右旋糖苷蔗糖酶的研究進(jìn)展
韓瑨,吳正鈞,徐曉芬,吳江(.乳業(yè)生物技術(shù)國(guó)家重點(diǎn)實(shí)驗(yàn)室,上海20046;2.上海乳業(yè)生物工程技術(shù)研究中心,上海20046;.光明乳業(yè)研究院,光明乳業(yè)股份有限公司,上海20046)
?
右旋糖苷蔗糖酶的研究進(jìn)展
韓瑨1,吳正鈞2,*,徐曉芬3,吳江3
(1.乳業(yè)生物技術(shù)國(guó)家重點(diǎn)實(shí)驗(yàn)室,上海200436;2.上海乳業(yè)生物工程技術(shù)研究中心,上海200436;3.光明乳業(yè)研究院,光明乳業(yè)股份有限公司,上海200436)
摘要:右旋糖苷蔗糖酶(dextransucrase)是一種典型的葡萄糖基轉(zhuǎn)移酶,可利用蔗糖為底物合成右旋糖苷,而后者在醫(yī)藥、食品等領(lǐng)域用途廣泛。從酶的來(lái)源、產(chǎn)量?jī)?yōu)化、制備方法和活性檢測(cè)的角度總結(jié)右旋糖苷蔗糖酶的研究進(jìn)展,并對(duì)其發(fā)展趨勢(shì)進(jìn)行的展望。
關(guān)鍵詞:右旋糖苷蔗糖酶;右旋糖苷;來(lái)源;產(chǎn)量?jī)?yōu)化;制備方法;活性檢測(cè)
右旋糖苷蔗糖酶(dextransucrase簡(jiǎn)稱DSR,EC 2.4.1.5)是一類典型的葡萄糖基轉(zhuǎn)移酶(glucosyltransferase,簡(jiǎn)稱GTF),屬于糖苷水解酶第70家族(glycoside-hydrolase family 70)[1]。DSR主要存在于胞外生境中或被錨定于胞壁上[2],其作用原理可分解為兩步反應(yīng),如圖1所示[3]。
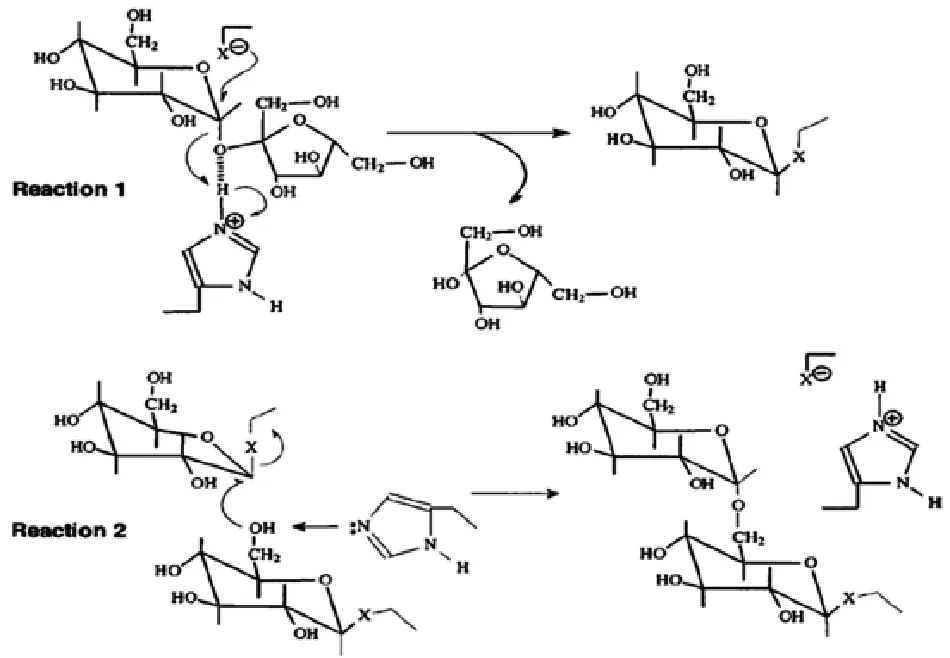
圖1右旋糖苷蔗糖酶對(duì)蔗糖的裂解和α-(1,6)-糖苷鍵的形成機(jī)制Fig.1 Mechanism for the cleavage of sucrose and the formation of an α-(1,6)-glycosidic bond by dextransucrase
反應(yīng)1是蔗糖中的果糖部分發(fā)生了親核取代和質(zhì)子化,從而形成了葡萄糖基與DSR的復(fù)合物,反應(yīng)2是C-6上的羥基對(duì)葡萄糖基DSR復(fù)合物的C-1部位發(fā)起進(jìn)攻形成了一個(gè)α-(1,6)-糖苷鍵,該反應(yīng)可通過(guò)咪唑基團(tuán)從羥基上抽取一個(gè)質(zhì)子被進(jìn)一步催化[4]。由于DSR的分子量相對(duì)較大,所以到目前為止,只有少數(shù)幾個(gè)DSR的3D結(jié)構(gòu)與活性位點(diǎn)被,腸膜明串珠菌(Leuconostoc mesenteroides)B-1299[5]便是其中之一。
DSR的這種葡萄糖基轉(zhuǎn)化能力被用于右旋糖苷(dextran)的工業(yè)化生產(chǎn),同時(shí)也可合成部分寡聚糖[6]。由于DSR的主要產(chǎn)物dextran在醫(yī)藥(代血漿)、食品(穩(wěn)定劑、增稠劑)等領(lǐng)域用途廣泛[7],因此競(jìng)相被國(guó)內(nèi)外學(xué)者的研究與報(bào)道。盡管前期羅靳等總結(jié)了L. mesenteroides來(lái)源的DSR結(jié)構(gòu)、作用機(jī)制、基因克隆和異源表達(dá)[8],然而為了更全面地了解DSR,本文將從DSR的來(lái)源、產(chǎn)量?jī)?yōu)化、制備方法以及活性檢測(cè)的角度進(jìn)行概述。
1 DSR的來(lái)源
早期研究認(rèn)為,自然界中的大部分dextran主要是由明串珠菌屬(Leuconostoc)、鏈球菌屬(Streptococcus)以及乳桿菌屬(Lactobacillus)菌種分泌的DSR催化蔗糖合成而來(lái)的[3]。近年來(lái),隨著對(duì)DSR研究領(lǐng)域的不斷深入和擴(kuò)大,片球菌屬(Pediococcus)[9]、魏斯氏菌屬(Weissella)和醋酸桿菌屬(Acetobacter)[10]的部分菌株也加入了DSR產(chǎn)生菌的行列。其中部分報(bào)道如表1所示。
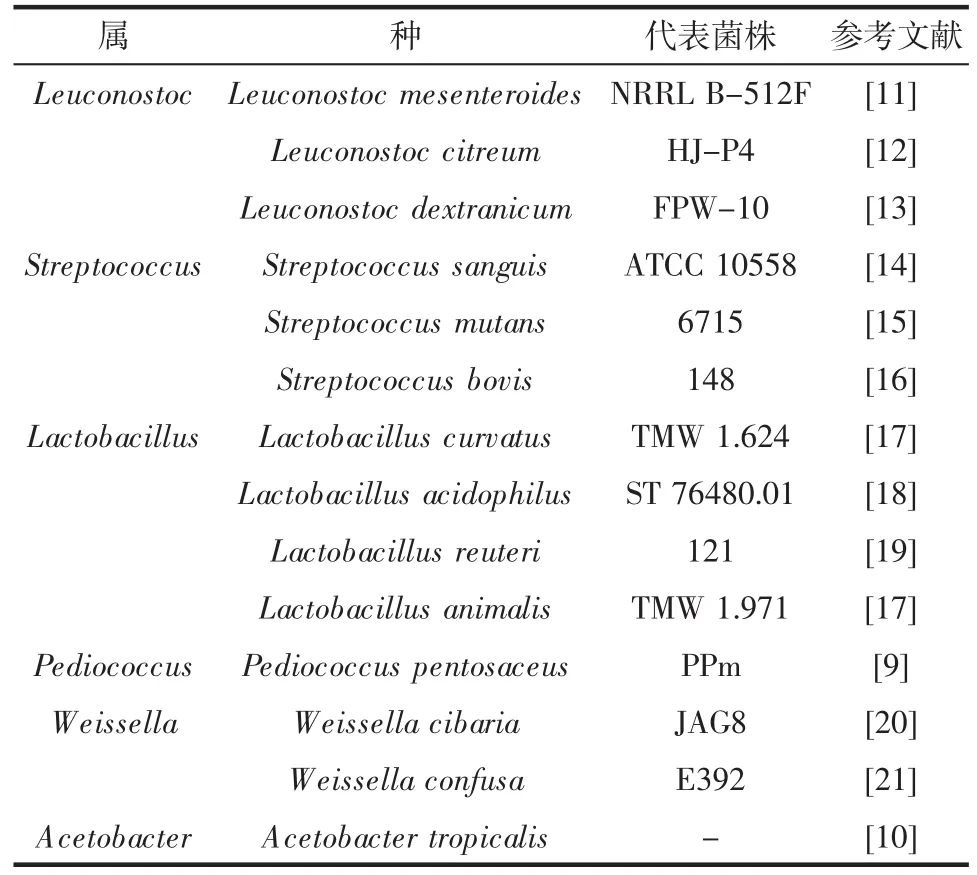
表1部分產(chǎn)DSR的代表菌株及其生物分類學(xué)地位Table 1 Biological taxonomies of partial representative strains producing DSR
Scheibler于1874年首次提出了dextran的概念[22],之后van Tieghem證明其產(chǎn)生菌為腸膜明串珠菌(Leuconostoc mesenteroides)[23]。鑒于dextran具有潛在的商業(yè)價(jià)值,以Leuconostoc DSR制備來(lái)dextran成為工業(yè)化生產(chǎn)的主要方法[24]。L. mesenteroides、L. citreum(檸檬明串珠菌)和L. dextranicum(葡聚糖明串珠菌)是目前主要產(chǎn)DSR的3種明串珠菌菌株。其中,L. mesenteroides NRRL B-512F和NRRL B-1299是最早用于研究DSR特性的菌株[25-27],L. citreum HJ-P4的DSR基因經(jīng)克隆后在大腸桿菌中成功被高水平表達(dá)[12]。更多的研究著眼于Leuconostoc DSR的穩(wěn)定性[28]、產(chǎn)量[29-30]、蛋白純化[31-32]及其活性[33-34]、基因克隆[35-36]、產(chǎn)物結(jié)構(gòu)特性[37]以及被固定化后的特性[38-39]。
Streptococcus DSR主要來(lái)自血鏈球菌(S. sanguis)、變異鏈球菌(S. mutans)和牛鏈球菌(S. bovis),它們中有的是早期研究不同類型的DSR的重要素材[40-41],有的參與了不同組分對(duì)DSR反應(yīng)速率影響的研究[42],有的是DSR作用機(jī)制的研究對(duì)象[43-44],還有的用于研究DSR合成寡聚糖的產(chǎn)量[45]。
