Optimization of Synthesis Process for Oleoyl-alginate Ester by Response Surface Methodology
LI Qian,LIU Chen-guang
(College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China)
李 倩,劉晨光*
(中國海洋大學海洋生命學院,山東 青島 266003)
Optimization of Synthesis Process for Oleoyl-alginate Ester by Response Surface Methodology
LI Qian,LIU Chen-guang*
(College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China)
Abstract:Box-Behnken experimental design and response surface methodology (RSM) were combinedly employed to evaluate the effects of the amounts of formic acid and oleoyl chloride as well as reaction temperature and time on the synthesis of oleoylalginate ester (OAE). The optimum synthesis conditions of OAE were found to be alginate amount of 0.5 g, formic acid amount of 4.95 mL, oleoyl chloride amount of 11.27 mL, reaction temperature of 50 ℃ and reaction time of 20 min. Under these conditions, the maximum degree of substitution (DS) and yield of OAE were observed to be respectively 4.93% and 92.27%, which were in accordance with the corresponding predicted values.
Key words:oleoyl-alginate ester (OAE);response surface methodology (RSM);synthesis process
李 倩,劉晨光*
(中國海洋大學海洋生命學院,山東 青島 266003)
Alginate, a natural linear polysaccharide, is widely used in food industry owing to its good properties such as biocompatibility, bioadhesiveness, and non-toxicity, et al[1]. Amphiphilic derivatives of alginate formed strong hydrogels that serve as a carrier for drugs and nutraceuticals[2-4]. In order to increase the hydrophobic properity, alginate has been modified by various hydrophobic groups such as noctylamine groups, alkyl chains (C12and C18), and cholesteryl by different procedures. However, these amphiphilic derivatives of alginate were synthesized in the presence of organic solvent, such as chloroform, dichloromethane, and dimethyl sulfoxide[4]. The organic solvents may irritate and damage the skin, eyes and respiratory passages[5]. Moreover, organic solvents cause fetal harm because it can readily cross the placenta and reach the fetal nervous system[6].
In our previous study acid (oleoyl chloride) has been successfully grafted to alginate without any organic solvents. The nanoparticles formed by oleoyl-alginate ester (OAE) has good size, morphic and thermodynamic stability and can be used as carriers for liposoluble nutraceuticals with controlled release in gastrointestinal fluid[4]. However, the OAE were synthesized with no attempt to optimize the synthesis protocols. The classical method of studying one variable at a time may be effective in some situations, but fails to consider the combined effects of all the factors involved[7]. Response surface methodology (RSM), a collection of statisti-cal and mathematical methods, is useful for developing, improving, and optimizing the processes[8]. The advantage of RSM is that it can reduce the number of experimental trials, evaluate the interactions between multiple parameters. Moreover, it is more effective and precise than many approaches[9]. RSM has already been successfully applied optimizing chemical reactions[10]. RSM could successfully optimize the chemical reaction conditions of various synthesis processes.
The objective of this study was to optimize the synthesis of OAE by RSM. The reaction variables investigated included the amount of formic acid, the amount of oleoyl chloride, reaction temperature and reaction time.
1 Materials and Methods
1.1Materials, reagents and instruments
Sodium alginate, formic acid, oleoyl chloride, and ethanol were purchased from Sigma chemicals (St. Louis, MO, USA). Low molecular weight alginate was obtained by the method of acid hydrolyze and its relative molecular mass was 1.72×104.
Design-Expert version 7.1 software (Stat-Ease Inc., USA), homothermal magnetic stirrer (Guosheng Co., China), and nuclear magnetic resonance (NMR) spectrometer (JEOL Co., Japan) were used in this study.
1.2Methods
1.2.1Preparation of oleoyl alginate ester (OAE)
OA was prepared by reacting low molecular alginate with oleoyl chloride derived from a previous work[4]. The reaction was carried out in a three-necked flask, which was placed in homothermal magnetic stirrer. The formylation reaction was carried out by impregnating 0.5 g alginate with formic acid (5-20 mL) for 10 min at 25 ℃ under stirring. Oleoyl chloride (8-45 mL) was then added dropwise. The reaction mixture was then heated, and the temperature (30-70 ℃) was maintained for times ranging from 10 to 40 min. At the end of the reaction, 100 mL of 95% ethanolwas added to terminate the reaction, and the mixture was filtered to obtain solid phase. The solid phase was washed with 95% ethanol several times and dried under vacuum at room temperature.
1.2.2Determination of the degree of substitution (DS)
The degree of substitution (DS), which represents the amount of oleoyl chains per 100 hexurunic acid residues, was calculated by comparing the ratio of methylene protons correlated with carbon 8 and 11 of oleoyl graft (δ = 2.0) to carbon 3-5 of alginate protons (δ = 3.3-3.7) in1H NMR spectra using equation 1[11]:

AOand AAcorrespond to the area of the methylene protons on oleoyl grafts and the carbon 3-5 of alginate protons on alginate main chains.
1.2.3Calculation of the percent yield
The percent yield of OAE was calculated as follows[12]:
Percent yield of OA/%= the solid obtained/initial alginate ×100
1.2.4Single factor analysis
Single-factor-test was employed to determine the preliminary range of the reaction variables including the amount of formic acid, the amount of oleoyl chloride, reaction temperature and reaction time.
