二維鋇配位聚合物[Ba(μ5-1,8-nap)]n的水熱合成、晶體結構和熒光性質
傅君丹 葉莉青 張春艷 溫一航
(浙江師范大學物理化學研究所,浙江省固體表面反應化學重點實驗室,金華 321004)
二維鋇配位聚合物[Ba(μ5-1,8-nap)]n的水熱合成、晶體結構和熒光性質
傅君丹 葉莉青 張春艷 溫一航*
(浙江師范大學物理化學研究所,浙江省固體表面反應化學重點實驗室,金華 321004)
通過水熱法合成得到1個以1,8-萘二酸(1,8-nap)為配體的Ba(Ⅱ)配合物[Ba(μ5-1,8-nap)]n(1)。X射線單晶衍射測定結果表明:配合物屬于單斜晶系,空間群P21/c。配合物最小不對稱單元由1個九配位的鋇原子和1個1,8-萘二酸配體構成。每個1,8-萘二酸配體連接五個鋇原子,每個鋇原子連接五個1,8-萘二酸配體,在(100)平面上形成二維雙層結構。此外,對配合物的固態熒光性質做了測定,結果顯示其在紫光區有熒光發射。
鋇(Ⅱ)配合物;1,8-萘二甲酸;晶體結構;水熱合成;熒光
0 Introduction
The ration design and synthesis of new metalorganic frameworks have attracted wide interest,not only because of their intriguing variety of architecture features and fascinating new topologies,but also because of their potential applications as functional solid materials in host-guest chemistry,ion exchange,fluore-scence,magnetism,and catalysis[1-5].To build these molecular architectures,researchers often employ aromatic polycarboxylate ligand to construct coordination polymers as their versatile coordination modes and high structural stability[6-7].At the same time,plenty of versatile and novel complexes of aromatic polycarboxylate groups containing naphthalene rings with various coordination modes have been synthesized and charac-terized[8-11].Among them,naphthalene-1,8-dicarboxylic anhydride,hydrolyzed under hydrothermal condition into the naphthalene-1,8-dicarboxylate ligand(1,8-nap),caused our attention.It has two carboxyl groups that may be completely or only partly deprotonated and thus results in multiple coordination sites,which may construct structures of higher dimensions.In our previous work,the features of naphthalene-1,8-dicarboxylate have been demonstrated in several complexes which have been reported[12-14].Herein,we wish to report the hydrothermal synthesis and crystal structure of the first barium complex with two-dimensional(2D)bilayer structure,[Ba(μ5-1,8-nap)]n(1).
1 Experimental
1.1 Materials and measurements
All starting materials and solvents for syntheses were obtained commercially and used without further purification.IR spectra were recorded on an FTIR-8700 spectrometer with KBr pellets in the range of 4 000~400 cm-1.Elemental analysis was performed on a PE-2400(Ⅱ)element analysis instrument.The crystal data collections were carried out on a Bruker SMART APEX-Ⅱ CCD diffractometer.Luminescence spectrum was performed on a HITACHI F-2500 Fluorescence Spectrometer in solid state at room temperature.
1.2 Synthesis of complex 1
A mixture of naphthalene-1,8-dicarboxylate anhydride(0.099 1 g,0.5 mmol),BaCl2·2H2O(0.122 1 g,0.5 mmol),Na2CO3(0.053 g,0.5 mmol)and waterethanol(18mL,V/V=1∶1)wassealedina25mLstainless-steel reactor with a Telflon liner and was heated at 433 K for 3 d.On completion of the reaction,the reactor was cooled slowly to room temperature and the mixture was filtered,giving pink single crystals suitable for X-ray analysis in yield 25%.Elemental Anal.Found(%):C,42.21;H,1.14.Calcd.For C12H6BaO4(%):C,41.16;H,1.19.IR(KBr,cm-1):3051(w),1619(m),1557(s),1437(s),1388(m),842(m),784(s).
