梭果黃芪的化學成分和生物活性研究
田新雁,韓冰洋,肖朝江,姜 北
大理學院藥學與化學學院 藥物研究所,大理 671000
Introduction
Genus Astraglus is the largest one in the Fabaceae family[1].As a member of the genus,Astragalus ernestii is mainly distributed in southwest China,including the northwest Sichuan,northwest Yunnan,and east Tibet,with an altitude between 3900-4500 m.This plant is often used as substitute of Chinese medicine“Huang Qi”[2-3]by local folks,and therefore to be thought to have similar medicinal function with Huangqi,such as accelerate the metabolism,antifatigue effects,adjust the body's immunological function,anti-hypoxic,radiation resistance,liver protection and so on[4-8].So far,only several chemical constituents have been reported from A.ernestii[9].As a part of the project to better understand chemical and bioactive properties of Astragalus plants,we recently investigated A.ernestii collected from northwest Yunnan.As a result,fourteen known compounds were isolated and identified.And one of the compounds showed cytotoxicities against the human gastric cancer cell line(MGC-803),the human hepatoma(HepG2),and the human ovarian cancer cell line(SKOV3).
Materials and Methods
Apparatus and reagents
NMR spectra were recorded on Bruker AM-400 with TMS as reference.Silica gel(200-300 mesh,300-400 mesh)used for column chromatography,and silica gel GF254TLC were purchased from Qingdao Marine Chemical Factory(Qingdao,China).Sephadex LH-20 and MCI-gel(CHP-20P)were purchased from Amersham Biosciences(Amersham,Sweden).Spots of TLC were colored by spraying with 10% H2SO4followed by heating.Reagents used in the studies were all of analytical purity.
Plant material
The plant sample was collected from Zhongdian(Yunnan province,China)and authenticated as Astragalus ernestii H.F.Comber by Dr.Zhang De-quan who was a botanist working at Dali University.A voucher specimen was deposited at Prof.Jiang Bei’s laboratory,College of Pharmacy and Chemistry,Dali University.
Extract preparation and compound isolation
The finely powdered roots of A ernestii(1.26 kg)were extracted six times with methanol at room temperature.The filtered solvent was evaporated to yield crude extract(168 g),which was suspended in H2O and partitioned with ethyl acetate.The EtOAc fraction(35 g)was subjected to silica gel column chromatography(200-300 mesh)and eluted with CHCl3-CH3COCH3(100:0-0:100)to afford fractions 1-10.Fr.2(2.0 g)was separated by silica gel column chromatography(CC)and eluted with petroleum-EtOAc system(150:1)to give compound 1(30 mg),9(20 mg),and 8(10 mg).Fr.3(1.5 g)was separated by silica gel CC and developed with petroleum-EtOAc system(20:1),and the appropriate subfractions were further purified by sephadex LH-20(eluted with MeOH),silica gel CC and recrystallization,and silica gel CC(eluted with CHCl3-MeOH,70:1),to yield compouds 12(6 mg),14(1.0 g),and 11(6 mg),respectively.Fr.8 was chromatography over an MCI column eluted with MeOH-H2O gradient system(20%-100%)to give compounds 2(800 mg),3(8 mg),4(10 mg),7(8 mg),5(10 mg),10(10 mg),and 13(6 mg).Fr.10 was chromatographed over an MCI column eluted with MeOH-H2O gradient system(10%-100%)to give compound 6(20 mg).
