Syntheses and Crystal Structures of Nickeand CopperComplexes with Schiff Base Ligand of 5-Chlorosalicylaldehyde
WU Qiong-JieCHEN Xiao-HuaCAI Bi-QiongXIE Yong-Ping
(1College of Life Science,Fujian Agriculture and Forestry University,Fuzhou 350002,China)
(2College of Chemistry and Materials Science,Fujian Normal University,Fuzhou 350007,China)
Syntheses and Crystal Structures of Nickeand CopperComplexes with Schiff Base Ligand of 5-Chlorosalicylaldehyde
WU Qiong-Jie*,1CHEN Xiao-Hua2CAI Bi-Qiong1XIE Yong-Ping1
(1College of Life Science,Fujian Agriculture and Forestry University,Fuzhou350002,China)
(2College of Chemistry and Materials Science,Fujian Normal University,Fuzhou350007,China)
Two polynuclear transition metal complexes with a monodentate heterocycle and tridentate Schiff base ligands have been synthesized and characterized by elemental analysis,IR,UV and X-Ray diffraction.One is nickel(Ⅱ) complex[Ni(L1)(Mf)](1)with 5-chlorosalicylaldehyde 4-nitrobenzoylhydrazone(H2L1)and morpholine (Mf),the other is copper(Ⅱ)complex[Cu2(L2)2(Py)2](2)with 5-chlorosalicylaldehyde salicyloylhydrazone(H2L2)and pyridine (Py).In compound 1,the Ni(Ⅱ) ion is coordinated by three donors (phenolate-O,imine-N and deprotonated amide-O atoms)from the ligand L12-and the N atom of the neutral morpholine,forming a squareplanar N2O2coordination geometry.In the crystal,every two molecules related by centrosymmetric operation in compound 1 are connected through intermolecular hydrogen bonds,forming one-dimensional chain.There are two crystallographically binuclear copper(Ⅱ)units in complex 2.The two metal centres are bridged by the phenolate O atoms of the neighboring ligand,forming two Cu2O2quadrangle.Each Cu(Ⅱ)ion has a square-pyramidal geometry. CCDC:846209,1;846210,2.
Schiff base;Nickel(Ⅱ)complex;Copper(Ⅱ)complex;crystal structure
The study of transition metal-hydrazone complexes,in which the hydrazone ligands are formed by condensing hydrazine with β-diketones,salicylaldehyde and their derivatives,has attracted considerableattention because ofthe biologicalactivity and chemical versatility of these complexes[1-3].Tridentate Schiff-bases are regarded as a good type of chelating ligands for transition metal. For example, aroylhydrazones of salicylaldehyde and its derivatives can bind to a given metal ion through phenolate oxygen, imine nitrogen and amide oxygen atoms[4-5].In addition, the phenolate-O site can function as a bridging site to furnish dinuclear complexes,which simultaneously having other ancillary ligands in their coordination positions[4,6-8].Neutral N-donor pyridine or morpholine has been used as the ancillary ligand in those reported complexes[6-7,9-10].In the following account,we have described the syntheses,characterizations and the crystal structures of a nickel complex and a binuclear copper complex with different aroylhydrazone ligands.
1 Experimental
1.1 Reagents and physical measurements
The Schiff base ligands 5-chlorosalicylaldehyde 4-nitrobenzoylhydrazone (H2L1)and 5-chlorosalicylaldehyde salicyloylhydrazone (H2L2)were synthesized according to the reported method[10].All starting chemicals were of analytical grade and used without further purification.Elemental analyses of carbon,hydrogen and nitrogen were carried with an Elementar Vario ELⅢ microanalyser.IR spectra were recorded on a Perkin-Elmer spectrum 2000 spectrophotometer with KBr pellets in the range of 4 000~400 cm-1.Electronic spectra were recorded on a UV-1900 UV/VIS spectrophotometer using ethanol as the solvent.
1.2 Synthesis of complex[Ni(L1)(Mf)](1)
H2L1(0.1 mmol)and NiCl2·2H2O(0.1 mmol)were dissolved in methanol(10 mL).After stirring for 15 min,morpholine(Mf,1 mL)was added to the solution, which was then stirred for another 1 h and filtered.Red single crystals of(1)were obtained after one week.Anal.Calcd.for C18H17ClN4NiO5(%):C 46.60,H 3.67,N 12.08;found(%):C 46.72,H 3.63,N 12.01.
