RIPK3基因轉染的SH-SY5Y細胞中HIF-1α基因及其信號通路相關基因表達變化
張國祿,程世翔,徐忠偉,衣泰龍,廖吉連,涂悅,張賽
(1武警后勤學院附屬醫院腦科醫院*,天津300162;2武警后勤學院中心實驗室)
?
RIPK3基因轉染的SH-SY5Y細胞中HIF-1α基因及其信號通路相關基因表達變化
張國祿1,程世翔1,徐忠偉2,衣泰龍1,廖吉連1,涂悅1,張賽1
(1武警后勤學院附屬醫院腦科醫院*,天津300162;2武警后勤學院中心實驗室)
摘要:目的觀察受體相互作用蛋白激酶3(RIPK3)基因轉染的神經母細胞瘤細胞系SH-SY5Y中低氧誘導因子1α(HIF-1α) mRNA及其信號通路相關基因表達變化。方法 構建表達RIPK3基因的pCMV6-AC-GFP質粒(重組質粒),培養SH-SY5Y細胞,分為實驗組及對照組,分別轉染重組質粒和空載質粒。采用Western blotting法檢測細胞中的RIPK3蛋白,分別于培養8、14、20、26、32、38 h后,通過MTT實驗檢測細胞增殖情況(OD值)。采用轉錄組測序技術(RNAseq)及Ingenuity Pathway Analysis(IPA)軟件檢測并篩選RIPK3-HIF1α下游信號通路中的關鍵基因。采用微滴式數字PCR(ddPCR)檢測兩組細胞中的HIF-1α mRNA。結果實驗組細胞中RIPK3蛋白相對表達量(0.806±0.097 5)高于對照組(0.455±0.088 6),P<0.05。隨培養時間延長,實驗組細胞增殖受到抑制。實驗組細胞中HIF-1α mRNA相對表達量(0.015 43±0.003 47)低于對照組(0.046 28±0.010 26),P<0.05。在HIF-1α為核心的相互作用關系網絡中,篩選出關鍵分子泛素綴合酶樣蛋白(UBC)、希佩爾-林道蛋白(VHL)、轉錄延伸因子B多肽1(TCEB1)、血管內皮生長因子A(VEGFA)。結論 RIPK3基因轉染SH-SY5Y后,細胞中HIF-1α mRNA表達下調,同時HIF-1α信號通路相關基因(UBC、VHL、TCEB1、VEGFA)的表達水平受到影響。
關鍵詞:神經母細胞瘤;受體相互作用蛋白激酶3;低氧誘導因子1α;泛素綴合酶樣蛋白;希佩爾-林道蛋白;轉錄延伸因子B多肽;血管內皮生長因子A
受體相互作用蛋白激酶3(RIPK3)屬于受體相互作用蛋白家族(RIPs)成員。作為腫瘤壞死因子(TNF)重要的下游效應分子,RIPK3廣泛表達于人體各組織,并被證實在細胞增殖、凋亡過程中發揮重要作用[1~4]。低氧誘導因子1α(HIF-1α)表達于所有已知的多細胞物種[5],是組織適應缺氧環境的重要轉錄因子[6],在缺血再灌注、腫瘤及黏膜炎癥等進程中發揮作用[7, 8]。本研究建立了穩定表達RIPK3的人神經母細胞瘤細胞模型,觀察細胞中HIF-1α及其下游信號分子轉錄水平的變化,探索RIPK3的潛在作用及其信號通路網絡。
1材料與方法
1.1實驗細胞與試劑人神經母細胞瘤細胞系SH-SY5Y購自美國標準生物品收藏中心(ATCC),細胞接種于含有10 %胎牛血清(Gibicol公司)、90% F12與DMEN配比為1∶1的完全培養基(Gibicol公司)中,37 ℃、5% CO2環境下培養,取對數生長期細胞用于實驗。質粒小提中量試劑盒(天根公司),Lipofectamine3000(Life公司),TRIzol(Invitrogen公司),SYBR Green試劑盒(Roche公司),β-actin抗體(Sigma公司),RIPK3抗體(Abcam公司),辣根過氧化物酶標記的羊抗兔、羊抗小鼠二抗(KPL公司),QX200 ddPCR EvaGreen Supermix(Bio-Rad公司)。
1.2表達RIPK3的pCMV6-AC-GFP質粒的獲取將RIPK3(NM_006871) Human cDNA ORF Clone質粒及對應空載質粒分別轉化DH5-α感受態大腸桿菌,菌液接種于含氨芐青霉素(50 μg/mL)的LB固體培養基中,37 ℃培養過夜。挑取單個菌落接種于含氨芐青霉素(50 μg/mL)的液體LB培養基中,37 ℃恒溫搖床震蕩16 h,提取質粒DNA,測定濃度后分裝,-80 ℃保存。
1.3質粒轉染及RIPK3蛋白檢測SH-SY5Y細胞接種于6孔板中,分為對照組與實驗組。將Lipofectamine3000試劑分別與空載質粒及RIPK3過表達質粒DNA混合構成質粒-脂質體復合體,孵育后的兩種質粒-脂質體復合體分別加入對照組與實驗組培養孔中孵育2~4 d。以含G418(1 000 μg/mL)的完全培養基篩選轉染細胞以獲取穩定轉染的SH-SY5Y細胞,傳代2次后G418濃度減為800 μg/mL維持培養,并凍存備用。取兩組對數生長期細胞分別提取蛋白,BCA定量法測定蛋白濃度后分裝置于-80 ℃保存。采用Western blotting法檢測RIPK3蛋白。

