Th22細胞因子協同促進小鼠輸卵管上皮細胞抗沙眼衣原體感染體外研究
徐玲娟,趙秀敏,朱丹陽,許 文
Th22細胞因子協同促進小鼠輸卵管上皮細胞抗沙眼衣原體感染體外研究
徐玲娟1,趙秀敏2,朱丹陽2,許 文3
目的 體外研究Th22細胞因子(IL-22、TNF-α)在小鼠輸卵管上皮細胞抵抗沙眼衣原體感染過程中的作用。方法 體外培養Th22細胞,ELISA檢測Th22上清中細胞因子含量及其對體外培養的小鼠輸卵管上皮細胞(MOECs)表達Th1趨化因子、抗菌肽的影響;Transwell實驗檢測MOECs上清對Th1細胞的趨化能力;分別用免疫熒光法、PCR及7-乙氧基試鹵靈(7-ethoxyresrufin, 7-ER)法檢測Th22上清對輸卵管上皮細胞抑制Chlamydiatrichomatis(Ct)能力、促進胰島再生源蛋白3g(regenerating islet-derived protein-3g,Reg3g)Reg3g表達及細胞活性的影響。結果 Th22細胞以分泌IL-22、TNF-α為主。Th22上清可誘導MOECs表達Th1細胞趨化因子CXCL9/10/11及抗菌肽mBD-2表達,活化MOECs對Th1細胞具有趨化作用并抑制Ct生長。同時,Th22上清可促進MOECsReg3g表達及增強其活性。結論 Th22細胞因子能增強MOECs介導的天然免疫功能,促進其損傷修復,在Ct及其它性傳播疾病中可能發揮重要作用。
細胞因子;IL-22;TNF-α;小鼠輸卵管上皮細胞;Th22細胞
Th22細胞是一類新發現的輔助性T細胞,以膜表面表達CCR10及分泌IL-22、TNF-α為主要特征,在監督和協調引起炎癥的免疫細胞過程中發揮作用[1-2]。研究表明,IL-22可單獨或協同IL-17或TNF-α誘導上皮細胞產生抗微生物肽及多種趨化因子,在皮膚黏膜損傷修復及抗微生物感染中發揮重要作用[3-4]。以IL-22還可做為分子佐劑,可有效增強DNA疫苗誘導的Th1免疫反應。在慢性以及過敏性炎癥疾病,如牛皮癬、過敏性濕疹等疾病中; Th22細胞功能的喪失將使慢性炎癥疾病惡化。因此,Th22細胞可能是治療慢性炎癥疾病的潛在靶標。在Ct、陰道毛滴蟲等性傳播病原體感染的患者陰道分泌物中IL-22明顯升高[5],因此,我們推測Th22細胞及其細胞因子在生殖道黏膜免疫中亦發揮重要作用。本實驗應用體外培養小鼠輸卵管上皮細胞模型, 分析兩種關鍵Th22細胞因子IL-22、TNF-α對小鼠輸卵管上皮細胞免疫功能的影響, 為時一步探討Th22細胞及其細胞因子在生殖道黏膜免疫中的作用。
1 材料與方法
1.1 鼠 雌性6~8周齡的BALB/c小鼠購自中科院上海實驗動物中心。