1.3 Synthesis of complex[Cu2(L2)2(Py)2](2)
To H2L2(0.1 mmol)in methanol(5 mL)was added an equimolar amount of CuCl2·2H2O (0.1 mmol)in methanol (5 mL).After stirring for 15 min,3 mL pyridine (Py)was added to the solution.The resulting mixture was stirred at room temperature for an additional period of 1 h and then filtered.Dark-blue prism-shaped crystals of(2)were obtained from the solution after two weeks.Anal.cald.for C38H28Cl2Cu2N6O6(%):C 53.13,H 3.98,N 9.39;found(%):C 52.86, H 3.25,N 9.74.
1.4 Crystal structure determination
Single crystals with suitable dimensions of 0.16 mm×0.24 mm×0.48 mm for complex 1 and 0.13 mm× 0.28 mm×0.51 mm for complex 2 were selected for single-crystal X-ray diffraction analysis.Crystallographic data were collected at a Rigaku RAPID Weissengberg IP diffractometer with graphitemonochromatized Mo Kα radiation (λ=0.071 073 nm)and the φ-ω scan mode.Both structures were solved by direct method with SHELXS-97[11]and refined by fullmatrix least squares calculations with SHELXL-97[12].All of the non-hydrogen atoms were refined anisotropically.All of the hydrogen atoms were located from the geometrical calculation and refined isotropically.Crystallographic data and structure refinement data for both complexes are listed in Table 1.
CCDC:846209,1;846210,2.
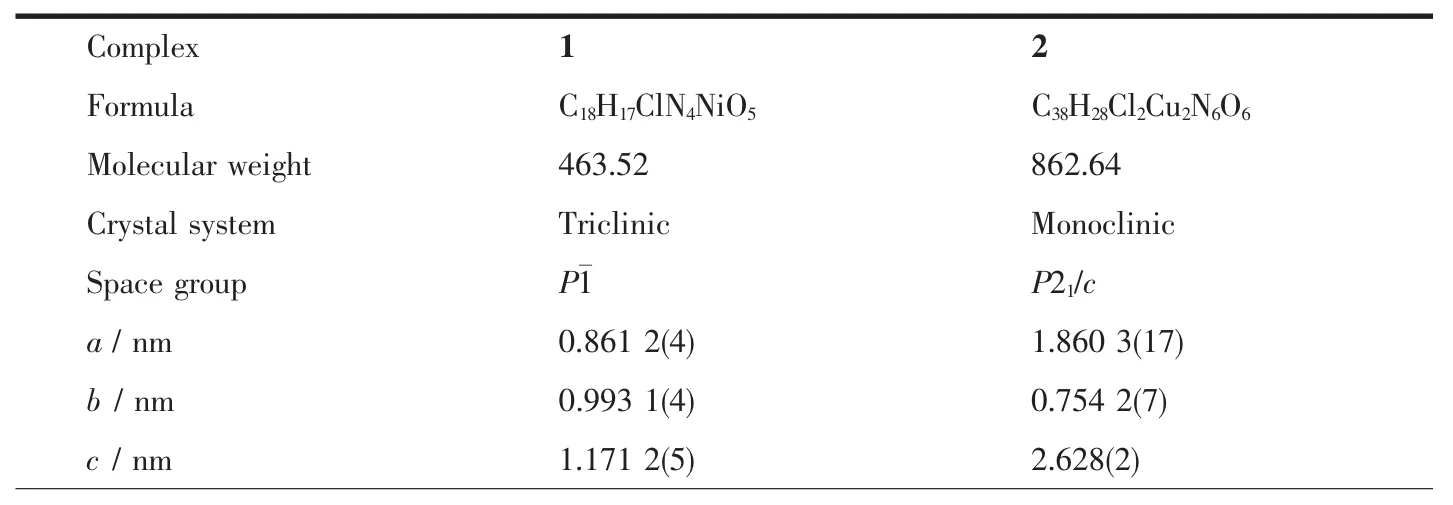
Table 1 Crystallographic data for complexes 1 and 2
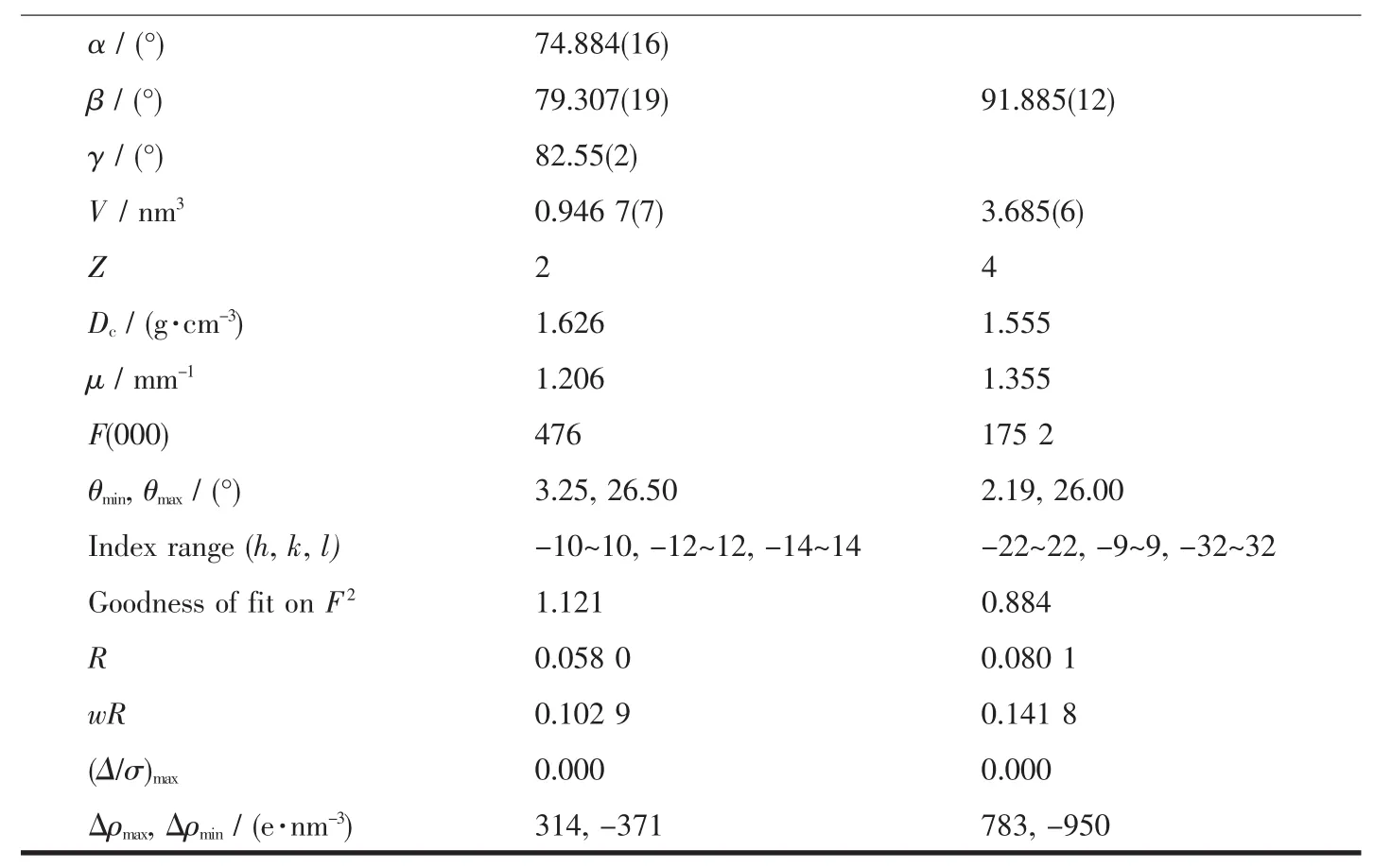
Continued Table 1
2 Results and discussion
2.1 Crystal structure of complex 1
The selected bond lengths and angles of complex 1 are given in Table 2.As shown in Fig.1,the nickel(Ⅱ) ion lies in a square-planar coordination environment with the hydrazone ligand(H2L1)coordinated to it as a tridentate chelating agent via the phenolic O2 atom,the imine N2 atom and the deprotonated amide O1 atom.The fourth coordination position is occupied by N4 atom of the morpholine ligand.There is nearly no deviation of the mental center from the N2O2squareplane.The maximum and minimum deviations from the mean plane constituted by O1,O2,N2,N4 and Ni1 is 0.0034(2)and 0.0010(1)nm,respectively.