早在1963年,Dunican等就發(fā)現(xiàn)Lactobacillus菌株RWM-13的DSR合成與溫度有關(guān)[46],之后直到21世紀(jì),有關(guān)Lactobacillus DSR的研究才取得進(jìn)展。研究表明,利用分子生物學(xué)的方法可將羅伊士乳桿菌(L. reuteri)121由原來(lái)表達(dá)reuteransucrase(一種以蔗糖為底物合成reuteran的酶,reuteran則是一種由葡萄糖以α-1,4/α-1,6糖苷鍵鏈合而成的葡聚糖)改為表達(dá)DSR[19],而Ruhmkorf等對(duì)彎曲乳桿菌(L. curvatus)TMW 1.624、L. reuteri TMW 1.106和動(dòng)物乳桿菌(L. animalis)TMW 1.971 DSR特性作了系統(tǒng)的橫向比較[18]。
與上述3種來(lái)源DSR的悠久歷史相比,對(duì)Pediococcus、Weissella和Acetobacter DSR的關(guān)注是在近幾年才開(kāi)始升溫的。憑借Collins等提出的分子生物學(xué)證據(jù),Weissella正式由原來(lái)的腸膜明串珠菌的一個(gè)亞種(L.paramesenteroides)被重新劃分為一個(gè)新的屬[47]。有關(guān)食竇魏斯氏菌(W. cibaria)JAG8 DSR穩(wěn)定性[20]與活性位點(diǎn)[48]的報(bào)道為Weissella來(lái)源DSR的研究提供了理論依據(jù)。戊糖片球菌(P. pentosaceus)變異株P(guān)Pm因其更穩(wěn)定DSR成為了一個(gè)Pediococcus研究的亮點(diǎn)[9],而來(lái)自A. tropicalis的報(bào)道證實(shí)了發(fā)酵液中DSR活力與生物量的正相關(guān)性[10]。
2 DSR產(chǎn)量的優(yōu)化
2.1培養(yǎng)基的優(yōu)化
培養(yǎng)基的優(yōu)化是提高微生物代謝產(chǎn)物產(chǎn)量的一種常規(guī)手段,研究表明,適當(dāng)調(diào)整發(fā)酵基料中蔗糖、酵母抽提物、牛肉浸膏等主要營(yíng)養(yǎng)成分的濃度可有效地達(dá)到DSR產(chǎn)量的最大化[49-52]。土溫80是另一種有助于DSR產(chǎn)量提高、活性增強(qiáng)的常用培養(yǎng)基組分[53],而且,在酶的濃縮過(guò)程中,土溫80可將失活的DSR多聚體分離為具有活性的DSR分子,當(dāng)其與氯化鈣共存時(shí),可促進(jìn)微生物合成有利于過(guò)濾濃縮收集的活性酶多聚體[29]。Dols等認(rèn)為,當(dāng)發(fā)酵液中含有大量蔗糖時(shí),大部分蔗糖被DSR酶解合成dextran的同時(shí),也積累了相當(dāng)濃度的代謝副產(chǎn)物—甘露醇和果糖,這些副產(chǎn)物可導(dǎo)致DSR低得率,但在發(fā)酵基料中加入低濃度(<8 g/L)的葡萄糖可消除副產(chǎn)物對(duì)DSR產(chǎn)量的負(fù)面影響[54],當(dāng)果糖不與G-1-P(葡萄糖-1-磷酸)同時(shí)被消耗時(shí),發(fā)酵液中DSR產(chǎn)量與細(xì)胞生長(zhǎng)呈正比例關(guān)系[2]。此外,DSR的合成速率還受到發(fā)酵基料中碳氮比濃度的影響[55]。
2.2培養(yǎng)條件的優(yōu)化
培養(yǎng)基的溫度、pH和通氣量也是影響DSR產(chǎn)量的主要因素。對(duì)明串珠菌而言,DSR合成的最適溫度通常在23℃~25℃,Mariana和Ravi Kiran等都曾指出,不適宜的溫度條件會(huì)延緩菌體生長(zhǎng),進(jìn)而降低DSR的合成速率,并最終導(dǎo)致DSR的低得率[30,56],而在低溫條件下(10℃~13℃),只要發(fā)酵時(shí)間足夠長(zhǎng)(72 h),同樣能達(dá)到DSR高產(chǎn)量的目的,這是因?yàn)榈蜏叵旅父灰资Щ睿娱L(zhǎng)的發(fā)酵時(shí)間也更有利于酶的積累[13]。有報(bào)道稱,在發(fā)酵過(guò)程維持在pH 5.5的條件下,DSR的活性最高,穩(wěn)定性最佳,因此,dextran的合成會(huì)比傳統(tǒng)不控pH的發(fā)酵體系更效率、更快速[57]。對(duì)于兼性厭氧或好氧的DSR產(chǎn)生菌而言(如Leuconostoc等),利用通氣或振蕩發(fā)酵可促進(jìn)菌體細(xì)胞的快速增殖,以此達(dá)到加速DSR合成的目的,上述理論很好地解釋了部分報(bào)道中在特定通氣量和振蕩速率下,DSR最大產(chǎn)率與菌體細(xì)胞最快生長(zhǎng)速率保持一致的現(xiàn)象[10,58],然而Goyal等對(duì)該理論執(zhí)不同的看法,他發(fā)現(xiàn)靜止發(fā)酵液中的酶活要比振蕩發(fā)酵液中的高30 %,對(duì)此現(xiàn)象他的解釋是:L. mesenteroides是微需氧的微生物,通過(guò)對(duì)培養(yǎng)基中底物的氧化作用獲取能量的。當(dāng)處于靜止培養(yǎng)時(shí),由菌體排出的CO2積累于發(fā)酵液表面和內(nèi)部,令細(xì)胞處于厭氧條件下,此時(shí)菌體為了氧化更多的底物來(lái)得到更多可支配的能量而誘導(dǎo)合成更大量的DSR[59]。
2.3誘變菌株
與培養(yǎng)基和培養(yǎng)條件的優(yōu)化不同,菌株誘變是通過(guò)改變菌體自身的DSR表達(dá)特性來(lái)達(dá)到提高酶產(chǎn)量的目的。Kim等利用磺酸甲乙酯(ethyl methane sulfonate)誘變L. mesenteroides B-742獲得了具有組成性DSR表達(dá)能力的變異菌株[60]。紫外照射是另一種行之有效的誘變方法,Schachtele等通過(guò)該方法得到了DSR產(chǎn)量大幅提高的S. mutans 6715變異株S19[61],憑借此法還可獲取比親代菌株DSR穩(wěn)定性更佳的菌株[9]。
2.4異源表達(dá)
異源表達(dá)是近幾年才興起的用于提高DSR產(chǎn)量的一種分子生物學(xué)手段,通常是將編碼DSR的基因進(jìn)行擴(kuò)增后克隆進(jìn)大腸桿菌中,由后者完成酶的組成性表達(dá)。克隆了L. citreum HJ-P4 DSR編碼基因的E. coli MC1061在低溫(15℃)下的產(chǎn)量可達(dá)19 178 U/L,比37℃時(shí)的產(chǎn)量高出330倍以上[12],同樣的增產(chǎn)現(xiàn)象被發(fā)現(xiàn)于L. citreum KM20 DSR的異源表達(dá)研究中[62]。