1.5SH-SY5Y細胞中HIF-1α mRNA檢測采用微滴式數字PCR(ddPCR)檢測兩組細胞中的HIF-1α mRNA。HIF-1α、GAPDH基因引物序列見表1。采用美國Bio-Rad公司生產的QX200 ddPCR系統,將由MILIQ水6 μL、cDNA 2 μL、上游引物1 μL、下游引物1 μL及ddPCR EvaGreen Supermix構成的反應體系進行微滴化處理,形成約20 000個微滴,將微滴轉移到PCR管中進行擴增,完成PCR反應后90 ℃維持5 min以穩定微滴,對擴增產物進行信號采集及數據分析,獲得基因絕對濃度,以目的基因濃度/GAPDH基因濃度計算目的基因相對表達量。

表1 HIF-1α、GAPDH基因引物序列
1.6HIF-1α信號通路相關基因的篩選與檢測提取兩組細胞總RNA,用帶有多加多聚T尾標記的磁珠及試劑盒處理,以獲取高純度mRNA。將純化的mRNA打斷為短片段,以此為模板合成雙鏈cDNA。純化cDNA并進行末端修復、鏈接多聚A尾及測序接頭,選擇合適長度的片段進行PCR反應,獲得最終的cDNA文庫。對cDNA進行檢測,確認符合實驗要求后進行RNAseq。測序結果經Ingenuity Pathway Analysis(IPA)軟件進行數據分析。篩選RIPK3-HIF-1α信號通路相關信號分子,將這些基因輸入到STRING10數據庫網站(http://string-db.org)中獲得其相互作用網絡。相關基因表達檢測通過RNAseq技術完成。

2結果
2.1兩組細胞中RIPK3蛋白表達變化實驗組與對照組細胞中RIPK3蛋白相對表達量分別為0.45±0.11、0.81±0.12,兩組相比,P<0.05。
2.2兩組細胞增殖情況比較隨培養時間延長,實驗組細胞增殖受到抑制,培養20、26、32、38 h時兩組OD值差異有統計學意義。見表2。
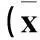
表2 兩組細胞增殖情況比較±s)
注:與實驗組相比,*P<0.05,**P<0.01。2.3兩組細胞中HIF-1α mRNA表達比較RNAseq結果顯示實驗組、對照組細胞中HIF-1α mRNA相對表達量分別為0.046 282 1、0.015 431 0。ddPCR結果顯示,實驗組、對照組細胞中HIF-1α mRNA相對表達量分別為0.004 00±0.000 347、0.001 11±0.000 163;實驗組細胞中HIF-1α mRNA相對表達量低于對照組(P均<0.05),與RNAseq結果一致。
2.4HIF-1α信號通路相關基因篩選及其表達在HIF-1α為核心的相互作用關系網絡中,共篩選出四種相關分子:泛素綴合酶樣蛋白(UBC)、希佩爾-林道蛋白(VHL)、轉錄延伸因子B多肽1(TCEB1)、血管內皮生長因子A(VEGFA)。
3討論
RIPK3作為細胞程序性壞死信號通路中的重要一環越來越引起學者的關注。作為TNF-α下游重要的信號分子,RIPK3參與天冬氨酸特異酶切的半胱氨酸蛋白酶88(Caspase-8)及核轉錄因子κB(NF-κB)活化等過程[9~12]。然而,RIPK3在神經系統中的作用機制尚待進一步研究。本實驗以SH-SY5Y細胞為基礎建立了穩定過表達RIPK3基因的神經元細胞模型[13],并通過RNAseq及IPA數據分析尋找潛在的RIPK3下游效應分子。本實驗發現,SH-SY5Y細胞轉染RIPK3基因后,細胞中HIF-1α的表達水平明顯下調。
HIF-1α是一種重要的氧敏感轉錄因子。HIF的一個重要作用是維持氧供需平衡,以減少活性氧簇(ROS)的生成[14]。在氧充足情況下,HIF-1α與VHL結合,后者能聚集泛素連接酶標記HIF-1α而使HIF-1α降解;UBC在此泛素化的過程中發揮重要作用,將泛素從泛素相關蛋白(UBA)的巰基位點轉移至蛋白底物上,進而進入泛素依賴性蛋白水解途徑[15]。有報道[16, 17]指出,HIF-1α可直接或間接調節血管內皮細胞中2%以上的基因。最近研究[18]顯示,HIF-1α在成人體內能通過諸多信號通路促進組織再生,其機制包括從轉錄水平激活修復基因及其受體,如VEGF、胎盤生長因子(PLGF)、血小板衍生因子(PDGF)、血管生成素1(ANGPT1)[19];通過調節促血管生成因子及其受體如1-磷酸神經鞘氨醇(S1P)[20]、趨化因子受體4(CXCR4),從而促進內皮祖細胞在缺氧部位聚集[21];通過調控參與細胞周期和DNA復制過程的基因從而促進內皮細胞增殖、分化[22]。本研究中,HIF-1α及其信號通路相關分子構成一個復雜的信號網絡。有學者[23]發現,海馬神經元損傷后,RIPK3表達明顯上調且集中分布于細胞膜,這種效應獨立于RIPK1的調控。本研究結果顯示,轉染RIPK3基因可對SH-SY5Y細胞中HIF-1α及其信號通路相關分子表達水平造成影響,推測RIPK3在神經系統損傷修復過程中發揮十分重要的作用,通過控制RIPK3的活性有望促進神經組織再生與修復。
由于客觀因素限制,我們僅從表觀對RIPK3過表達引起的HIF-1α及其信號通路相關分子表達變化進行觀察,且僅對HIF-1α進行驗證,未能進一步研究RIPK3與HIF-1α具體的相互作用關系,這仍需通過后續實驗進一步驗證。
參考文獻:
[1] Yu PW, Huang BC, Shen M, et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB[J]. Curr Biol, 1999,9(10):539-542.