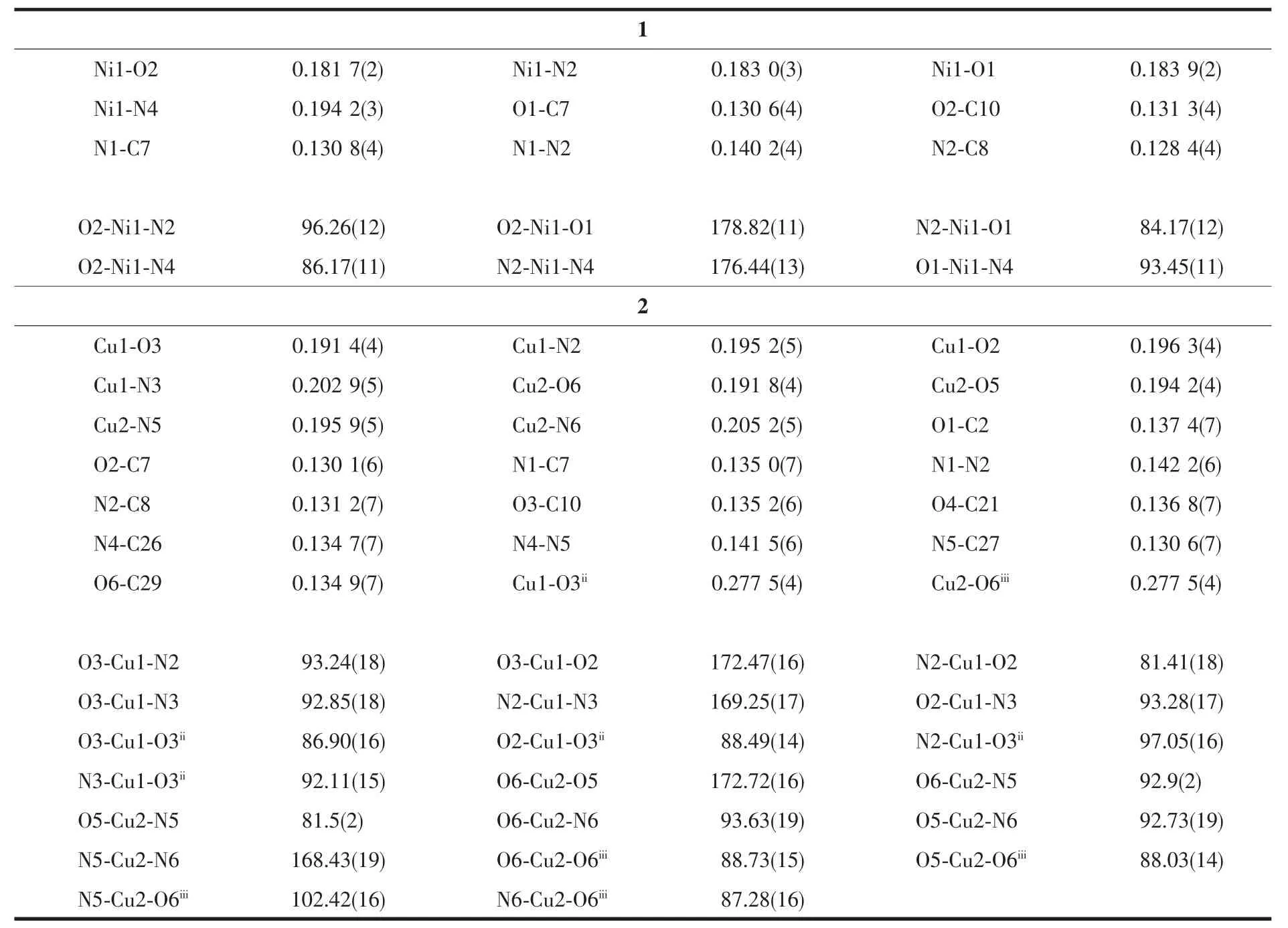
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
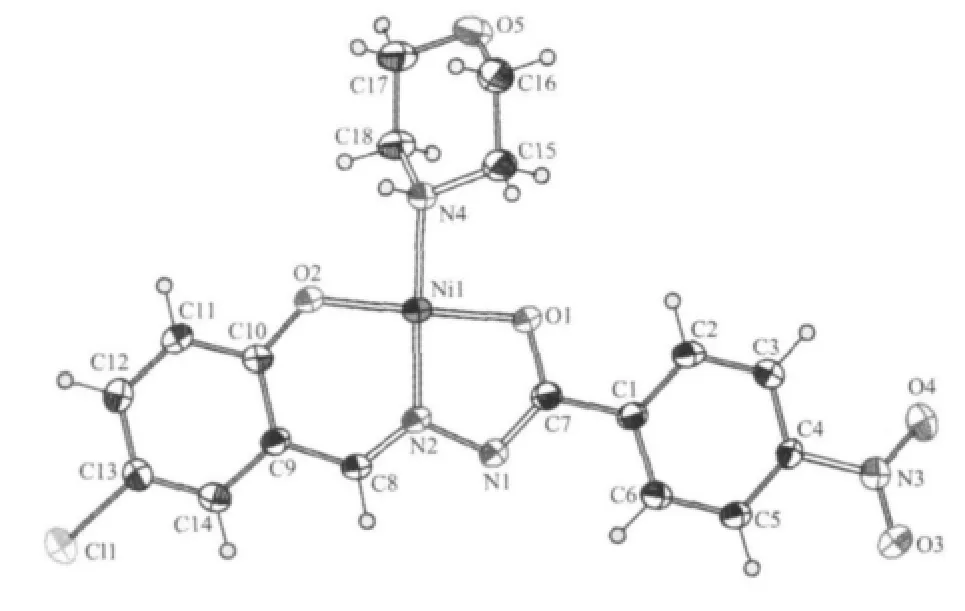
Fig.1 Molecular structure of complex 1 with 30% thermal ellipsoids
The N-N,N-C and C-O bond distances in the=NNC(O-)-fragment of L12-are consistent with the enolate form of the amide functionalities[9-10,13].The Ni1-O1 (amide),Ni1-O2(phenolic)and Ni1-N2(imine)bond distances are similar to those observed in nickel(Ⅱ)complexes having the same coordinating atoms[9,10,13]. The Ni1-N4(morpholine)bond length is comparable to the value observed in the only three examples of a tetracoordinated Ni(Ⅱ) complex containing monodentated neutral morpholine moiety[9-10,13].Both the sixmembered chelating ring (r.m.s deviation=0.000 6(2) nm)and the five-membered chelating ring(r.m.s deviation=0.0015(4)nm)are close to planar,and the dihedral angle between them is 0.6(2)°.The dihedral angle between the two benzene rings of the hydrazone ligand is 5.8(2)°,indicating a slight twist of the whole ligand.