1.3 X-ray crystallography
Crystal of 1 (0.31 mm×0.18 mm×0.06 mm)was mounted on glass fiber using epoxy resin.Data collection was performed on a Bruker SMART APEX-Ⅱ CCD diffractometer(Mo Kα radiation,λ=0.071073 nm).The data of 1 was collected up to 2θ maximum of 55.12°using the φ-ω scan technique.Data intensity was corrected by Lorentz-polarization factors and empirical absorption.The structure was solved by direct methodsand expanded with Fouriertechniques.Anisotropic displacement parameters were applied to all non-hydrogen atoms in full-matrix least-squares refinements based on F2.The hydrogen atoms were assigned with isotropic displacementfactorsand included in the final refinement cycles by the use of geometrical restrains.All calculations were performed with SHELX-97 package[15].All pertinent crystallographic data for 1 is summarized in Table 1.The select bond distances and bond angles are listed in Table 2.
CCDC:778610.
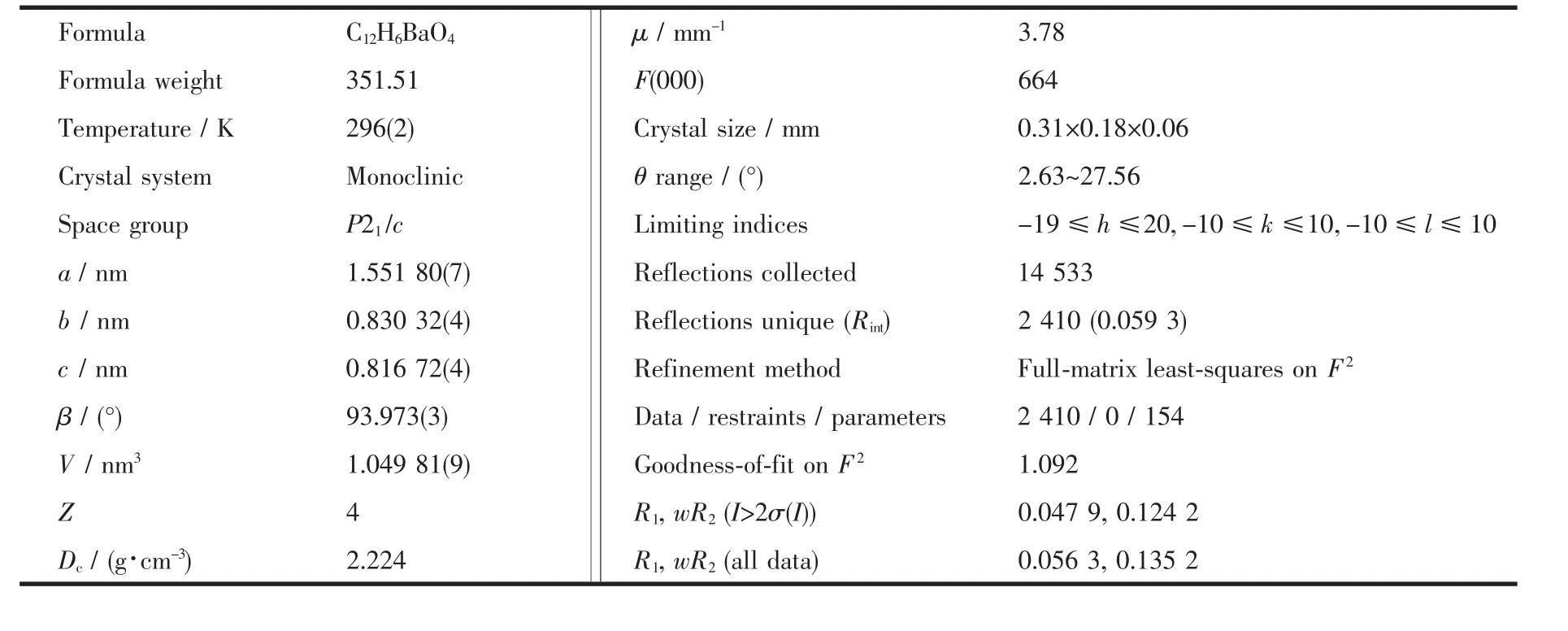
Table 1 Crystallographic data for complex 1
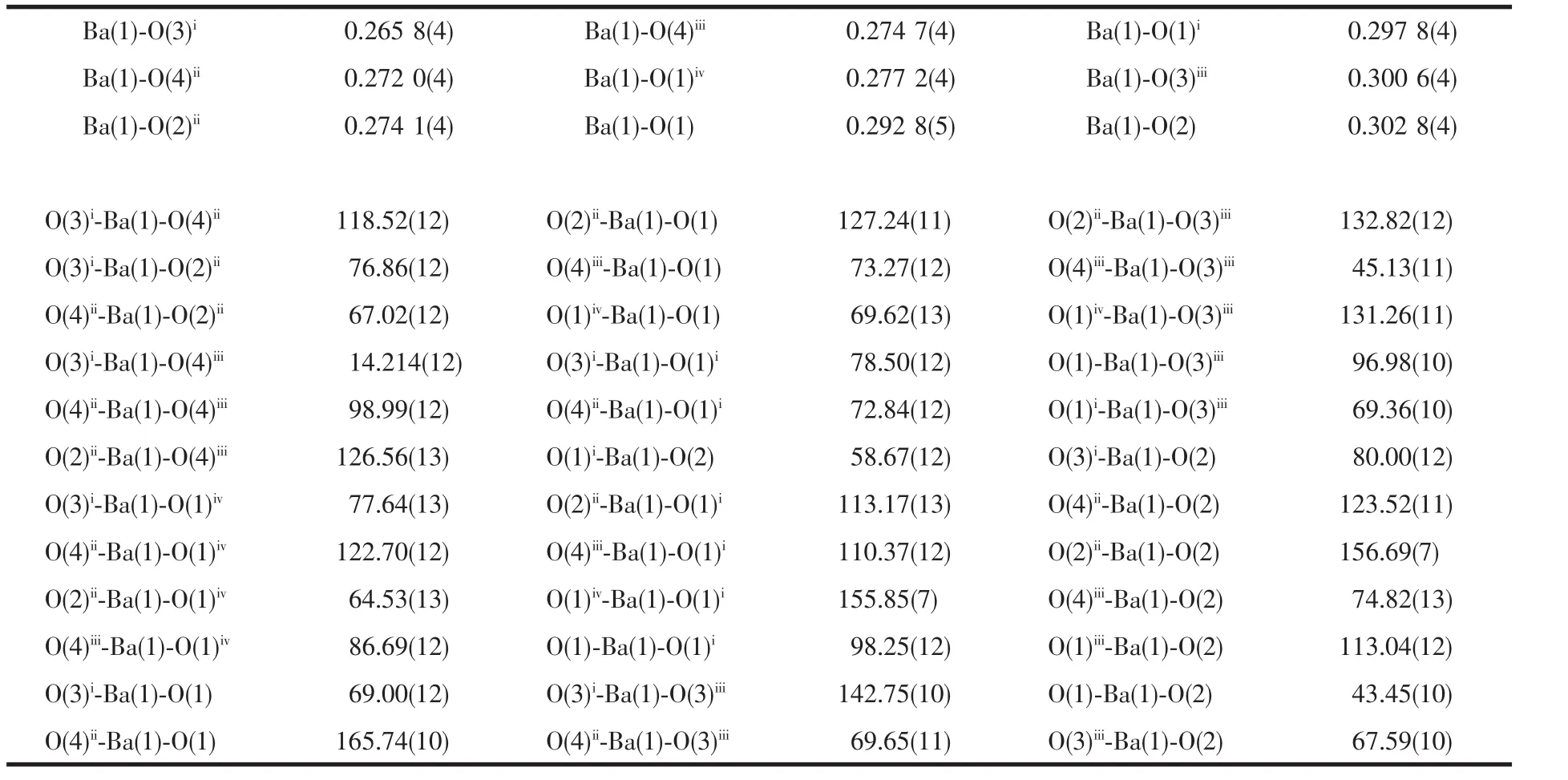
Table 2 Data of selected bond distances(nm)and bond angles(°)of the complex
2 Results and discussion
2.1 IR spectrum
The weak peaks 3 051 cm-1of complex 1 are assigned to the C-H stretching vibrations.The conspicuous carboxylate stretching at 1 619,1 557,1 437,1 388 cm-1present two groups of the antisymmetric Ⅴas(COO-)and symmetric stretching frequency Ⅴs(COO-).The strong band appeared in the 778 cm-1in 1 is ascribed to the asymmetric stretching vibration of the aromatic ring.These data clearly show the formation of the complex 1.