3 DSR的制備方法
3.1聚乙二醇沉淀法
聚乙二醇(polyethylene glycol,PEG)是一種無(wú)電荷的線性大分子聚合物,其較強(qiáng)的脫水能力能夠破壞蛋白質(zhì)分子表面的水化層而使蛋白發(fā)生沉淀。此方法的優(yōu)點(diǎn)是成本低廉,操作簡(jiǎn)便,一次可處理大量樣品。其缺點(diǎn)是分離結(jié)果易受過(guò)量的脂類、離心溫度及pH變化的影響,并且單獨(dú)使用時(shí)非特異結(jié)合高,所以常與其它方法聯(lián)合使用。PEG法作為主流的蛋白質(zhì)制備與純化的方法之一至今仍運(yùn)用于DSR的研究中[11,63]。
3.2硫酸銨沉淀法
硫酸銨沉淀法,又稱為鹽析,其原理是溶液中的離子強(qiáng)度不同時(shí),不同蛋白質(zhì)的溶解度不同。高濃度的鹽離子在蛋白質(zhì)溶液中可與蛋白質(zhì)競(jìng)爭(zhēng)水分子,從而破壞蛋白質(zhì)表面的水化膜,降低其溶解度,使之從溶液中沉淀出來(lái)。由于不同蛋白質(zhì)溶解度各異,因而可利用不同濃度的鹽溶液來(lái)沉淀不同的蛋白質(zhì)。硫酸銨沉淀法因其溶解度大,溫度系數(shù)小和不易使蛋白質(zhì)變性而應(yīng)用最廣[64]。
3.3羥基磷灰石吸附法
羥基磷灰石是哺乳動(dòng)物骨骼和牙齒的主要無(wú)機(jī)成分,具備優(yōu)良的生物兼容性和特殊的表面性能,因其卓越的物理吸附能力而被廣泛用于蛋白質(zhì)純化工藝中[64]。
4 DSR的活性檢測(cè)
根據(jù)測(cè)定的目標(biāo)物的不同,用于測(cè)定DSR活性的方法主要可分為直接法(測(cè)定dextran合成量)和間接法(測(cè)定副產(chǎn)物fructose釋放量)。直接法包括了稱重法、同位素法和TLC法,而DNS法、鐵氰化物/砷鉬酸鹽法、銅/雙喹啉法以及酶法屬于間接法,這些測(cè)定技術(shù)的方法特性見(jiàn)表2。
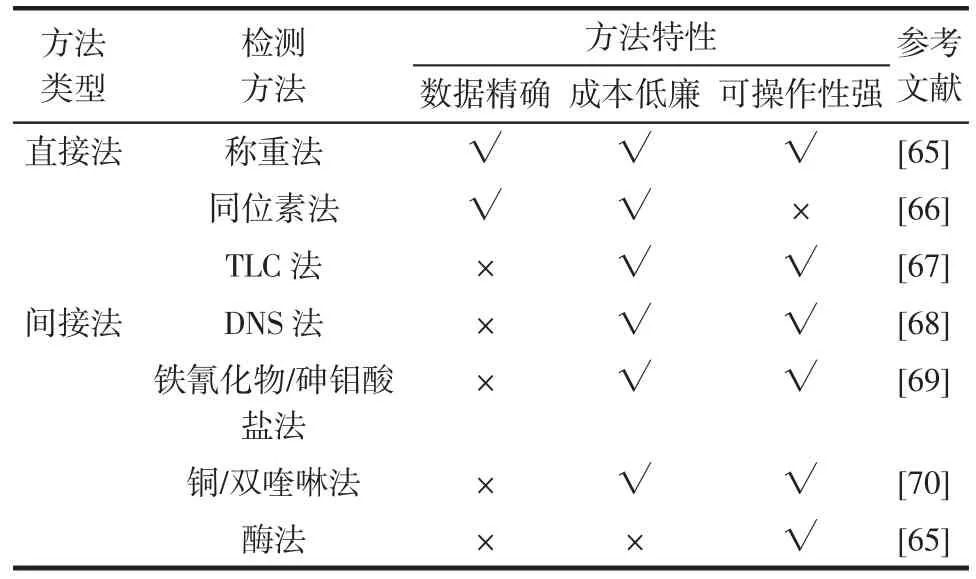
表2 DSR活性測(cè)定的主要方法及其特性Table 2 Characterizations of main methods for DSR activity assay
Mary Helen比較了上述DSR測(cè)定方法后發(fā)現(xiàn),由稱重法和同位素法測(cè)定的活性最準(zhǔn)確[65]。但后者對(duì)實(shí)驗(yàn)材料與設(shè)備的要求較高(14C-蔗糖和液體閃爍計(jì)數(shù)儀等),因此,實(shí)驗(yàn)室更多地采用稱重法來(lái)獲取DSR活性數(shù)據(jù)。雖然與直接法相比,間接法測(cè)定的DSR活性數(shù)據(jù)不夠精確,但其中的DNS法因其簡(jiǎn)便的操作流程受到研究人員的一致青睞,尤其在高通量篩選時(shí)具有快速、準(zhǔn)確的優(yōu)點(diǎn)。
5 展望
眾所周知,右旋糖苷蔗糖酶是催化分解蔗糖,合成dextran的關(guān)鍵性酶,其分子量因存在形式(單體或多聚體)而異,通常為64 kDa~245 kDa[27],Ca2+、Mg2+、Co2+等金屬離子和一些抑制劑(尿素、EDTA等)可對(duì)DSR活性造成顯著的影響[59]。DSR所合成的多聚糖和寡聚糖用途廣泛,前者以Dextran為例,被應(yīng)用于臨床、制藥、食品、攝影膠片以及精細(xì)化工等領(lǐng)域[71],而后者被證實(shí)可作為益生元來(lái)改善腸道菌群的結(jié)構(gòu)與數(shù)量[72]。此外,作為酶反應(yīng)副產(chǎn)物之一的果糖,是一種低卡路里的單糖,在食品工業(yè)中也有一定的應(yīng)用價(jià)值。DSR巨大的商業(yè)價(jià)值使其成為炙手可熱的研究對(duì)象之一。
盡管對(duì)于DSR的研究早在20世紀(jì)50年代[25]就已展開(kāi),并且隨著研究手段的飛速發(fā)展,關(guān)于DSR的報(bào)道涵蓋了DSR的酶學(xué)特性[46]、產(chǎn)量?jī)?yōu)化[29]、作用機(jī)理[43]、克隆表達(dá)[12]等多個(gè)領(lǐng)域,但是,依然存在一些技術(shù)問(wèn)題需要解決:一、現(xiàn)有培養(yǎng)基中DSR的產(chǎn)量普遍較低,因此有必要利用培養(yǎng)基組成、培養(yǎng)條件、變異誘導(dǎo)以及異源表達(dá)等方法將產(chǎn)量進(jìn)一步提高;二、DSR是蔗糖誘導(dǎo)細(xì)胞的產(chǎn)物,低濃度蔗糖對(duì)DSR的誘導(dǎo)效果較差,而過(guò)量的蔗糖往往會(huì)因?yàn)閐extran的大量合成而使發(fā)酵液粘度增加,從而影響DSR的提取,所以發(fā)酵基料中蔗糖添加量的平衡點(diǎn)有待于進(jìn)一步研究;三、通過(guò)對(duì)環(huán)境因素(溫度、pH、離子強(qiáng)度)的研究最大限度地提高DSR的穩(wěn)定性,從而更好地服務(wù)于工業(yè)化生產(chǎn);四、篩選與應(yīng)用天然的發(fā)酵基料。到目前為止,用于制備DSR的培養(yǎng)基都是人工合成的,伴有健康概念的天然發(fā)酵基料的篩選與應(yīng)用將打破原有狹隘的制備工藝,為DSR的獲取提供新的渠道。
參考文獻(xiàn):
[1] HENRISSAT B, DAVIES G. Structural and sequence-based classification of glycoside hydrolases[J]. Current opinion in structural biology, 1997, 7(5): 637-644
[2] DOLS M, CHRAIBI W, REMAUD-SIMEON M, et al. Growth and energetics of Leuconostoc mesenteroides NRRL B -1299 during metabolism of various sugars and their consequences for dextransucrase production[J]. Applied and environmental microbiology, 1997, 63(6): 2159-2165
[3] NAESSENS M, CERDOBBEL A, SOETAERT W, et al. Leuconostoc dextransucrase and dextran: production, properties and applications [J]. J Chem Technol Biotechnol, 2005, 80(8): 845-860