In the crystal structure,the asymmetric units are linked by a week intermolecular N4-H4A(morpholine)…O4i(nitro)(symmetry code:ix-1,y+1,z)hydrogen bonds,leading to a one-dimensional chain(Fig.2).The N4…O4 distance and N4-H4A…O4 angle are 0.335(4) nm and 161.68°,respectively.The Ni…Ni distance in this uniform arrangement is 1.23 (1)nm,which is longerthanthat(0.8533(3)nm)inthesimilarcomplex[10].
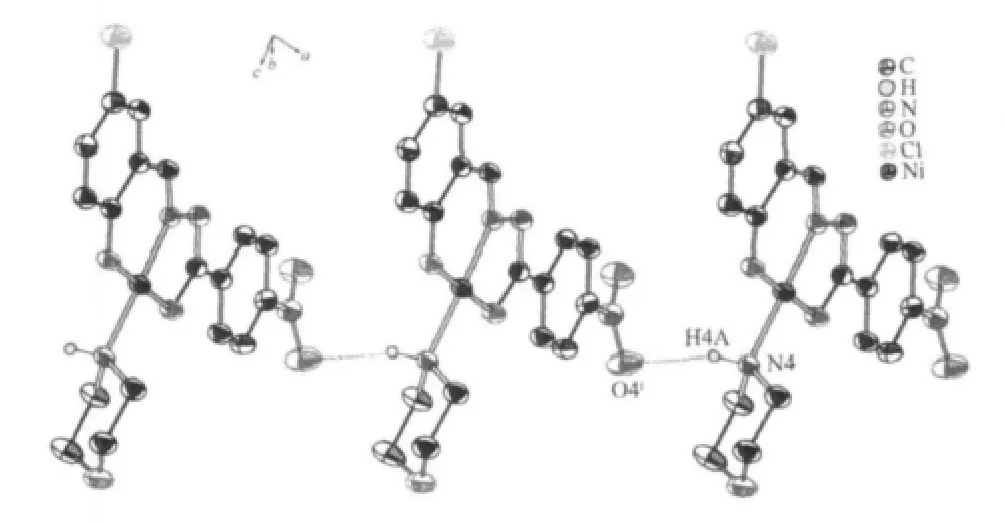
Fig.2 One-dimensional chain structure of complex 1
2.2 Crystal structure of complex 2
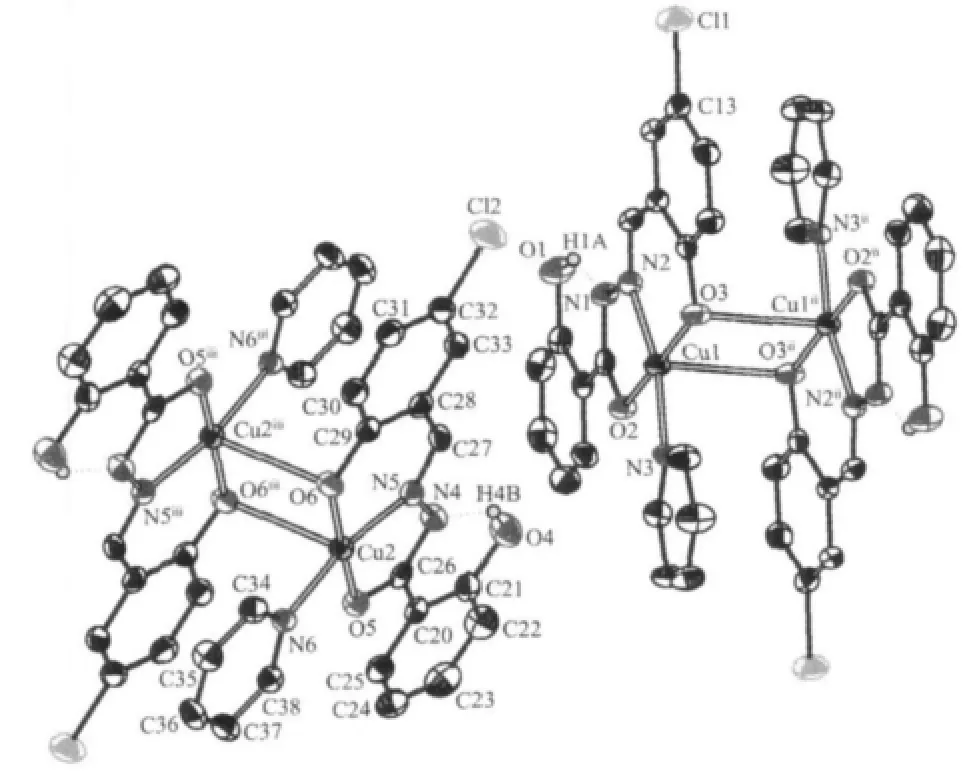
Fig.3 Molecular structure of complex 2 with 30% thermal ellipsoids
The selected bond lengths and angles of complex 2 are given in Table 2.Single crystal X-ray diffraction analysis reveals that in compound 2 each unit cell consists of two molecules[Cu(L2)(Py)]2(named molecule A and molecule B),which are crystallographically independent but chemically similar as shown in Fig.3. Either A or B has a coplanar Cu2(μ-O)2fragment with the Cu1…Cu1ii(symmetry code:ii1-x,2-y,-z)and Cu2…Cu2iii(symmetry code:iii-x,-y,-z)separations of 0.345 2(3)nm and 0.340 7(3)nm,respectively.The environment around Cu1 and Cu2 in molecules A and