2.2 Crystal structure of complex 1
The X-ray diffraction study shows that complex 1 crystallizes in a centrosymmetric space group P21/c,and the asymmetric unit is composed of one Ba atom,one naphthalene-1,8-dicarboxylate ligand.
As shown in Fig.1,the center Ba(Ⅱ)ion is 9-coordinated to seven carboxylate groups from five 1,8-nap ligands,the Ba-O distances range from 0.265 8(4)to 0.302 8(4)nm are similar to the reported[16].As to 1,8-nap ligand,the carboxylic groups in complex 1 are all deprotonated,in agreement with the IR spectrum,where no a absorption peak around 1730 cm-1for protonated carboxylate group is observed.Two carboxylate groups of 1,8-nap represent different coordination modes:bis-bidentate and quinquidentate (Scheme 1).As a result,the whole 1,8-nap ligand acts as μ5-bridgelinking five Ba(Ⅱ)atoms.
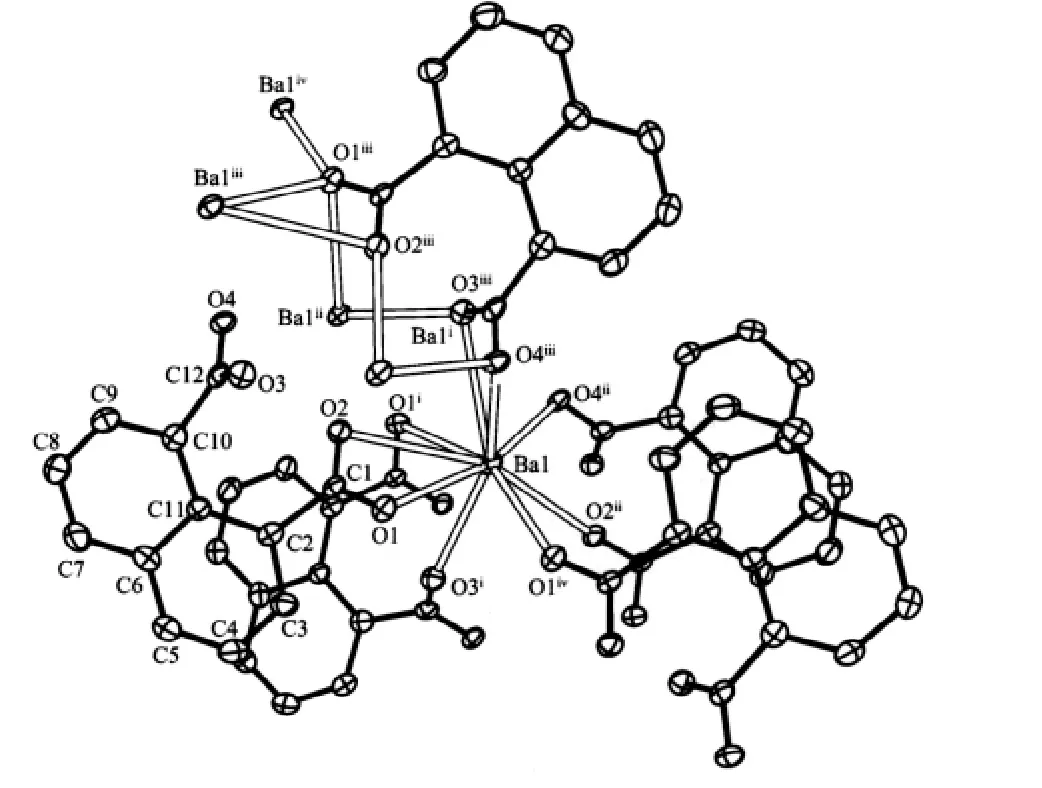
Fig.1 Coordination environment of the barium atom in the complex 1 showing 30%probability ellipsoids and the atom-labeling scheme
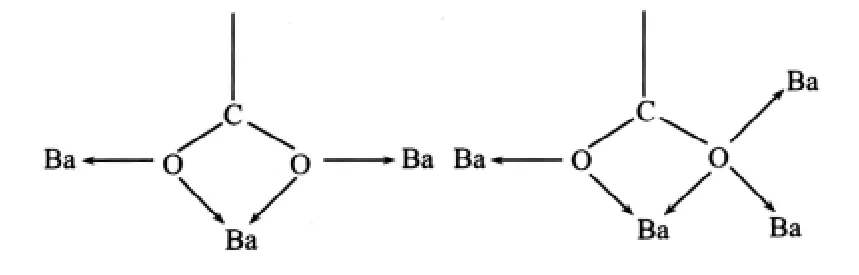
Scheme 1 Coordination modes of the carboxylate groups in complex 1
Each 1,8-nap ligand links five Ba(Ⅱ)atoms and each Ba(Ⅱ)atom attaches to five ligands to form 1D double chainsalong c axis (Fig.2a),both consisting of 4-metal grids with the sizes of 0.453 40(4)nm×0.431 09(4)nm(Ba1-Ba1i×Ba1-Ba1ii).Meanwhile,naphthalene rings exist on sides of double