1.2 主要試劑 HeLa 229 cells及CtE型株購自ATCC。抗沙眼衣原體LPS多克隆抗體購自Abcam。抗鼠IL-22、TNF-α抗體及鼠IgG1同型對照抗體(BD 公司,美國)。抗鼠CD3、CD28, IFN-γ、IL-4 單抗及重組鼠TNF-α, IL-6, and IL-2;CXCL-9, -10, 及-11 ELISA kits 均購自 R&D公司。mBD-2試劑盒購自Phoenix Pharmaceuticals公司。FITC標記抗鼠CD4單抗、PE標記抗鼠CXCR3單抗購自Pharmingen。7-乙氧基試鹵靈(7-ethoxyresrufin , 7 -ER )購自Sigma公司。
1.3 Th22細胞誘導及小鼠輸卵管上皮細胞的原代培養 磁珠分離BALB/c小鼠脾CCR10+CD4+T細胞,以1 μg/mL抗CD3、2 μg/mL抗CD28、5 μg/mL IFN-γ、5 μg/mL IL-4 單抗及細胞因子50 ng/IL-6、20 ng/mL TNF-α100 U/mL IL-2 (Th22 條件)刺激[3],誘導Th22細胞極化,流式分析表型(BD FACS Calibur)。于第7 d,以抗CD3、抗CD28抗體刺激,收集上清,-80 ℃凍存。
MOECs的原代培養參見文獻[6]。1×105MOECs/孔,接種接種于24孔板中,加入培養基2 mL, 細胞貼壁生長至85% 時,準備Th22上清干預。干預前基礎培養基饑餓培養細胞過夜, 次日上午于各培養皿中分別加入Th22上清,分別于24、48、72 h 后分離上清,-80 ℃凍存。
1.4 ELISA檢測細胞因子 依說明書操作,檢測Th22上清中IL-22, TNF-α, IL-17, IFN-γ、IL-4 含量及 MOECs上清中mBD-2 and CXCL-9, -10及-11含量。
1.5 Transwell遷移實驗 磁珠分離BALB/c 小鼠脾細胞中CD4+T 細胞,抗CD3 (1 μg/mL), 抗CD28 (2 μg/mL), 5 μg/mL 抗鼠IL-4及25 ng/mL IL-12 (Th1 條件) 刺激48 h后,離心、移除上清,補新鮮完全RPMI-1640,繼續培養48 h,以誘導CXCR3表達。2×105CD4+T細胞加于Transwell上室,下室加MOECs培養上清。37 ℃,5% CO2培養4 h 后置于 4 ℃ 20 min以分離膜上細胞,流式分析遷移的CD4+T數及其CXCR3表達。
遷移阻斷實驗:抗CXCR3 抗體(5 μg/ml)或同型對照抗體預先與激活的淋巴細胞在37 ℃孵育30 min, 然后用所收集的細胞上清液做遷移實驗。實驗重復3 次。
1.6 免疫熒光檢測活化MOECs抑制Ct增殖能力 3×104MOECs/孔置于96孔板中,1.2×103CtEB與100 μL MOECs培養上清(見上述)于37 ℃孵育45 min后加入上述孔中,室溫250×g離心40 min,棄上清,補200 μL完全1640(含10%胎牛血清)。37 ℃,5% CO2繼續培養40 h,10% 甲醇固定10 min, 加入兔抗Ct特異性脂多糖抗體200 μL, 37 ℃ 1 h, PBS洗滌后加入羊抗兔IgG-FITC 200 μL 30 min, PBS洗滌后甘油封片,熒光顯微鏡下(40×)計數檢測包涵體。