Structural identification results
Lupineketene(1),colorless needle crystal(CHCl3);1H NMR(CDCl3,400 MHz)δ:4.69(1H,br s,H-29b),4.57(1H,br s,H-29a),1.68(3H,s,H-30),1.08(6H,s,H-23,26),1.04(3H,s,H-24),0.96(3H,s,H-27),0.94(3H,s,H-25),0.80(3H,s,H-28);13C NMR(CDCl3,100 MHz)δ:217.9(s,C-3),150.8(s,C-20),109.4(t,C-29),54.9(s,C-5),49.8(d,C-9),48.3(d,C-18),47.9(d,C-19),47.3(s,C-4),43.0(s,C-17),42.8(s,C-14),40.8(s,C-8),40.0(t,C-22),39.6(t,C-1),37.4(d,C-13),36.9(s,C-10),35.6(t,C-16),34.1(t,C-2),33.6(t,C-7),29.8(t,C-21),27.4(t,C-15),26.6(q,C-23),25.1(t,C-12),21.5(t,C-11),21.0(q,C-24),19.7(t,C-6),19.3(q,C-30),18.0(q,C-28),15.9(q,C-25),15.8(q,C-26),14.5(q,C-27).These data are consistent with the literature values[10].Thus,1 was determined to be lupineketene.
β-D-Glucopyranoside 3,4-dihydro-3-(2-hydroxy-3,4-dimethoxyphenyl)-2H-1-benzopyran-7-yl(2),colorless needle crystal(MeOH).1H NMR(400 MHz,CD3OD)δ:6.98(1H,d,J=8.5 Hz,H-5),6.77(1H,d,J=9.0 Hz,H-6'),6.62(1H,dd,J=8.5,2.5 Hz,H-6),6.54(1H,d,J=2.5 Hz,H-8),6.46(1H,d,J=9.0 Hz,H-5'),4.85(1H,d,J=8.0 Hz,H-1''),4.20(1H,ddd,J=10.5,3.5,1.5 Hz,H-2),3.96(1H,t,J=10.5 Hz,H-2),3.75(3H,s,4'-OCH3),3.69(1H,dd,J=12.0,2.0 Hz,H-7''),3.69(3H,s,3'-OCH3),3.46(1H,dd,J=12.0,6.0 Hz,H-6''),3.36(1H,dddd,J=10.5,3.5,11.0,5.0 Hz,H-3),3.29(1H,ddd,J=9.0,6.0,2.0 Hz,H-5''),3.26(1H,d,J=9.0 Hz,H-3''),3.20(1H,dd,J=9.0,8.0 Hz,H-2''),3.15(H,t,J=9.0 Hz,H-4''),2.81(1H,ddd,J=16.5,5.0,1.5 Hz,H-4);13C NMR(100 MHz,CD3OD)δ:158.4(s,C-7),156.3(s,C-9),153.2(s,C-4'),149.5(s,C-3'),137.6(s,C-2'),131.1(d,C-5),122.8(d,C-6'),122.3(s,C-1'),117.0(s,C-10),110.1(d,C-6),105.6(d,C-8),104.4(d,C-5'),102.5(d,C-1''),78.2(q,C-5''),78.0(s,C-3''),74.9(d,C-2''),71.9(q,C-4''),71.0(t,C-2),62.5(t,C-6''),61.0(q,-OCH3),56.2(q,-OCH3),33.5(d,C-3),31.1(t,C-4).These data are consistent with the reported values[11].Thus,2 was determined to be the title compound.
Liquiritigenin(3)was obtained as yellow powder(MeOH).1H NMR(400 MHz,CD3OD)δ:8.16(1H,d,J=8.8 Hz,H-5),7.53(2H,br.d,J=8.7 Hz,H-2',6'),7.21(2H,br.d,J=8.16 Hz,H-3',5'),6.88(1H,dd,J=2.0,8.8 Hz,H-6),6.80(1H,d,J=2.0 Hz,H-8),5.55(1H,dd,J=2.8,13.0 Hz,H-2β),3.25(1H,dd,J=13.6,16.5 Hz,H-3α),2.75(1H,dd,J=2.8,16.9 Hz,H-3β);13C NMR(100 MHz,CD3OD)δ:190.4(s,C-4),166.5(s,C-7),164.5(s,C-9),159.3(s,C-4'),130.2(s,C-1'),129.5(d,C-5),128.7(d,C-2',6'),116.5(d,C-3',5'),114.9(s,C-10),111.5(d,C-6),103.7(d,C-8),80.3(d,C-2),44.4(t,C-3).These data for 3 are consistent with the literature values for liquiritigenin[12].