chain,the centroids distance between adjacent naphthalene rings are 0.433 79(2),0.404 68(2)nm,respectively.Furthermore,the 4-metal grids are extended by the carboxylate group of 1,8-nap ligands along b axis,form double chains (Fig.2b).The dihedral angle between the two adjacent naphthalene rings of the two adjacent chains is 0.000(178)°and the distance between their centroids is 0.830 32(4)nm,which means that the two planes are parallel but no π-π stacking interactions exists between them.As a result,such double chains are further extended by the carboxylic groups of 1,8-nap ligands running in the direction of the b axis and c axis to form a 2D bilayer framework along the(100)plane(Fig.3).
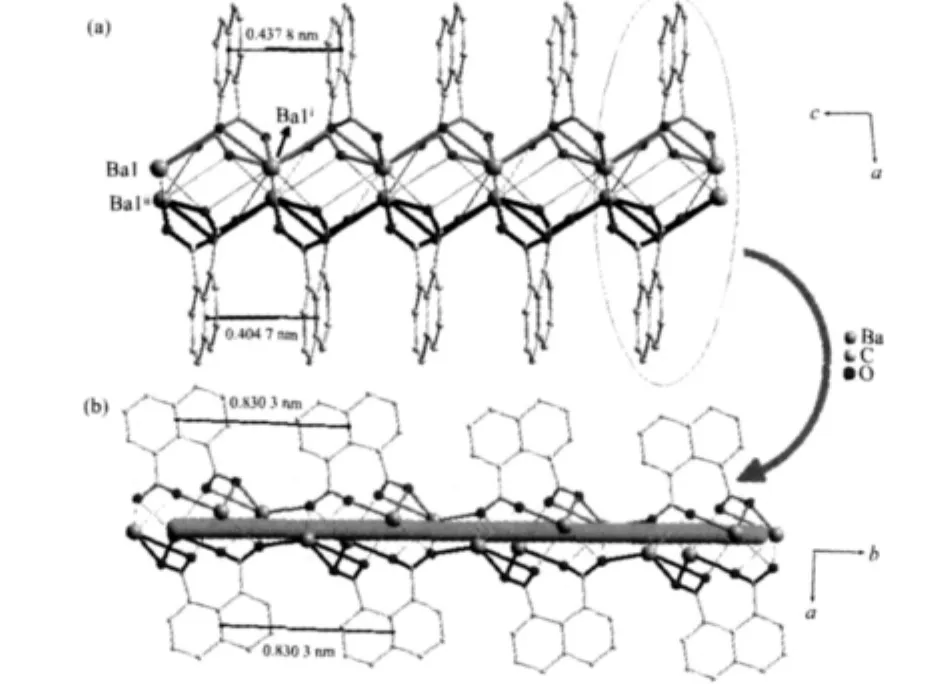
Fig.2 (a)A view of the double chains of complex 1;(b)4 grids along b axis
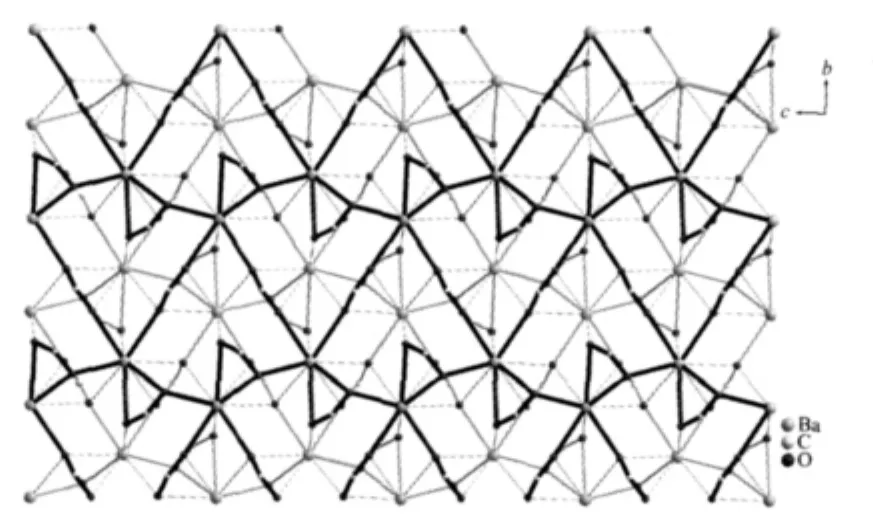
Fig.3 A view of 2D bilayer framework of complex 1
2.3 Luminescence property
The solid state luminescence spectrum of complex 1 reveals a emission maximum at approximately 409 nm in the voilet region,while a fluorescent emission band is observed at 449 nm for the naphthalene-1,8-dicarboxylate anhydride,upon excited at 339 nm (Fig.4).It is clear that the blue-shift of emission occurs in complex 1,which is probably because of the coordination of the 1,8-nap ligand breaks the coplanar effect of the naphthalene-1,8-dicarboxylic anhydride molecule.Photoluminescence behavior is closely associated with the local environment around metal ions[13,17].
