and bpdc2-ligand with ellipsoids at the 50% probability level for 2; (b) Perspective view along c-axis of the 1D water-carboxylic group hydrogen bonding chain; (c) 1D coordination chains stacking via π-π interactions; (d) Three dimensional supramolecular structure of 2
2.2 Thermogravimetric analysis
Thermogravimetric (TG) analysis was carried out to examine the thermal stabilities and confirm the structures of complexes 1 and 2 together with elemental analysis. The purity of all the powder samples are confirmed by PXRD (Supporting information). All the complexes are heated in nitrogen from room temperature to 700℃. For 1 (Fig.3a), a weight loss occuring at the beginning is related to the poor crystal stability. The weight loss before 150℃of 38.10% corresponds to the weight of the coordinated DMF and water molecules. And then there is a long plateau emerging until the temperature reachs 450℃at which the organic ligands begin to decompose. Fig.3b shows that complex 2 can be stable up to the temperature of 110℃, and then a quick weight loss of 16.17% from 110 to 150 ℃corresponds to the loss of three coordinated water molecules (16.85%). There is a long plateau before 420 ℃at which the bpdc2-ligands begin to decompose.
2.3 Infrared spectra
As is shown in Fig.4, in the high-frequency region, the spectra of 1 and 2 are dominated by an intense and wide absorption centered at 3 430 and 3 400 cm-1, which arise from ν(O-H) stretching modes of lattice water molecules[21]. Moreover, there are similar absorption peaks in two spectra, 1 670, 1 250 cm-1for 1 and1 685, 1 298 cm-1for 2, which are corres-ponding to the νaand νsabsorption of -COO- groups[22], and the Δν of 420 cm-1for 1 and 387 cm-1for 2 indicate the unidentate coordination mode of the carboxylate groups in 1 and 2[23]. The absorption bands of pyridyl rings are assigned to the peaks of 1 590 and 1 410 cm-1. For 1, there are extra peaks of DMF molecules at 2 922 cm-1and 2 854 cm-1.
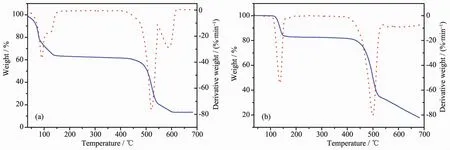
Fig.3 Thermogravimetric analysis curves of 1 (a) and 2 (b)

Fig.4 IR spectra of 1 and 2
2.4 Luminescent properties
The photoluminescent properties have also been measured in solid state at room temperature (Fig.5). The emission peaks of 1 (432 and 500 nm) and 2 (442 nm), which could be attributed to the π→π* transition within the pyridyl rings of the ligand, are hypochromatic shift compared with that of the ligand (455 and 547 nm). It′s interesting that there is only one emission peak for 2 whereas H2bpdc ligand and 1 exhibit two emission peaks. Since the PXRD data of raw materials is in accordance with the simulated PXRD data of the reported crystal structure[18], we confirmed that the conformation of H2bpdc ligand is trans-mode structure. So, the disappearance of the emission peak for 2 might be associated with the cisconformation of the ligand in the crystal structure of 2, which is in accordance with the literature[24].
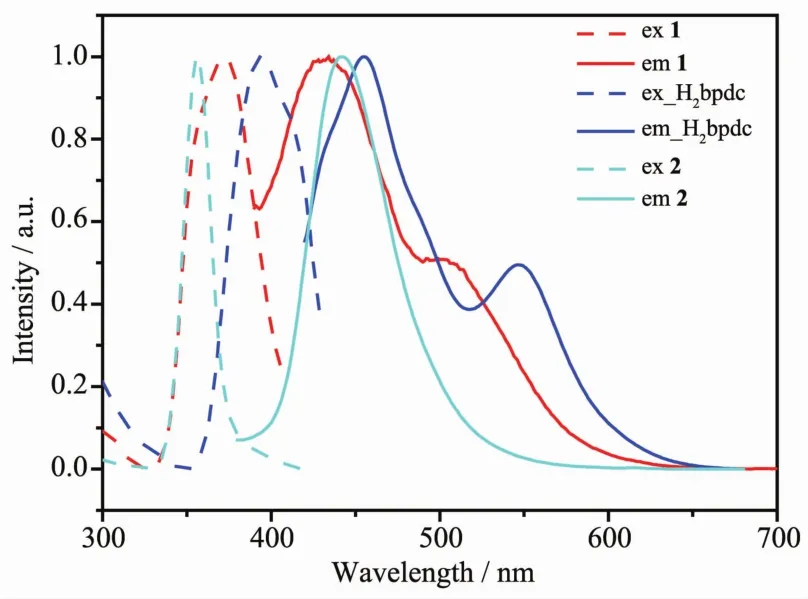
Fig.5 Solid-state luminescent spectra of H2bpdc, 1 and 2
3 Conclusions
In summary, two alkaline-earth metal complexes have been obtained via solvothermal conditions. Both of them are 1D coordination chain structures where complex 2 contain interesting hydrogen bonding chains. Luminescent properties show that emission peaks of 2 are different from that of 1, which are similar to the emission peaks of organic ligand.