(3R)-8,2'-Dihydroxy-7,4'-dimethoxy isoflavane(4)was obtained as white crystal(CH3COCH3).1H NMR(acetone-d6,400 MHz)δ:6.88(1H,d,J=8.4 Hz,H-6'),6.82(1H,d,J=9.0 Hz,H-5),6.49(1H,d,J=9.0 Hz,H-6),6.35(1H,dd,J=8.5,2.6 Hz,H-5'),6.26(1H,d,J=2.4 Hz,H-3'),4.24(1H,brd,J=10.2 Hz,H-2β),3.97(1H,t,J=10.2 Hz,H-2α),3.81(1H,s,4'OMe),3.78(1H,s,7-OMe),3.45(1H,m,H-3),2.96(1H,dd,J=16.2,10.8 Hz,H-4β),2.82(1H,ddd,J=16.2,5.2,1.9 Hz,H-4α);13C NMR(100 MHz,acetone-d6)δ:157.5(s,C-7),156.0(s,C-4'),152.6(s,C-9),148.9(s,C-2'),136.8(s,C-8),130.9(d,C-6'),122.4(d,C-5),121.5(s,C-1'),114.1(s,C-10),108.7(d,C-5'),104.2(d,C-6),103.6(d,C-3'),70.3(t,C-2),60.7(4'-OCH3),50.0(7-OCH3),32.9(d,C-3),30.8(t,C-4).These data for 4 are highly consistent with those reported values for(3R)-8,2'-dihydroxy-7,4'-dimethoxy isoflavane[13].
Isoliquiritigenin(5)was obtained as yellow powder(MeOH).1H NMR(CD3OD,400 MHz)δ:8.01(1H,d,J=8.9 Hz,H-6'),7.82(1H,d,J=14.5 Hz,Hα),7.65(3H,overlap,H-2,6,β),6.87(2H,d,J=8.5 Hz,H-3,5),6.44(1H,dd,J=9.1,2.7 Hz,H-5'),6.30(1H,d,J=2.3 Hz,H-3');13C NMR(CD3OD,100 MHz)δ:192.1(CO),166.1(s,C-4'),164.9(s,C-2'),160.2(s,C-4),144.3(d,C-β),131.9(s,C-6'),130.4(d,C-2,6),126.4(s,C-1),116.9(d,C-α),115.5(d,C-2,5),113.3(d,C-1'),107.7(d,C-5'),102.4(d,C-3').These data are consistent with those reported values[14].Therefore,5 was determined to be isoliquiritigenin.
Sucrose(6)was obtained as colorless crystal(DMSO).1H NMR(DMSO-d6,400 MHz,)δ:5.42(1H,d,J=3.3 Hz,H-1),4.23(1H,dd,J=8.7,2.5 Hz,H-3'),4.06(1H,td,J=8.4,2.4 Hz,H-4'),3.57(1H,dd,J=9.8,3.5 Hz,H-2);13C NMR(DMSO-d6,100 MHz)δ:103.6(d,C-2'),92.1(s,C-1),81.3(d,C-5'),76.3(d,C-3'),73.9(d,C-4'),72.3(t,C-5),72.5(d,C-3),71.0(d,C-2),69.1(d,C-4),62.3(t,C-6'),61.2(d,C-1'),60.0(t,C-6).These data for 6 are consistent with the literature values for sucrose[15].