1.7 PCR檢測Reg3β表達Ct感染MOECs 24 h、48 h后,RT-PCR檢測再生蛋白(regenerating protein 3β,Reg3β) mRNA表達,引物序列為 5′-CGCATTAGTTGCCCCAAGG-3′ and 5′-TCCAGGCCTCTTTTGGCAG-3′. 提取Th22上清處理的MOECs總RNA,合成 cDNA。
1.8 細胞活性檢測Ct感染MOECs 24 h、48 h后,依說明書操作,熒光分光光度計檢測試鹵靈吸光度。
2 結 果
2.1 Th22細胞誘導 Th22 細胞是新發現的CD4+T 細胞,以膜表達CCR10及分泌IL-22為特征。因此,在本研究中采用抗CD3、CD28、IFN-γ、IL-4單抗及細胞因子IL-6、TNF-α 、IL-2組合刺激BALB/c小鼠來源的CCR10+CD4+T細胞誘導Th22細胞極化。7 d后,以抗CD3、CD28刺激活化后,流式分析表明所得CD4+T 細胞表達Th22標志CCR10 及IL-22 及 TNF-α(圖1). ELISA分析上清進一步表明,所得T細胞高表達Th22特征細胞因子IL-22、TNF-α而低表達Th1、Th2、Th17細胞因子IFN-γ、IL-4及IL-17(表1)。
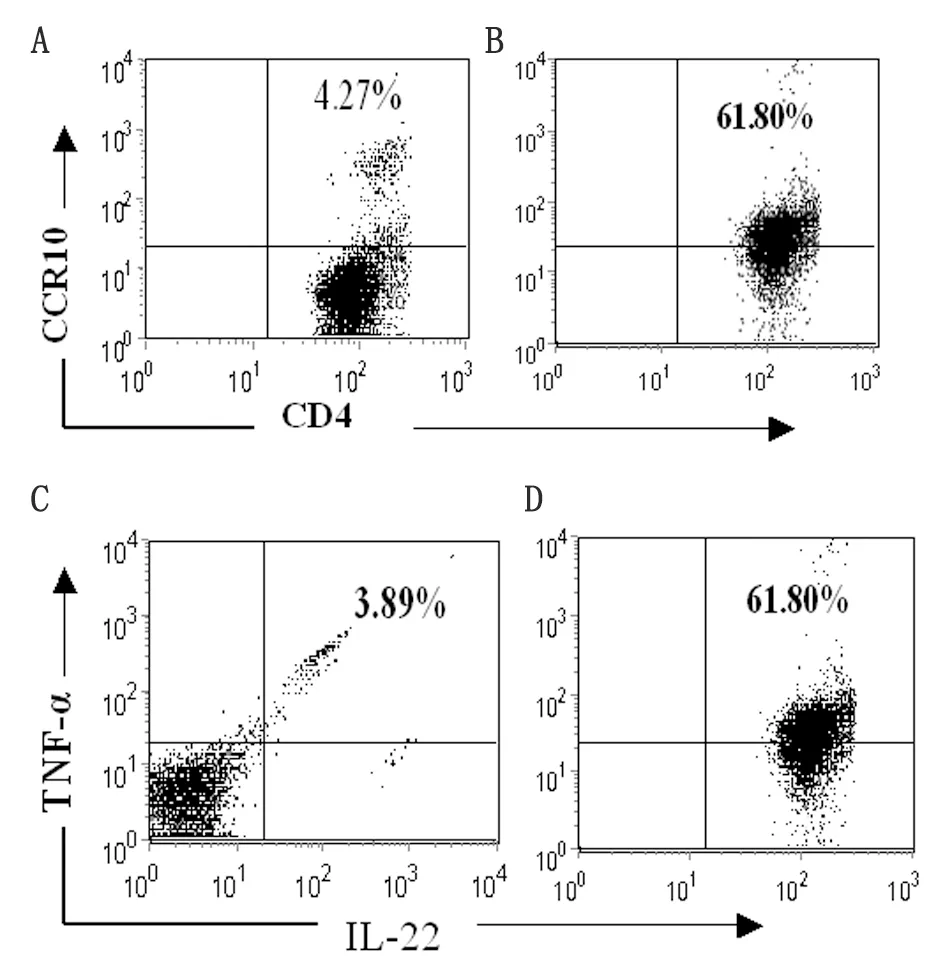
By flow cytometry, freshly isolated CCR10+CD4+T cells from the spleens of normal mice (left panel) and polarized Th22-type cells (right panel) were stained with anti-CCR10, anti-CD4, anti-IL-22, and anti-TNF-α Abs after permeabilization. The percentage of cells that were double-positive for CCR10 and CD4 (A and B) or IL-22 and TNF-α are shown (C and D).
圖1 Th22細胞誘導
Fig.1 Polarization of CD4+T cells into IL-22- and TNF-α-producing cells
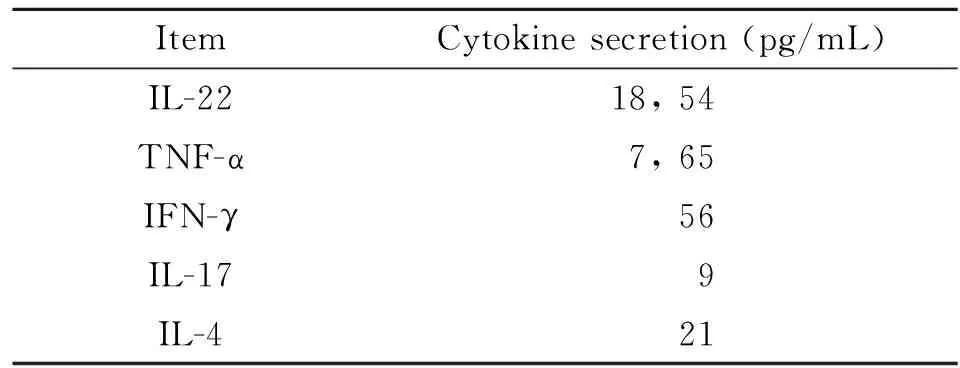
表1 Th22細胞因子檢測
Note: We set up Th22-type cells from spleens of BALB/c mice. Supernatants were measured 48 hrs after anti-CD3/CD28 stimulation.