[2] Vanden Berghe T, Linkermann A., Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways[J]. Nat Rev Mol Cell Biol, 2014,15(2):135-147.
[3] Kikuchi M, Kuroki S, Kayama M, et al. Protease activity of procaspase-8 is essential for cell survival by inhibiting both apoptotic and nonapoptotic cell death dependent on receptor-interacting protein kinase 1 (RIP1) and RIP3[J]. J Biol Chem, 2012,287(49):41165-41173.
[4] Moriwaki K, Chan FK. Necrosis-dependent and independent signaling of the RIP kinases in inflammation[J]. Cytokine Growth Factor Rev, 2014,25(2):167-174.
[5] Loenarz C, Coleman ML, Boleininger A, et al. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens[J]. EMBO Rep, 2011,12(1):63-70.
[6] Semenza GL. Hypoxia-inducible factors in physiology and medicine[J]. Cell, 2012, 148(3):399-408.
[7] Ruggieri MR Sr. Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction[J]. Nat Clin Pract Urol, 2006, 3(4):206-215.
[8] Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia[J]. Nat Med, 2012, 18(5):774-782.
[9] Belizario J, Vieira-Cordeiro L, Enns S. Necroptotic cell death signaling and execution pathway: lessons from knockout mice[J]. Mediators Inflamm, 2015, 2015:128076.
[10] Amini P, Stojkov D, Wang X, et al. NET formation can occur independently of RIPK3 and MLKL signaling[J]. Eur J Immunol, 2016,46(1):178-184.
[11] Cuda CM, Misharin AV, Khare S, et al. Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner[J]. Arthritis Res Ther, 2015,17:291.
[12] Moriwaki K, Balaji S, McQuade T, et al. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair[J]. Immunity, 2014, 41(4):567-578.
[13] Jia DP, Wang S, Zhang BC, et al. Paraptosis triggers mitochondrial pathway-mediated apoptosis in Alzheimer′s disease[J]. Exp Ther Med, 2015, 10(2):804-808.
[14] Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations[J]. J Clin Invest, 2013,123(9):3664-3671.
[15] Miro-Murillo M, Elorza A, Soro-Arnaiz I, et al. Acute VHL gene inactivation induces cardiac HIF-dependent erythropoietin gene expression[J]. PLoS One, 2011, 6(7):e22589.
[16] Park C, Kim TM, Malik AB. Transcriptional regulation of endothelial cell and vascular development[J]. Circ Res, 2013,112(10):1380-1400.