7α-Hydroxysitosterol(7),colorless needle crystal(CHCl3),EIMS m/z(rel.int.%):412[M-H2O].1H NMR(CDCl3,400 MHz)δ:5.60(1H,d,J=5.1 Hz,H-6),3.85(1H,brs,H-7),3.59(1H,m,H-3),0.99(3H,s,Me-19),0.92(3H,d,J=6.5 Hz,Me-21),
0.86 (3H,overlap,Me-26),0.84(3H,overlap,Me-29),0.80(3H,overlap,Me-27),0.70(3H,s,Me-18);13C NMR(CDCl3,100 MHz)δ:146.2(s,C-5),123.8(d,C-6),71.3(d,C-3),65.3(d,C-7),55.7(d,C-17),49.4(d,C-14),45.8(d,C-24),42.3(s,C-13),42.2(d,C-9),42.0(t,C-4),39.2(t,C-12),37.5(d,C-8),37.4(s,C-10),37.0(t,C-1),36.1(d,C-20),33.9(t,C-22),31.4(t,C-2),29.7(t,C-16),29.0(d,C-25),28.3(t,C-23),24.3(t,C-15),23.1(t,C-28),20.7(t,C-11),19.9(q,C-27),19.0(q,C-19),18.8(q,C-26),18.2(q,C-21),11.9(q,C-29),11.6(q,C-18).These data for 7 are consistent with the literature values for 7α-hydroxysitosterol[16].
5α,8α-Epidioxy-(22E,24R)-ergosta-6,22-dien-3βol(8),white powder(CHCl3);1H NMR(CDCl3,400 MHz)δ:6.53(1H,d,J=8.4 Hz,H-7),6.22(1H,d,J=8.7 Hz,H-6),5.24(1H,dd,J=7.9,15.3 Hz,H-22),5.24(1H,dd,J=7.3,15.1 Hz,H-23),3.99(1H,m,H-3),1.09(3H,s,H-19),1.00(3H,d,J=6.6 Hz,H-21),0.90(3H,s,H-l8),0.89(3H,m,H-28),0.84(3H,d,J=6.5 Hz,H-26),0.82(3H,d,J=4.1 Hz,H-27);13C NMR(CDCl3,100 MHz)δ:135.4(d,C-6),135.1(d,C-22),132.3(d,C-23),130.6(d,C-7),82.2(s,C-5),79.4(d,C-8),66.5(d,C-3),56.2(d,C-17),51.6(d,C-l4),51.0(d,C-9),44.5(s,C-13),42.7(d,C-24),39.5(d,C-20),39.3(t,C-12),37.0(t,C-4),36.9(s,C-10),34.6(t,C-1),33.0(d,C-25),30.0(t,C-2),28.6(t,C-16),23.3(t,C-11),20.6(t,C-15),20.6(21-CH3),19.8(26-CH3),19.6(27-CH3),18.1(19-CH3),17.5(28-CH3),12.9(18-CH3).These data are consistent with the literature values[17].Thus,8 was determined to be 5α,8α-epidioxy-(22E,24R)-ergosta-6,22-dien-3βol.
Trilinolein(9),colorless oil(CHCl3),1H NMR(CDCl3,400 MHz)δ:5.31-5.41(12H,m),5.26(1H,m),4.28(2H,dd,J=4.2,11.9 Hz),4.13(2H,dd,J=6.0,12.0 Hz),2.75(4H,t,J=6.5 Hz),2.29(2H,overlap),2.28(4H,overlap),2.00-2.07(12H,overlap),1.60(6H,m),1.22-1.38(overlap),0.87(9H,t,J=6.7);13C NMR(CDCl3,100 MHz)δ:173.1(s,C-1',1'''),172.7(s,C-1''),130.1(d,C-10',10'',10'''),129.9(d,C-12',12'',12'''),128.0(s,C-13',13'',13'''),127.8(s,C-9',9'',9'''),68.8(d,C-1,3),62.0(t,C-2),34.1(t,C-2',2'''),33.9(t,C-2''),31.4(t,C-3',3'',3'''),29.0-29.6(t,C-4'-7',4''-7'',4'''-7''',15',15''15'''),27.1(t,C-8',8'',8'''),25.5(t,C-14',14'',14'''),24.8(t,C-11',11'',11'''),24.7(t,C-16',16'',16'''),22.5(t,C-17',17'',17'''),14.0(q,C-18',18'',18''').These data for 9 are agreed with the literature values for trilinolein[18].