2.2 Th22上清誘導MOECs產生Th1趨化因子CXCL9/10/11及抗菌肽mBD-2 為檢測Th22上清能否刺激MOECs天然免疫功能,MOECs經Th22上清作用24、48及72 h后,收集培養上清,ELISA檢測CXCL9、CXCL10、CXCL11及mBD-2濃度。如圖2所示,Th22上清能夠誘導小鼠輸卵管上皮細胞產生CXCL9、CXCL10、CXCL11及mBD-2, anti-IL-22/TNF-α抗體顯著抑制Th22上清的刺激作用,排除了其它細胞因子干擾的可能。

MOECs were stimulated with medium alone, IL-22, TNF-α, IL-22+TNF-α, or Th22 cell supernatant for 24, 48, or 72 hrs. For neutralization experiments, Th22 cell supernatants were pretreated with anti-IL-22+TNF-α mAbs for 30 min and then were used to stimulate MOECs. The levels of (A) CXCL-9, (B) CXCL-10, (C) CXCL-11, and (D) mBD-2 over a time-course in cell-free supernatants were quantified using ELISA kits. *P<0.05, **P<0.01, compared with the untreated control group; #P<0.05, ##P<0.01, compared with corresponding Th22 supernatant untreated by anti-IL-22/TNF-α mAbs.
圖2 ELISA檢測Th22上清刺激MOECs表達Th1趨化因子CXCL9/10/11及抗菌肽mBD-2表達
Fig.2 MOECs producing Th1 cell-associated chemokines and mBD-2 after IL-22+TNF-α stimulation
2.3 活化MOECs具有趨化Th1細胞的作用 T細胞介導的細胞免疫在清除Ct等性傳播病原中發揮關鍵作用。CXCL9、CXCL10、CXCL11可與效應T細胞表面CXCR3受體結合募集T細胞進入炎癥部位。本研究中我們通過Transweell實驗觀察Th22上清活化的MOECs趨化CXCR3+T細胞的能力。結果顯示活化的MOECs具有趨化活化T細胞的能力,與未刺激對照組比較差異有統計學意義。抗anti-IL-22及TNF-α抗體顯著抑制Th22上清誘導的趨化作用。
2.4 Th22細胞因子具有促進MOECs損傷修復的作用 由于IL-22 在上皮細胞的損傷修復中扮演重要作用,在本研究中,我們以體外感染Ct的MOECs為模型,進一步檢測了Th22上清對生殖道上皮細胞的保護作用。首先,我們檢測了Th22上清活化的MOECs能否抑制Ct生長。結果表明,以Th22上清活化的MOECs具有抑制生長的能力,與未處理對照組比較具有顯著差異。以anti-IL-22及TNF-α抗體封閉的Th22上清處理的MOECs抑制Ct生長的能力顯著下降。 接著,我們檢測了Th22
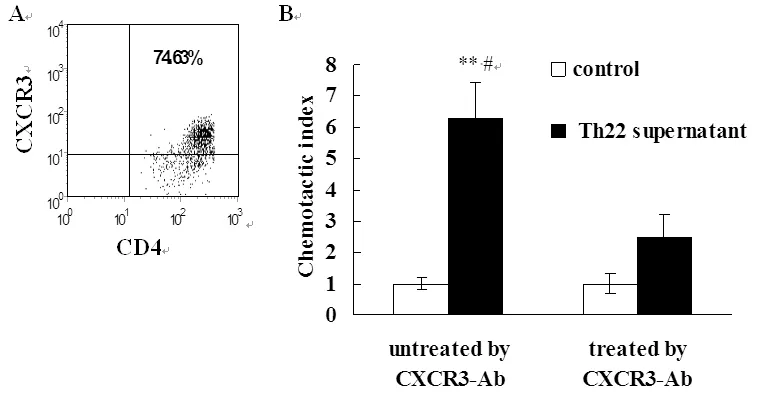
MOECs were stimulated by Th22 supernatant for 48 hrs. Then, supernatants were collected and analyzed by chemotaxis assay to assess the chemotactic activity of supernatants for Th1 cells. **P<0.01, compared with control; #P<0.05, compared with corresponding T cells that were not treated with CXCR3 antibody.
圖3 Th22上清活化的MOECs誘導Th1細胞遷移
Fig.3 Migration of Th1 cells induced by supernatants of MOECs treated with Th22 cell supernatant
上清對MOECs表達Reg3β的影響。Reg3β是促進上皮組織損傷修復的一個重要蛋白。結果表明,Th22上清具有促進Reg3β表達的作用。另外,我們的結果還證明了Th22上清具有促進MOECs活性的作用。以上結果表明,Th22細胞因子具有促進MOECs損傷修復的作用。

(A) MOEC supernatants from Th22 cell supernatant stimulation significantly inhibitedCtgrowth. Data are expressed relative values compared with the IFU/well resulting from infection with untreatedCt; (B) 24 and 48 h later, cell viability was quantified using Resorufin; (C)REG3βmRNA copy numbers were measured by quantitative RT-PCR.