Supporting information is available at http://www.wjhxxb.cn
References:
[1] Zhang W X, Liao P Q, Lin R B, et al. Coord. Chem. Rev., 2015,293-294:263-278
[2] Lin Z J, Lü J, Hong M C, et al. Chem. Soc. Rev., 2014,43: 5867-5895
[3] Cui Y J, Yue Y F, Qian G D, et al. Chem. Rev., 2012,112: 1126-1162
[4] Liu K, Shi W, Cheng P. Coord. Chem. Rev., 2015,289-290: 74-122
[5] Horcajada P, Gref R, Baati T, et al. Chem. Rev., 2012,112: 1232-1268
[6] Kobayashi A, Ohba T, Saitoh E, et al. Inorg. Chem., 2014,53: 2910-2921
[7] Darkhijav B(達(dá)日賀扎布), SUO Quan-Ling(索全伶), GAO Yuan-Yuan(高媛媛), et al. Chinese J. Struct. Chem.(結(jié)構(gòu)化學(xué)), 2014,33:585-590
[8] Lee E Y, Suh M P. Angew. Chem. Int. Ed., 2004,43:2798-2801
[9] Liu D, Huxford R C, Lin W. Angew. Chem. Int. Ed., 2011, 50:3696-3700
[10]Huang S L, Jia A Q, Jin G X. Chem. Commun., 2013,49: 2403-2405
[11]Sun Y G, Sun D, Yu W, et al. Dalton Trans., 2013,42:3957-3967
[12]Huh S, Jung S, Kim Y, et al. Dalton Trans., 2010,39:1261-1265
[13]Min Z, Singh-Wilmot M A, Cahill C L, et al. Eur. J. Inorg. Chem., 2012:4419-4426
[14]Zhao J, Shi D, Cheng H, et al. Inorg. Chem. Commun., 2010,13:822-827
[15]Blake A J, Champness N R, Easun T L, et al. Nat. Chem., 2010,2:688-694
[16]Ou Y C, Wang J, Leng J D, et al. Dalton Trans., 2011,40: 3592-3600
[17]Ou Y C, Liu W T, Leng J D, et al. CrystEngComm, 2010, 12:3748-3757
[18]Wang C. Acta Crystallogr., 2009,E65:o2081
[19]Blessing R H. Acta Crystallogr., 1995,A51:33-38
[20]SHELXTL 6.10, Bruker Analytical Instrumentation, Madison, Wisconsin, USA, 2000.
[21]Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. 6th Ed. New Jersey: John Wiley and Sons, Inc., 2009.
[22]Droz.dz.ewski P, Broyna A, Kubiak M. J. Mol. Struct., 2004, 707:131-137
[23]Deacon G B, Phillips R. Coord. Chem. Rev., 1980,33:227-250
[24]Wang J, Luo J, Zhi B, et al. CrystEngComm, 2014,16:9810-9816
Two 1D Chain Structures Derived from Alkaline-Earth Metal Complexes and 2,2′-Bipyridine-5,5′-dicarboxylate Ligand: Syntheses, Crystal Structures and Luminescent Properties
OU Yong-Cong*ZHONG Jun-Xing SONG Ying-Yi ZHU Lan-Lan WU Jian-Zhong
(Guangzhou Key Laboratory of Materials for Energy Conversion and Storage, School of Chemistry and Environment, South China Normal University, Guangzhou 510006, China)
Abstract:Two alkaline-earth metal coordination complexes, [Ca(bpdc)(DMF)2(H2O)2]n(1) and [Mg(bpdc)(H2O)3]n(2) (H2bpdc=2,2′-Bipyridine-5,5′-dicarboxylic acid), have been obtained via solvothermal conditions and characterized by elemental analysis, IR spectra, thermogravimetric analysis and photoluminescent measurement. Single crystal X-ray diffractions reveal that complexes 1 and 2 are 1D chain structures which are further connected into 3D supramolecular structures by hydrogen bonding interactions and π-π stacking interactions. It′s noted that the solid-state fluorescent emissions of 1 and 2 are different in related to the different conformation modes of the bpdc(2-)ligand. CCDC: 1444695, 1; 1444696, 2.
Keywords:alkaline-earth metal complexes; solvothermal syntheses; conformation; photoluminescence
收稿日期:2015-12-30。收修改稿日期:2016-03-01。
DOI:10.11862/CJIC.2016.092
中圖分類號(hào):O614.23+1;O614.22
文獻(xiàn)標(biāo)識(shí)碼:A
文章編號(hào):1001-4861(2016)04-0738-07
 無機(jī)化學(xué)學(xué)報(bào)2016年4期
無機(jī)化學(xué)學(xué)報(bào)2016年4期
- 無機(jī)化學(xué)學(xué)報(bào)的其它文章
- 基于二氰根鉻的一系列氰根橋聯(lián)CrⅢ-CuⅡ-CrⅢ三核配合物的合成、結(jié)構(gòu)與磁性
- Co與甜菜堿類衍生物形成的兩種配合物的結(jié)構(gòu)和磁性特征
- 4d-4f異金屬配位聚合物的合成、結(jié)構(gòu)和熒光性質(zhì)
- 兩個(gè)基于雙(4-吡啶-4-苯基)胺的鈷、鋅配合物的合成和晶體結(jié)構(gòu)
- 以5-(6-羧酸-2-萘基)-間苯二羧酸為配體構(gòu)筑的兩個(gè)鎘的金屬-有機(jī)骨架化合物的合成、晶體結(jié)構(gòu)及熒光性質(zhì)
- 一系列基于雜多酸構(gòu)筑的配合物的合成,晶體結(jié)構(gòu)及光催化性能