[4] ROBYT J. Dextran[J]. Encyclopedia of polymer science and engineering, 1986, 4: 752-767
[5] LEEMHUIS H, PIJNING T, DOBRUCHOWSKA J M, et al. Glucansucrases: Three-dimensional structures, reactions, mechanism, aglucan analysis and their implications in biotechnology and food applications[J]. Journal of biotechnology, 2013, 163(2): 250-272
[6] ROBYT J F, EKLUND S H. Stereochemistry involved in the mechanism of action of dextransucrase in the synthesis of dextran and the formation of acceptor products[J]. Bioorganic Chemistry, 1982, 11(2): 115-132
[7] BAHAVANI A, NISHA J. Dextran—the polysaccharide with versatile uses[J]. Int J Pharm Biol Sci, 2010, 1(4): 569-573
[8]羅靳,楊雅麟,王建華.腸膜明串珠菌右旋糖苷蔗糖酶的研究進(jìn)展[J].微生物學(xué)通報(bào), 2007, 34(4): 787-790
[9] KOTHARI D, TYAGI A, PATEL S, et al. Dextransucrase from the mutant of Pediococcus pentosaceus (PPm) is more stable than the wild type[J]. 3 Biotech, 2011, 1(4): 199-205
[10] CHAUHAN S, NISHA A W. Oxygen transfer rate modulates the dextransucrase production by Acetobacter tropicalis[J]. J Biochem Microb Technol, 2013, 1(1): 1-7
[11] ROBYT J F, WALSETH T F. Production, purification, and properties of dextransucrase from Leuconostoc mesenteroides NRRL B-512F[J]. Carbohydrate research, 1979, 68(1): 95-111
[12] YI A-R, LEE S-R, JANG M-U, et al. Cloning of dextransucrase gene from Leuconostoc citreum HJ-P4 and its high-level expression in E. coli by low temperature induction[J]. Journal of Microbiology and Biotechnology, 2009, 19(8): 829-835
[13] SHAMALA T, PRASAD M. Preliminary studies on the production of high and low viscosity dextran by Leuconostoc spp[J]. Process Biochemistry, 1995, 30(3): 237-241
[14] HUANG S, HEU C L, MAYERS R M. The purification and properties of dextransucrase from Streptococcus sanguis ATCC 10558[J]. Carbohydrate research, 1979, 74(1): 287-300
[15] CHLUDZINSKI A M, GERMAINE G R, SCHACHTELE C F. Purification and properties of dextransucrase from Streptococcus mutans [J]. Journal of bacteriology, 1974, 118(1): 1-7
[16] TAKAGI K, IOROI R, UCHIMURA T, et al. Purification and some properties of dextransucrase from Streptococcus bovis 148[J]. Journal of fermentation and bioengineering, 1994, 77(5): 551-553
[17] R HMKORF C, BORK C, MISCHNICK P, et al. Identification of Lactobacillus curvatus TMW 1.624 dextransucrase and comparative characterization with Lactobacillus reuteri TMW 1.106 and Lactobacillus animalis TMW 1.971 dextransucrases[J]. Food microbiology, 2013, 34(1): 52-61
[18] ABEDIN R M, ALIAA M, SHALL M A, et al. Optimization and Statistical Evaluation of Medium Components Affecting Dextran and Dextransucrase Production by Lactobacillus acidophilus ST76480.01[J]. Life Science Journal, 2013, 10(1): 1346-1353
[19] KRALJ S, VAN GEEL-SCHUTTEN I G, FABER E J, et al. Rational transformation of Lactobacillus reuteri 121 reuteransucrase into a dextransucrase[J]. Biochemistry, 2005, 44(25): 9206-9216