Data are normalized to the expression ofβ-actin, and are expressed as the fold-increase over the average gene expression in untreated MOECs.
*P<0.05, **P<0.01, compared with the control group; #P<0.05, ##P<0.01, compared with the corresponding Th22 cell supernatant untreated by anti-IL-22 or TNF-α mAbs group.
圖4 IL-22 and TNF-α 協同促進MOECs損傷修復
Fig.4 IL-22 and TNF-α co-operate to protect MOECs againstCt
3 討 論
Th22細胞主要通過協調其它炎癥細胞從而在慢性炎癥疾病中發揮重要作用。IL-22、TNF-α是兩個關鍵的Th22細胞因子。IL-22受體由IL-22R1與IL-10R2組成。 由于IL-22R1主要表達于皮膚黏膜細胞并決定細胞對IL-22的反應性,故上皮細胞等組織細胞為IL-22的主要靶細胞[7]。IL-22可單獨或協同TNF-α還可誘導上皮細胞表達抗菌肽(如防御素、S100A7等)及Th1趨化因子CXCL9 /10/11的表達,具有抑制細菌生長及募集效應T細胞進入炎癥部位的作用[4, 8]。IL-22還可促進上皮細胞損傷修復[4]。
包括我們前期研究在內的結果表明Ct等性傳播病原體感染患者陰道分泌物中IL-22水平明顯上升且輸卵管上皮細胞可表達IL-22特異受體IL-22R1[9],因此我們推測Th22細胞及其細胞因子在生殖道黏膜免疫中亦發揮重要作用。由于mBD-2及CXCL9/10/11具有抗菌及趨化Th1細胞的作用,因此,在本研究中,我們建立了Th22細胞系并觀察其培養上清對MOECs產生mBD-2及CXCL9/10/11的影響。結果表明,Th22細胞上清能有效誘導MOECs產生mBD-2及趨化因子CXCL9/10/11。中和IL-22、TNF-α可有效抑制抗菌肽及趨化因子的表達,因而排除了其它細胞因子干擾的可能。
女性生殖道是HIV、HPV、HSV-2及Ct等性傳播病原體的入口[10]。T細胞介導的細胞免疫在清除這些性傳播病原體的過程中發揮重要作用。然而由于特殊的解剖屏障,T細胞難于進入生殖道。而趨化因子CXCL9/1011可與效應T細胞及記憶T細胞上CXCR3受體結合促進其進入生殖道發揮效應作用[11]。有證據表明,沙眼衣原體感染小鼠輸卵管組織中CXCL9、CXCL10 CXCL11表達明顯升高,且與沙眼衣原體的清除有關[12]。本研究中,通過Transwell實驗,我們亦證實了Th22活化的MOECs具有趨化CXCR3+T細胞的作用。另外,Th22活化的MOECs培養上清可顯著抑制Ct的生長。這些結果表明,Th22細胞因子具有調節MOECs免疫功能的效應。
Reg3g蛋白家族屬于C-類凝集素家族,具有抗菌、促進細胞增殖、抑制炎癥和細胞凋亡的作用[13-14]。IL-22可活化STAT3信號途徑,進而調節Reg3g蛋白的表達,促進上皮細胞損傷修復。我們前期的研究表明,IL-22可上調MOECs STAT3表達,因此在本研究中我們進一步觀察了Th22細胞因子對MOECs表達Reg3g的影響。結果顯示,IL-22、TNF-α可協同促進Reg3g的表達并增加MOECs的活性。這表明,Th22細胞因子具有促進MOECs損傷修復的作用。
綜上所述,本研究認為,Th22細胞因子具有調節MOECs免疫功能并促進其損傷修復的作用,這為進一步研究Th22細胞及其細胞因子在生殖道黏膜免疫中的作用提供了實驗依據。
[1]Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling[J]. J Clin Invest, 2009, 119(12): 3573-3585.
[2]Fujita H, Nograles KE, Kikuchi T, et al. Human Langerhans cells induce distinct IL-22-producing CD4+T cells lacking IL-17 production[J]. Proc Natl Acad Sci U S A, 2009, 106(51): 21795-21800.
[3]Sugita S, Kawazoe Y, Imai A, et al. Role of IL-22- and TNF-alpha-producing Th22 cells in uveitis patients with Behcet’s disease[J]. J Immunol, 2013, 190(11): 5799-5808.