n-Tritriacontane(10)was obtained as white powder(CHCl3).EI-MS:464[M+,6.2],449(8.8),435(10.0),421(12.5),407(21.9),393(28.1),379(43.8),113(25.0),99(28.8),85(68.8),71(90.6),57(100).These data are consistent with the literature values[19].Thus,10 was determined to be ntritriacontane.
n-Octadecane(11)was obtained as white powder(CHCl3).EI-MS:254[M+,8.1],239(8.1),225(8.1),211(8.1),197(8.1),149(29.4),111(32.5),97(38.8),71(62.5),57(100).These data are consistent with the literature values[20].Thus,11 was determined to be n-octadecane.
Octacosanol(12),white powder(CHCl3);EI-MS:410[M+,12.5],392(6.3),364(19),336(12.5),308(6.3),280(6.3),195(6.3),181(9.4),167(12.6),153(17),139(25),125(34.4),111(46.9),97(81.3),83(87.5),71(62.5),57(100).These data are consistent with the literature values[21].Therefore,12 was determined to be octacosanol.
n-Heptacosane(13),white powder(CHCl3);EI-MS:380[M+,16.5],365(6.5),351(6.5),337(6.55),323(6.5),309(6.5),295(6.5),71(81),57(100).These data are agreed with the literature values[22].Thus,13 was determined to be n-heptacosane.
β-Sitosterol(14),white needle crystal(CHCl3);1H NMR(CDCl3,400 MHz)δ:5.36(1H,dd,J=4.5,2.8 Hz,H-6),3.53(1H,m,H-3a),1.01(3H,s,H-19),0.93(3H,d,J=6.6 Hz,H-21),0.86(3H,t,J=6.0 Hz,H-26),0.83(3H,d,J=6.8 Hz,H-29),0.81(3H,d,J=6.6 Hz,H-28),0.68(3H,s,H-18);13C NMR(CDCl3,100 MHz)δ:140.7(s,C-5),121.6(d,C-6),71.8(d,C-3),56.8(d,C-14),56.0(d,C-17),51.0(d,C-9),45.9(d,C-24),42.4(s,C-13),42.0(t,C-4),39.8(t,C-12),37.3(t,C-1),36.5(s,C-10),36.1(d,C-20),33.9(t,C-22),31.9(d,C-8),31.6(t,C-2),32.0(t,C-7),29.1(q,C-27),28.3(t,C-16),26.1(t,C-23),24.1(t,C-15),23.0(d,C-25),21.1(t,C-11),19.7(q,C-29),19.4(q,C-19),19.2(t,C-28),18.6(q,C-21),11.8(q,C-18),11.0(q,C-26).These data for 14 are consistent with the literature values for β-sitosterol[23].
Cytotoxic assay
Cell lines
The human gastric cancer cell line(MGC-803),the human hepatoma cell line(HepG2),and the human ovarian cancer cell line(SKOV3)was obtained from the Key Laboratory of Medical Insects and Spiders Resources for Development and Utilization,Yunnan Province.
Cell culture
Cell line(MGC-803)was maintained in RPMI-1640(GIBCO)and HepG2,SKOV3 were maintained in DMEM(GIBCO),supplement with 10% fetal bovine serum FBS(GIBCO),100 IU/mL penicillin and 100 μg/mL streptomycin(Life Technologies).Cells were grown in 25 cm2tissue culture flasks in a humidified atmosphere containing 5% CO2at 37 ℃.Once the cells reach 80% confluence,1 mL of trypsin-EDTA solution was added to the flask for 5 min to detach the monolayer cells.The cells were occasionally observed under the inverted microscope until the cell layer was dispersed.Then,2 mL of complete growth medium was added to the flask followed by repeated gentle pipetting to split apart the cell clumps.Approximately 0.5-1×106cells were sub cultured into a new 25 cm2flask containing 8 mL of fresh medium.