[20] MOHAN RAO T J, GOYAL A. Purification, optimization of assay, and stability studies of dextransucrase isolated from Weissella cibaria JAG8[J]. Preparative Biochemistry and Biotechnology, 2013, 43(4): 329-341
[21] MAINA N H, TENKANEN M, MAAHEIMO H, et al. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreumand Weissellaconfusa[J].Carbohydrate Research,2008,343(9): 1446-1455
[22] SCHEIBLER C. Investigation on the nature of the gelatinous excretion (so-called frog's spawn) which is observed in production of beet-sugar juices[J]. Zeitschrift fur Versuch-wesen Deutsche Zucker-Industrie, 1874, 24: 309-319
[23] VAN TIEGHEM P. On sugar-mill gum[J]. Ann Sci Nature Bot Biol Veg, 1878, 7: 180-203
[24] ALSOP R. Industrial production of dextrans[J]. Progress in Industrial Microbiology, 1983, 18: 1-44
[25] TSUCHIYA H M, KOEPSELL H J, CORMAN J, et al. The effect of certain cultural factors on production of dextransucrase by Leuconostoc mesenteroides[J]. Journal of bacteriology, 1952, 64(4): 521-526
[26] KOBAYASHI M, MATSUDA K. Purification and characterization of two activities of the intracellular dextransucrase from Leuconostoc mesenteroides NRRL B-1299[J]. Biochimica et Biophysica Acta (BBA)-Enzymology, 1975, 397(1): 69-79
[27] KOBAYASHI M, MATSUDA K. Purification and properties of the extracellular dextransucrase from Leuconostoc mesenteroides NRRL B-1299[J]. Journal of biochemistry, 1976, 79(6): 1301-1308
[28] RABELO M C, FONTES C M L, RODRIGUES S. Stability study of crude dextransucrase from Leuconostoc citreum NRRL B-742[J]. Indian Journal of Microbiology, 2011, 51(2): 164-170
[29] KITAOKA M, ROBYT J F. Large-scale preparation of highly purified dextransucrase from a high-producing constitutive mutant of Leuconostoc mesenteroides B-512FMC[J]. Enzyme and microbial technology, 1998, 23(6): 386-391
[30] CORTEZI M, MONTI R, CONTIERO J. Temperature effect on dextransucrase production by Leuconostoc mesenteroides FT 045 B isolatedfromAlcoholandSugarMillPlant[J].AfricanJournalofBiotechnology, 2005, 4(3): 279-285
[31] PURAMA R K, GOYAL A. Identification, effective purification and functional characterization of dextransucrase from Leuconostoc mesenteroides NRRL B-640[J]. Bioresource technology, 2008, 99 (9): 3635-3642
[32] COTE G L. The use of immobilized concanavalin a for the separation of alternansucrase from dextransucrase in culture broth ofLeuconostoc mesenteroides NRRL B-1355[J]. Biotechnology techniques, 1992, 6(1): 45-48
[33] CHELLAPANDIAN M, LARIOS C, SANCHEZ-GONZALEZ M, et al. Production and properties of a dextransucrase from Leuconostoc mesenteroides IBT-PQ isolated from `pulque', a traditional Aztec alcoholic beverage [J].Journalof Industrial Microbiologyand Biotechnology,1998,21(1/2):51-56