B,respectively,are a(4+1)distorted square-pyramidal geometry,asevidenced by the structuralindex parameter τ[14]which are 0.06 and 0.07,respectively. The value is consistent with that of 0.012 in the copper(Ⅱ)complex[Cu(HSBzh)(HPz)Cl]·H2O[15]with the same coordination geometry.The basal plane is defined by the phenolic oxygen atom,the imine nitrogen atom and the deprotonated amide oxygen atom from the tridentate dinegative ligand()and pyridine nitrogen atom.The average deviations from the mean planes in A and B are 0.011 93 nm and 0.011 95 nm,respectively with the Cu(Ⅱ)atoms 0.0039(2)nm and 0.0053(2)nm out of the relevant planes.The corresponding r.m.s.deviation from the mean plane is 0.006 6 nm[16].Another phenolate oxygen atom O3iior O6iiiof the neighboring hydrazone ligand completes the five-coordinated square pyramidal geometry in the axial direction,respectively, resulting the Cu1ii-O3,Cu1-O3iibonds of 0.277 5(4)nm and Cu2iii-O6,Cu2-O6iiibonds of 0.277 5(4)nm.The long Cu-O bonds may be explained by Jahn-Teller effect[17].The torsion angle Cu1-O3-Cu1ii-O3iiand Cu2-O6-Cu2iii-O6iiiare 0.00°.