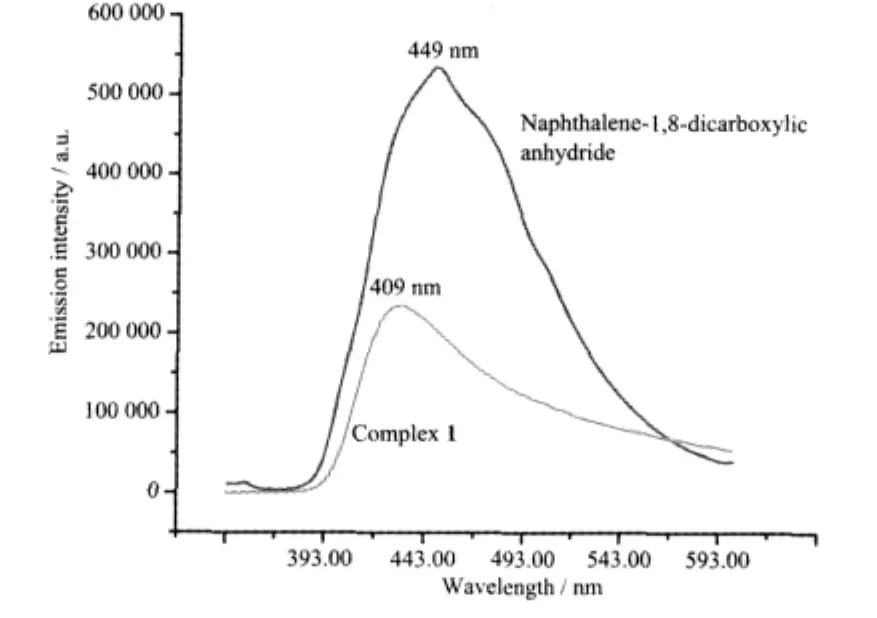
Fig.4 Fluorescence emission spectrum of complex 1 in the solid state at room temperature
[1]Chandler B D,Cramb D T,Shimizu G K H.J.Am.Chem.Soc.,2006,128:10403-10412
[2]Chen X M,Tong M L.Acc.Chem.Res.,2007,40(20):162-170
[3]Kupplera R J,Timmonsb D J,Fang Q R,et al.Coord.Chem.Rev.,2009,253:3042-3066
[4]Gao X M,Li D S,Wang J J,et al.J.CrystEngComm,2008,10:479-482
[5]Du M,Jiang X J,Zhao X J.Chem.Commun.,2005,44:5521-5523
[6]Li D S,Wu Y P,Zhang P,et al.Cryst.Growth Des.,2010,10:2037-2040
[7]Ma L F,Wang Y Y.Cryst.Growth Des.,2009,9(5):2036-2038
[8]Moon H R,Kobayashi N,Suh M P.Inorg.Chem.,2006,45:8972-
[9]Chen L F,Zhang J,Song L J,et al.Inorg.Chem.Comm.,2005,8(6):555-558
[10]Zou R Q,Liu C S,Shi X S,et al.CrystEngComm,2005,118:722-727
[11]Maji T k,Kaneko W,Ohba M,et al.Chem.Commun.,2005,36:4613-4615
[12]Wen Y H,Feng X,Feng Y L,et al.Inorg.Chem.Commun.,2008,11:659-661
[13]FENG Xia(封霞),TANG Zhi-Wei(唐治煒),FENG Yun-Long(馮云龍),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24:1713-1717
[14]Wen Y H,Feng X,He Y H,et al.Acta Cryst.Sect.C,2007,C63:m504-m506
[15]Sheldrick G M.SHELXS-97 and SHELXL-97,Program for the Solution and the Refinement of Crystal Structure,University of G?ttingen,Germany,1997.