[34] SHUKLA R, ILIEV I, GOYAL A. Purification and characterization of dextransucrase from Leuconostoc mesenteroides NRRL B-1149 [J]. Biotechnology & Biotechnological Equipment, 2010, 24(sup1): 576-580
[35] KIM H, KIM D, RYU H-J, et al. Cloning and sequencing of the α-1→6 dextransucrase gene from Leuconostoc mensenteroides B-742CB[J]. Journal of microbiology and biotechnology, 2000, 10(4): 559-563
[36] FRAGA VIDAL R, MOULIS C, ESCALIER P, et al. Isolation of a gene from Leuconostoc citreum B/110-1-2 encoding a novel dextransucrase enzyme[J]. Current Microbiology, 2011, 62(4): 1260-1266
[37] KOTHARI D, GOYAL A. Structural characterization of enzymaticallysynthesizeddextranandoligosaccharidesfromLeuconostocmesenteroides NRRL B-1426 dextransucrase[J]. Biochemistry (Moscow), 2013, 78(10): 1164-1170
[38] GUPTA A, PRABHU M. Immobilization and properties of dextransucrase from Leuconostoc mesenteroides culture, LM[J]. Journal of General and Applied Microbiology, 1995, 41(5): 399-407
[39] UL Q S, AMAN A, SYED N, et al. Characterization of dextransucrase immobilized on calcium alginate beads from Leuconostoc mesenteroides PCSIR-4[J]. The Italian journal of biochemistry, 2007, 56(2): 158-162
[40] GRAHAME D A, MAYER R M. The origin and composition of multipleformsofdextransucrasefromStreptococcussanguis[J]. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 1984, 786(1): 42-48
[41] GRAHAME D A, MAYER R M. Purification, and comparison, of two forms of dextransucrase from Streptococcus sanguis[J]. Carbohydrate research, 1985, 142(2): 285-298
[42] NEWBRUN E, CARLSSON J. Reaction rate of dextransucrase from Streptococcus sanguis in the presence of various compounds[J]. Archives of oral biology, 1969, 14(5): 461-468
[43] ROBYT J F, CORRIGAN A J. The mechanism of dextransucrase action: activation of dextransucrase from Streptococcus mutans OMZ 176 by dextran and modified dextran and the nonexistence of the primer requirement for the synthesis of dextran[J]. Archives of biochemistry and biophysics, 1977, 183(2): 726-731
[44] MCCABE M M, SMITH E E. The dextran acceptor reaction of dextransucrase from Streptococcus mutans K1-R[J]. Carbohydrate research, 1978, 63: 223-239
[45] IOROI R, HAYASHI T, OHARA N, et al. Oligosaccharide production by dextransucrase of Streptococcus bovis no. 148 isolated from bovine rumen[J]. Journal of the Japanese Society for Food Science and Technology, 1990, 27(5): 355-362
[46] DUNICAN L, SEELEY H. Temperature-sensitive dextransucrase synthesis by a lactobacillus[J]. Journal of bacteriology, 1963, 86(5): 1079-1083
[47] COLLINS M, SAMELIS J, METAXOPOULOS J, et al. Taxonomic studies on some leuconostoc -like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species[J]. Journal of Applied Bacteriology, 1993, 75(6): 595-603
[48] RAO T J M, GOYAL A. Identification of active site residues in dextransucrase from Weissella cibaria JAG8[J]. Journal of Proteins & Proteomics, 2013, 4(3): 223-230