The bond distances,Cu-N and Cu-O,are comparable to those found in the similar compound [Cu2(C16H14O4N2)2(C5H5N)2]·2CH4O[16]. Double-bond character is present in C7-N1,C8-N2,C26-N4 and C27-N5,as judged from their bond lengths(0.1350(6), 0.131 2(6)nm,0.134 9(6)nm,0.130 8(6)nm),which is in agreement with an enolic form of H2L[18-19].The C7-O2 and C26-O5 bond lengths of 0.129 9(6)nm and 0.1316(6)nm are a little longer than the value of 0.1273 nm expected for an enolic form of the hydrazone ligand[19].
Each tridentate hydrazone ligand combines with one Cu(Ⅱ)atom resulting in one planar five-membered and one planar six-membered chelating rings,with mean devition of 0.00084~0.00186 nm and 0.00387~0.00572 nm,respectively.The two phenyl rings in each ligand,C1-C6 and C9-C15,C20-C25 and C28-C33, make dihedral angles of 5.5(3)°and 1.5(3)°,respectively,indicating each ligand in complex 2 is planar with mean devition of 0.00366~0.00774 nm.
In the crystal structure there are intramolecular O1-H1A…N1 and O4-H4B…N4 hydrogen bondings between the uncoordinated phenolate O atom and hydrazine N atom of the hydrazone ligand.The O…N distances and the O-H…N angles are 0.2628(5)nm, 0.2635(6)nm and 145.55°,146.39°,respectively.
2.3 IR spectra
IR spectra of the ligand show stretching bands attributed to PhO-H,C=O and N-H at 3 445~3 427, 1 679~1 671 and 3 329~3 302 cm-1,respectively[17].Bands at 1231~1225cm-1are assigned to the stretching vibration of ν(Ph-O).For the title complexes,the absence of the N-H and C=O stretching vibration bands suggested the deprotonation and enolization of the CONH group and coordination to the metal ions[20].A new band appearing in 1 246 cm-1in the complex was assigned to the ν(C-O)(enolate)mode[21].The deprotonation and coordination can also be confirmed by the bands at 563~686 cm-1(M-O linkages)and 419~626 cm-1(M-N linkages)[17,22],respectively.
2.4 Electronic spectra
The electronic spectra were recorded in ethanol solution for the title complexes(Fig.4).They both have absorptions locating in the range of 300~350 nm corresponding to n-π*transitions[23].The bands between 200 and 300 nm are assigned to π-π*transitions of the phenyl ring[23]ligands.They also display an electronic spectral bands at ca.400 nm,which may be assigned to the ligand-to-metal charge transfer(LMCT)transition[10].
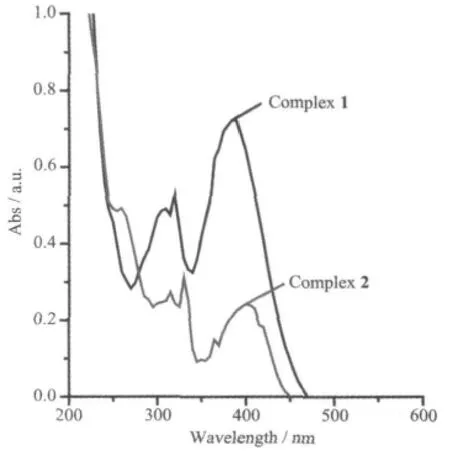
Fig.4 UV spectra of complexes 1 and 2
[1]Dutta S,Manivannan V,Giri Babu L,et al.Acta Cryst.,1995, C51:813-815
[2]Gao S,Weng Z Q,Liu S X.Polyhedron,1998,17:3595-3606
[3]Liu S X,Gao S.Polyhedron,1998,17:81-84
[4]Yin H.Acta Cryst.,2008,C64:m324-m326
[5]Chakraborty J,Thakurta S,Pilet G,et al.Polyhedron,2009,28: 819-825
[6]HU Zong-Qiu(胡宗球),DING Yu(丁瑜),JIA Bing(加兵), et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006, 22:925-929
[7]Chan S C,Koh L L,Leung P H,et al.Inorg.Chim.Acta,1995, 236:101-108
[8]Iskander M F,Khalil T E,Werner R,et al.Polyhedron,2000, 19:1181-1191
[9]Lian Z X,Liu P,Zhang J M,et al.Chinese J.Struct.Chem., 2008,27:639-644
[10]CHEN Xiao-Hua(陳小華),WU Qiong-Jie(吳瓊潔),CHEN Shun-Yu(陳順玉),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26:1573-1576
[11]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Solution,University of G?ttingen,Germany,1997.