[16]Liu W L,Zou Y,Lu C S,et al.Inorg.Chem.Comm.,2004,7:434-436
[17]Fu Z Y,Wu X T,Dai J C,et al.Eur.J.Inorg.Chem.,2002,41:2730-2735
Hydrothermal Synthesis,Crystal Structure and Luminescence Property of a 2D Ba(Ⅱ) Coordination Polymer[Ba(μ5-1,8-nap)]n
FU Jun-Dan YE Li-Qing ZHANG Chun-Yan WEN Yi-Hang*
(Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces,Institute of Physical Chemistry,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
The title complex,[Ba(μ5-1,8-nap)]n(1),was the first barium complex based on naphthalene-1,8-dicarboxylate(1,8-nap)ligand,has been synthesized by hydrothermal method.It belongs to monoclinic system with space group P21/c,a=1.551 80(7)nm,b=0.830 32(4)nm,c=0.816 72(4)nm,β=93.973(3)°,V=1.049 81(9)nm3,Dc=2.224 g·cm-3,Z=4,F(000)=664,the final R1=0.0479,wR2=0.1242.The crystal structure of complex 1 consists of a 9-coordinated Ba(Ⅱ) atom and a 1,8-nap ligand.Each 1,8-nap ligand links five Ba(Ⅱ) atoms and each Ba(Ⅱ)atom attaches to five 1,8-nap ligands to form a 2D bilayer framework along the(100)plane.The luminescence of complex 1 has also been investigated,and results reveals that it displays luminescent property in the voilet region.CCDC:778610.
Ba(Ⅱ)complex;naphthalene-1,8-dicarboxylate;hydrothermally synthesis;luminescence property
O614.23+2
A
1001-4861(2011)01-0179-05
2010-06-21。收修改稿日期:2010-08-11。
*通訊聯系人。E-mail:wyh@zjnu.edu.cn;會員登記號:S060017686M。