[17] Mistry IN, Smith PJ, Wilson DI, et al. Probing the epigenetic regulation of HIF-1alpha transcription in developing tissue[J]. Mol Biosyst, 2015,11(10):2780-2785.
[18] Pan XY, Zhang ZH, Wu LX, et al. Effect of HIF-1a/VEGF signaling pathway on plasma progesterone and ovarian prostaglandin F(2)a secretion during luteal development of pseudopregnant rats[J]. Genet Mol Res, 2015, 14(3):8796-8809.
[19] Zou D, Zhang Z, He J, et al. Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1alpha mediated BMSCs[J]. Biomaterials, 2012,33(7):2097-2108.
[20] Kalhori V, Kemppainen K, Asghar MY, et al. Sphingosine-1-Phosphate as a Regulator of Hypoxia-Induced Factor-1alpha in Thyroid Follicular Carcinoma Cells[J]. PLoS One, 2013,8(6):e66189.
[21] Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1[J]. Nat Med, 2004,10(8):858-864.
[22] Nauta TD, van Hinsbergh VW, Koolwijk P. Hypoxic signaling during tissue repair and regenerative medicine[J]. Int J Mol Sci, 2014,15(11):19791-19815.
[23] Yin B, Xu Y, Wei RL, et al. Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury[J]. Brain Res, 2015,1609:63-71.
Expression changes of HIF-1α and its signaling pathway related gene in SH-SY5Y cells transfected by RIPK3 gene
ZHANGGuolu1,CHENGShixiang,XUZhongwei,YITailong,LIAOJilian,TUYue,ZHANGSai
(1Neurology&NeurosurgeryHospitalAffiliatedtoLogisticalCollegeofChineseArmedPoliceForces,Tianjin300162,China)
Abstract:ObjectiveTo observe the expression changes of hypoxia-inducible factor 1α (HIF-1α) and its signaling pathway related gene in the neuroblastoma cell line SH-SY5Y transfected by receptor interacting serine/threonine kinase 3 (RIPK3) gene. MethodsThe pCMV6-AC-GFP plasmid expressing RIPK3 gene (recombinant plasmid) was constructed. SH-SY5Y cells were cultured and then were transfected with the recombinant plasmid and empty vector plasmid as the experimental group and control group respectively. Expression of RIPK3 protein was detected by Western blotting, and the OD value was detected by MTT assay at 8, 14, 20, 26, 32 and 38 h. RNA transcriptome sequencing (RNAseq) and Ingenuity Pathway Analysis (IPA) was used to detect and screen the key genes in the downstream signaling pathway of RIPK3-HIF-1α. Droplet Digital PCR (ddPCR) was used to detect the HIF-1α mRNA in the two groups. ResultsThe RIPK3 expression in the experimental group (0.806±0.097 5) was higher than that of the control group (0.455±0.088 6), P<0.05. The proliferation of SH-SY5Y was inhibited as the cell incubation time was prolonged. The expression of HIF-1α mRNA in the experimental group (0.01543±0.00347) was strongly down-regulated as compared with that of the control group (0.04628±0.01026) (P<0.05). In the interaction network of HIF-1α which was taken as the core, we screened the key molecules such as ubiquitin-conjugating enzyme (UBC), von Hippel-Lindau (VHL), transcription elongation factor B polypeptide 1 (TCEB1) and vascular endothelial growth factor A (VEGFA). ConclusionAfter the SY5Y cells were trasfected by RIPK3, the HIF-1α mRNA expression was down-regulated in SH, and the expression levels of HIF-1α signaling pathway-related genes (UBC, VHL, TCEB1 and VEGFA) were affected.
Key words:neuroblastoma; receptor interacting serine/threonine kinase 3; hypoxia-inducible factor 1α; ubiquitin-conjugating enzyme-like protein; Von Hippel-Lindau protein; transcription elongation factor B polypeptide 1; vascular endothelial growth factor A
(收稿日期:2015-09-28)
中圖分類號:R34
文獻標志碼:A
文章編號:1002-266X(2016)07-0013-04
doi:10.3969/j.issn.1002-266X.2016.07.005
通信作者簡介:張賽(1956-),男,醫學博士、教授、主任醫師,主要研究方向為神經創傷及危重癥。E-mail: zhangsai718@vip.126.com
作者簡介:第一張國祿(1990-),男,碩士研究生在讀,從事神經外科專業的研究。E-mail: zhangguolu.wjyxy@163.com
基金項目:國家自然科學基金項目(31200809);武警部隊后勤科研項目(WJHQ2012-20)。
*暨天津市神經創傷修復重點實驗室、武警部隊腦創傷與神經疾病研究所。