MTT colorimetric assay
The MTT assay is commonly used in the screening of anti-cancer compounds,and this method was first developed in 1983.The tetrazolium salt(MTT)is used as a developing dye.The tetrazolium ring of MTT can be cleaved by dehydrogenases in the mitochondria of living cells to produce a purple formazan.The MTT soluble formazan reaction was only partially soluble in the medium,and so the[10% SDS-5% isobutanol-0.012 mol/L HCl(w/v/v)]was used to dissolve the formazan,and the optical densities at 570 nm are read by a scanning multi-well spectrophotometer[26].
Briefly,exponentially growing cells were seeded into 96-well plate at a density of approximately 1×105cells/90 μL/well and allowed to adhere overnight,Treatments in the final concentration range between 3.0 and 300 μg/mL were introduced.Meanwhile,the control wells were treated with 0.3% of DMSO equivalent to the amount of DMSO used as a vehicle in the sample treated wells.After 48 h of incubation,15 μL of MTT solution(5.0 mg/mL)was added and incubation for an addition 4 h.Medium and excessive MTT were aspirated and formazan formed was solubilized by the addition of 100 μL[10% SDS-5% isobutanol-0.012 mol/L HCl(w/v/v)].The optical densities at 570 nm are read by a scanning multi-well spectrophotometer.The results were listed in the table 1.
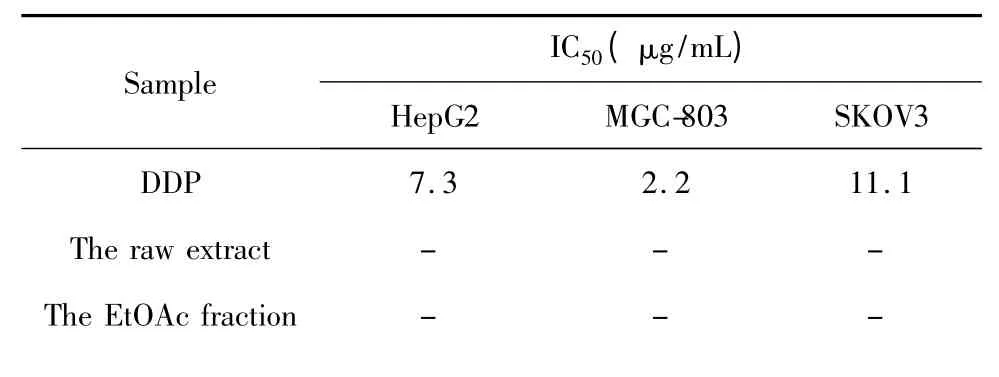
Table 1 Cytotoxicities of the samples from A.ernestii

Conclusion
Among the fourteen compounds obtained from A.ernestii,compound 2 and β-sitosterol were the major constituents of the EtOAc fraction.According to the cytotoxic experiments on the samples including raw extract,ethyl acetate fraction,butanol fraction,water fraction,and compound 2,2 showed moderate cytotoxicities against human gastric cancer cell line(MGC-803),human hepatoma cancer cell line(HepG2),and human ovarian cancer cell line(SKOV3).However,the other samples didn’t have activities on these cell lines.
1 Avunduk S,Mitaine-Ofer AC,Alankus-Caliskan O,et al.Triterpene glycosides from the roots of Astragalus flavescens.J Nat Prod,2008,71:141-145.
2 Flora of China Editorial Committee of Chinese Academy of Sciences.Flora of China.Vol 42.Beijing:Science Press,1993.188.
3 China National Group Corporation of Traditional and Herbal Medicine.Compendium of Chinese Medicinal Resources.Beijing:Science Press,1994.550.