[12]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Refinement,University of G?ttingen,Germany, 1997.
[13]Chen X H,Wu Q J,Liang Z Y,et al.Acta Cryst.,2009,C65: m190-m194
[14]Addison A W,Rao T N,Reedijk J,et al.J.Chem.Soc., Dalton Trans.,1984:1349-1356
[15]Iskander M F,Khalil T E,Haase W,et al.Polyhedron,2001, 20:2787-2798
[16]HuoLH,LuZZ,GaoS.ActaCryst.,2004,E60:m1636-m1638 [17]Li D C,Wang S N,Xu H,et al.Inorg.Chim.Acta,2011,365: 85-95
[18]DENG Zhao-Peng(鄧兆鵬),GAO Shan(高山),ZHAO Hui(趙輝),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2007,23:173-176
[19]CHEN Xiao-Hua(陳小華),LIU Shi-Xiong(劉世雄).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2004,23:33-37
[20]Ghosh T,Mondal B,Ghosh T,et al.Inorg.Chim.Acta,2007, 360:1753-1761
[21]Seena E B,Mathew N,Kuriakose M,et al.Polyhedron,2008, 27:1455-1462
[22]Bekheit M M,Ibrahim K M.Synth.React.Inorg.Met.-Org. Chem.,1986,16:1135-1147
[23]Hu X,Li Y P,Wang Y J,et al.J.Chem.Crystallogr.,2010,40: 846-851
含5-氯水楊醛Schiff堿配體的鎳和銅配合物的合成及晶體結構
吳瓊潔*,1陳小華2蔡碧瓊1謝勇平1
(1福建農林大學生命科學學院,福州 350002)
(2福建師范大學化學與材料學院,福州 350007)
合成了2個含三齒Schiff堿配體和單齒N-雜環分子的多核過渡金屬配合物:1個含5-氯水楊醛縮對硝基苯甲酰腙(H2L1)和嗎啡啉(Mf)的鎳(Ⅱ)配合物[Ni(L1)(Mf)](1),1個含5-氯水楊醛縮水楊酰腙(H2L2)和吡啶(Py)的銅(Ⅱ)配合物[Cu2(L2)2(Py)2](2),并通過元素分析、紅外光譜、紫外光譜以及單晶衍射等手段進行表征。在配合物1中,中心Ni(Ⅱ)與酰腙配體(L12-)的酚氧、亞胺氮、去質子酰胺氧原子以及中性嗎啡啉氮原子配位形成平面四方形的N2O2配位構型,相鄰配合物通過分子間氫鍵作用構筑成一維超分子鏈狀結構。配合物2中含有2個晶體學上獨立的雙核銅(Ⅱ)配合物,相鄰配合物分子的酚氧原子分別橋聯2個[Cu(L2)(Py)]基本單元,形成2個含有Cu2(μ-O)2核心的配合物。每個Cu(Ⅱ)原子具有五配位的NONO(O)四角錐配位構型。
Schiff堿;鎳(Ⅱ)配合物;銅(Ⅱ)配合物;晶體結構
O614.81+3;O614.121
A
1001-4861(2012)01-0201-06
2011-04-29。收修改稿日期:2011-08-29。
福建農林大學校青年教師基金(No.08B10)資助項目。*
。E-mail:wqiongj_jane@163.com
- 無機化學學報的其它文章
- 碳酸鹽共沉淀法制備Li[Li0.2Co0.13Ni0.13Mn0.54]O2中加料方式對產物性能的影響
- Sonochemical Synthesis of Different Morphological CaF2Microstructures
- Synthesis,Crystal Structure and Properties of a Manganese(Ⅱ)Complex with an Asymmetrical Substituted Triaryltriazole
- S2-控制劑對Ag納米產物的形貌及光學性能的影響
- Hydrothermal Synthesis,Crystal Structure and Catalytic Properties of a Polyoxovanadate Organicamine
- Tumor-Imaging Core-Shell Nano-Models for Catalase