[4]Eyerich S, Wagener J, Wenzel V, et al. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection withCandidaalbicans[J]. Eur J Immunol, 2011, 41(7): 1894-1901.
[5]Makinde HM, Zariffard R, Mirmonsef P, et al. IL-22 levels are associated withTrichomonasvaginalisinfection in the lower genital tract[J]. Am J Reprod Immunol, 2013, 70(1): 38-44.
[6]Li X,Xu W. The expression of murin β-defensin-2 in murine oviduct epithelial cells induced by IL-22[J]. Prog Obstet Gynecol,2014, 23(08): 627-629.
[7]Pham TA, Clare S, Goulding D, et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen[J]. Cell Host Microbe, 2014, 16(4): 504-516.
[8]Guilloteau K, Paris I, Pedretti N, et al. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis[J]. J Immunol, 2010, 184(9): 5263-5270.
[9]Frazer LC, Scurlock AM, Zurenski MA, et al. IL-23 induces IL-22 and IL-17 production in response toChlamydiamuridarumgenital tract infection, but the absence of these cytokines does not influence disease pathogenesis[J]. Am J Reprod Immunol, 2013, 70(6): 472-484.
[10]Huo Z, Yu P, Cheng W. Preliminary study of primary and secondary infection in mice genital withChlamydiamuridarum[J]. Chin J Zoonoses, 2013, 29(10): 1000-1005.
[11]Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells[J]. Nature, 2012, 491(7424): 463-467.
[12]Maxion HK, Kelly KA. Chemokine expression patterns differ within anatomically distinct regions of the genital tract duringChlamydiatrachomatisinfection[J]. Infect Immun, 2002, 70(3): 1538-1546.
[13]Rendon JL, Li X, Akhtar S, et al. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury[J]. Shock, 2013, 39(1): 11-18.
[14]Gessner MA, Werner JL, Lilly LM, et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense againstAspergillusfumigatus[J]. Infect Immun, 2012, 80(1): 410-417.
Potential protective role of the Th22 cytokines againstChlamydiatrichomatisinfection in genital tract
XU Ling-juan1,ZHAO Xiu-min2,ZHU Dan-yang2,XU Wen3
(1.DepartmentofLaboratoryMedicine,HospitalofTraditionalChineseMedicineofJinhuaCity,Zhejiang318300,China;2.DepartmentofObstetricsandGynecology,TaizhouFirstPeople’sHospital,Taizhou318020,China;3.DepartmentofMicrobiologyandImmunology,WengzhouMedicalUniversity,Wengzhou325035,China)
Th22 cells are a novel class of leukocytes characterized by the secretion of both IL-22 and TNF-α. Th22 cells have little or no direct impact on other immune cells, but exert selective effects on epithelia. It is not known, however, whether Th22 cells play a role in genital mucosal immunity. Here, we demonstrate that IL-22 and TNF-α synergistically induce several immunomodulatory molecules, such as the antimicrobial peptide mBD-2 (murine β-defensin 2) and the antimicrobial chemokines CXCL-9, -10, and -11 in primary murine oviduct epithelial cells (MOECs). The induction of innate immunity is relevant in aninvitroinfection model, in which MOECs stimulated with Th22 cell supernatants effectively inhibit the growth ofChlamydiatrichomatisand maintain the survival of the epithelia compared with untreated control. In summary, we demonstrate that the Th22 cell cytokines IL-22 and TNF-α play important roles in genital tract infection. The potential for Th22 cell cytokines to modulate innate immune mediators may lead to the development of new topical agents to treat and/or prevent immune-mediated STDs.
IL-22; defensin; chemokine; oviduct epithelial cell; Th22 cell
10.3969/cjz.j.issn.1002-2694.2015.08.012
許文,Email:wenxu.cn@gmail.com
1.浙江金華市中醫院檢驗科,金華 321017; 2.浙江臺州第一人民醫院婦產科,臺州 318300; 3.溫州醫科大學微生物學與免疫學教研室,溫州 325035
Correponding author: Xu Wen, Email: wenxu.cn@gmail.com
R374
A
1002-2694(2015)08-0742-05
2014-10-30;
2015-03-26
浙江省自然科學基金((Y2 110670);溫州市科技局項目(Y20110126)聯合資助
Supported by the Zhejiang Provincial Natural Science Foundation (No. Y2 110670) and the Science and Technology Planning Project of Wenzhou Municipal (No. Y20110126)