4 Qiu PY,Xu ZC,Chen ZY,et al.Study on immunomodulatory effect of Astragalus polysaccharide in mice.J Xinxiang Med Colle,2006,23:585-685.
5 Kong LM.Immunological regulating effect of the Astragalus.Inner Mongolia Medical Journal,2007,39:73-74.
6 Wang RT,Shan BE,Li QX,et al.Extracorporeal experimental study on immuno-modulatory activity of Astragalus memhranaceus extract.Chin J Integrative Med,2002,22:453-456.
7 Xu M,Hu XP,Zhu H,et al.Immunomodulatory effects of total Astragalus extract.Pharmacol Clin Chin Mater Med,2005,21(3):27-29.
8 Lu JT,Yang Y,Wei W,et al.Protective effects of compound Astraglus extract on chemical and immunalogical liver injury in mice.Chinese Journal of Information on TCM,2008,15:33-34.
9 Sun LM,Wang XL,Deng WL,et al.Chemical constituents from Astragalus ernestii.Chin J Nat Med,2011,9:38-41.
10 Wang XY,Liu LP,Kang TG,et al.Chemical constituents from Euphorbia tirucalli.China Tradit Herb Drugs,2011,42:2398-2401.
11 Wang HK,He K,Xu HX,et al.The structure of astrachrysosid A and the study of 2D NMR on astragsieverisianin XV and 7,2'-dihydroxy-3',4'-dimethoxy-iso flavane-7-O-β-Dglucoside.Acta Pharm Sin,1900,25:445-450.
12 Lai GF,Zhao PJ,Ni ZW,et al.A new fructofuranoside from Helwingia chinensis.Acta Botanica Yunnanica,2008,30:115-120.
13 Li RF,Zhou YZ,Qiao L,et al.Chemical constituents of Astragalus membranaceus Bge.var.Mongholicus(Bge.)Hsiao.J Shenyang Pharm Univ,2007,24:20-24.
14 Yang H,Wang D,Tong L,et al.Flavonoid aglycones of Oxytropis falcate.Chem Nat Compd,2009,2:239-240.
15 Zhou L,Wang N,Miao F,et al.Chemical consitituents of Gentiana apiata N.E.Br..Chin J Org Chem,2004,24:1249-1252.
16 Wang Y,Zou ZM.Study on steroids from the stem of Croton caudatus Geisel.var.tomentosus Hook.Chin Pharm J,2008,43:897-899.
17 Ma BJ,Shen JW,Yu HY,et al.Chemical composition of theFruiting Bodies of Helvella elastica.Acta Bot.Boreal.-Occident.Sin,2009,29:2115-2117.
18 Yin W,Wang G.Chemical constituents in fermentation liquid of the Fungus Lycoperdon Fuscum.J Anhui Tradit Chin Med Colle,2010,29(6):67-70.
19 Tang RJ,Bi NJ.Studies on the chemical constituents of Euonymus Fortunei(Turcz.)Hand-Mazz.West China Journal of Pharmaceutic Sciences,1989,4(2):76-78.
20 Yao QQ,Zuo CX.Chemical studies on the constituents of Phyllanthus urinaria L..Acta Pharm Sin,1993,28:829-835.
21 Su L,Lou FC,Zheng WP,et al.Studies on the constituents from the branch bark of Ginkgo biloba L..Pharmaceutical Biotechnology,1999,4(6):1-5.
22 Wei H,Wen DX,Liu XD,et al.Constituents in petroleum ether and ethyl acetate extract fractions of Dracaena cochinensis(Lour.)S.C.Chen(Ⅱ).Chin J Chin Mater Med,1998,10:616-618.
23 Zhou JS,Zhang TT,Chen JJ,et al.Chemical constituents from the roots of Streptocaulon griffithii.Chin J Nat Med,2009,7:108-110.
24 Lv QJ.Methodology of new drug research in pharmacology.Beijing:Chemical Industry Press,2007.96-247.