[49] PURAMA R K, GOYAL A. Screening and optimization of nutritional factors for higher dextransucrase production by Leuconostoc mesenteroides NRRL B-640 using statistical approach[J]. Bioresource technology, 2008, 99(15): 7108-7114
[50] SAWALE S D, LELE S. Increased dextransucrase production by response surface methodology from Leuconostoc species;isolated from fermented idli batter[J]. Global Journal of Biotechnology and Biochemistry, 2009, 4(2): 160-167
[51] LELE S, SAWALE S D. Enhanced production of dextransucrase by L. mesenteroides MTCC 867 using response surface methodology[J]. Recent Research in Science and Technology, 2009, 1(3): 94-99
[52] PURAMA R K, GOYAL A. Application of response surface methodology for maximizing dextransucrase production from Leuconostoc mesenteroides NRRL B-640 in a bioreactor[J]. Applied biochemistry and biotechnology, 2008, 151(2/3): 182-192
[53] GOYAL A, KATIYAR S S. Effect of certain nutrients on the production of dextransucrase from Leuconostoc mesenteroides NRRL B-512F[J]. Journal of basic microbiology, 1997, 37(3): 197-204
[54] DOLS M, REMAUD-SIMEON M, MONSAN P F. Dextransucrase production by Leuconostoc mesenteroides NRRL B-1299. Comparison with L. mesenteroides NRRL B-512F[J]. Enzyme and microbial technology, 1997, 20(7): 523-530
[55] LOPRETTI M, MARTINEZ E, TORRES L, et al. Influence of nitrogen/carbon ratio and complementary sugars on dextransucrase production by Leuconostoc mesenteroides NRRL B512 (f)[J]. Process biochemistry, 1999, 34(9): 879-884
[56] PURAMA R K, GOYAL A. Optimization of conditions of Leuconostoc Mesenteroides NRRL B-640 for production of a dextransucrase and its assay[J]. Journal of food biochemistry, 2009, 33(2): 218-231
[57] LAZIC M, VELJKOVIC V, SAVIC D, et al. pH control and the production of extracellular dextransucrase byLeuconostoc mesenteroides[J]. World Journal of Microbiology and Biotechnology, 1991, 7(1): 25-28
[58] MICHELENA G L, MART NEZ A, BELL A, et al. Scale-up of dextransucrase production by Leuconostoc mesenteroides in fed batch fermentation[J]. Brazilian Archives of Biology and Technology, 2003, 46(3): 455-459
[59] GOYAL A, NIGAM M, KATIYAR S S. Optimal conditions for production of dextransucrase from Leuconostoc mesenteroides NRLL B-512F and its properties[J]. Journal of basic microbiology, 1995, 35(6): 375-384
[60] KIM D, ROBYT J F. Production, selection, and characteristics of mutants of Leuconostoc mesenteroides B-742 constitutive for dextransucrases[J]. Enzyme and microbial technology, 1995, 17(8): 689-695
[61] SCHACHTELE C F, GERMAINE G R, HARLANDER S K. Production of elevated levels of dextransucrase by a mutant of Streptococcus mutans[J]. Infection and immunity, 1975, 12(4): 934-937
[62] KO J-A, JEONG H J, RYU Y B, et al. Large increase in Leuconostoc citreum KM20 dextransucrase activity achieved by changing the strain/inducer combination in an E. coli expression system[J]. Journal of Microbiology and Biotechnology, 2012, 22(4): 510-515
[63] VASILEVA T, IVANOVA I, ILIEV I. Purification and characterization of glucosyltransferases from new strains Leuconostoc mesenteroides[J]. Biotechnology & Biotechnological Equipment, 2009, 23 (Sup1): 693-697
[64] KIM D, ROBYT J F. Properties of Leuconostoc mesenteroides B-512FMC constitutive dextransucrase[J]. Enzyme and microbial technology, 1994, 16(12): 1010-1015
[65] VETTORI M H P B, MUKERJEA R, ROBYT J F. Comparative study of the efficacies of nine assay methods for the dextransucrase synthesis of dextran[J]. Carbohydrate Research, 2011, 346(9): 1077-1082
[66] GERMAINE G R, SCHACHTELE C F, CHLUDZINSKI A M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans[J]. Journal of dental research, 1974, 53(6): 1355-1360
[67] MUKERJEA R, KIM D, ROBYT J F. Simplified and improved methylation analysis of saccharides, using a modified procedure and thin-layerchromatography[J].Carbohydrateresearch,1996, 292: 11-20
[68] MILLER G L. Use of dinitrosalicylic acid reagent for determination of reducing sugar[J]. Analytical chemistry, 1959, 31(3): 426-428
[69] TING S. Fruit Juice Assay, Rapid Colormetric Methods for Simultaneous Determination of Total Reducing Sugars and Fructose in Citrus Juices[J]. Journal of Agricultural and Food Chemistry, 1956, 4(3): 263-266
[70] FOX J D, ROBYT J F. Miniaturization of three carbohydrate analyses using a microsample plate reader[J].Analytical biochemistry, 1991, 195(1): 93-96
[71] MAJUMDER A, PURAMA R K, GOYAL A. An overview of purification methods of glycoside hydrolase family 70 dextransucrase[J]. Indian journal of microbiology, 2007, 47(3): 197-206
[72] CHEN H-L, LU Y-H, LIN JR J, et al. Effects of fructooligosaccharide on bowel function and indicators of nutritional status in constipated elderly men[J]. Nutrition Research, 2000, 20(12): 1725-1733
Progress in the Research and Development of Dextransucrase
HAN Jin1,WU Zheng-jun2,*,XU Xiao-fen3,WU Jiang3
(1. State Key Laboratory of Dairy Biotechnology,Shanghai 200436,China;2. Shanghai Engineering Research Center of Dairy Biotechnology,Shanghai 200436,China;3. Dairy Research Institute,Bright Dairy & Foods Co. Ltd.,Shanghai 200436,China)
Abstract:Dextransucrase is a typical glucoseyltransferase. Dextran synthesized from sucrose by dextransucrase is wildly used in pharmaceutical and food fields. In this article,the progress in the research and development of dextransucrase source,productivity optimization,preparation method and activity assay was reviewed and the future perspective is also predicted.
Key words:dextransucrase;dextran;source;productivity optimization;preparation method;activity assay
收稿日期:2014-09-02
DOI:10.3969/j.issn.1005-6521.2016.02.049
*通信作者
作者簡(jiǎn)介:韓瑨(1980—),男(漢),高級(jí)工程師,碩士,研究方向:乳品科學(xué)。
基金項(xiàng)目:“十二五”國(guó)家科技支撐計(jì)劃課題:發(fā)酵乳制品乳酸菌菌種與發(fā)酵劑的研究與開(kāi)發(fā)(2013BAD18B01);“十二五”國(guó)家863項(xiàng)目:優(yōu)良益生菌高效篩選與應(yīng)用關(guān)鍵技術(shù)(2011AA100901)