1.2.5The Response Surface Method
A three-level four-factor RSM along with Box-Behnken design was used in this study to optim ize the reaction conditions. The DS and reaction y ields were treated as responses. Design-Expert version 7.1 software was used to generate the experimental designs, statistical analysis and regression model.
2 Results and Discussions
2.1Single factor analysis
The effects of the amount of formic acid or oleoyl chloride to alginate, reaction temperature and reaction time on the DS and yield of OAE are demonstrated in figure 1a, 1b, 1c and 1d, respectively. Besides the calculation of DS, the new peaks and increased peaks in1H NMR spectra also confirmed the presence of oleoyl graft linked to alginate in the former article[4]. The former includedδ2.3= CH2(carbon 2 of oleoyl graft), δ2.0= CH2(carbon 8 and 11 of oleoyl graft) and δ1.7= CH2(carbon 3 of oleoyl graft), and the latter includedδ1.2= CH2(carbon 4-7 and 12-17 of oleoyl graft) and δ0.8= CH3(carbon 18 of oleoyl graft).

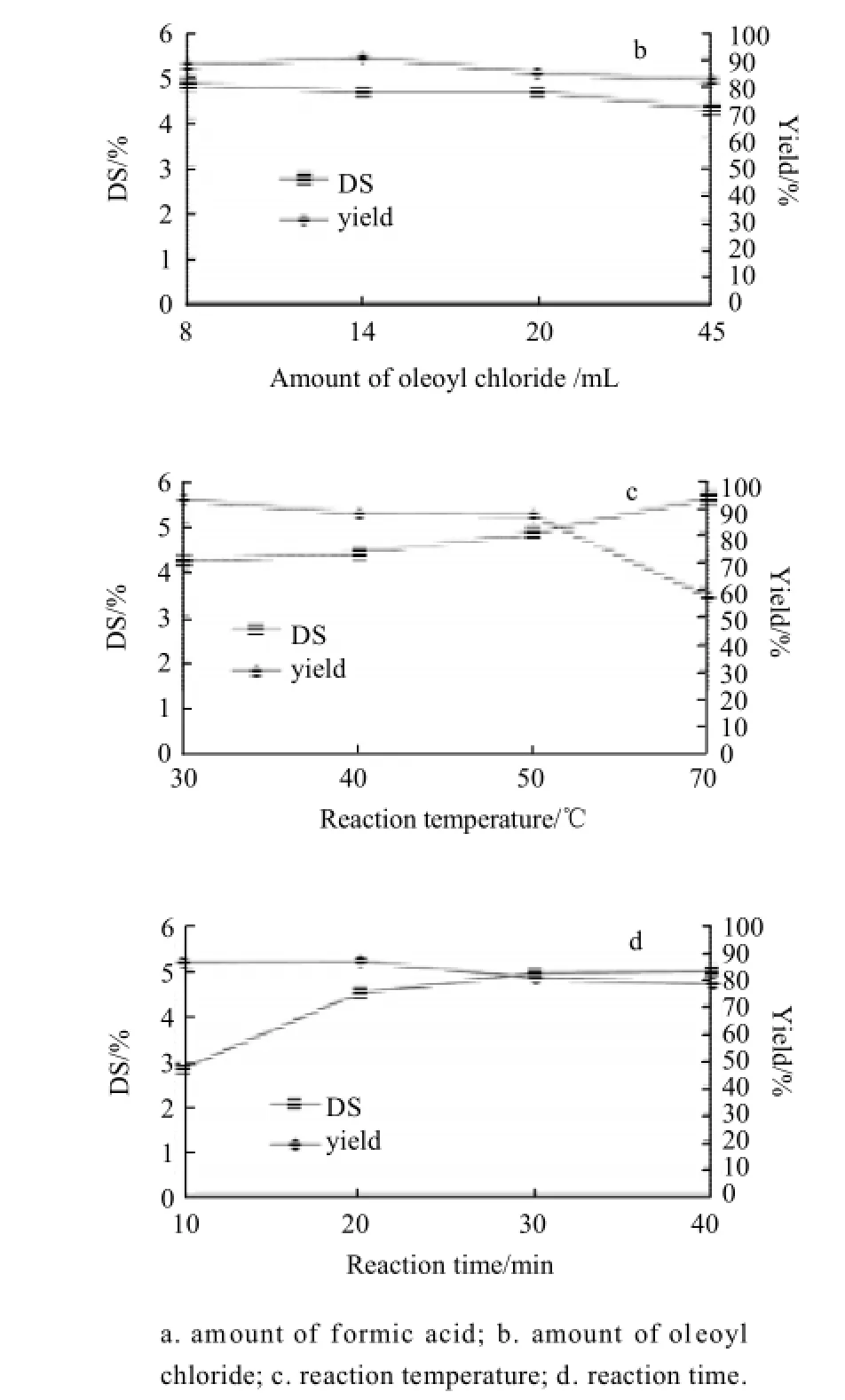
Fig.1 Effect of the variables on the DS and yield of OAE
2.1.1Effect of the amount of formic acid
As shown in Fig.1a, the effect of the amount of formic acid (2.5 to 10 mL) was investigated, while other variables were set as follows: 8 mL oleoyl chloride, reaction temperature 50 ℃, and reaction time 20 min. The DS of OAE increased with the amount of formic acid until 5 mL and began to decrease sharply. The maximum yield was (31.89 ± 91)% at 3.5 and decreased rapidly beyond 5 mL. So, we adopted 2.5-5 mL formic acid.
2.1.2Effect of the amount of oleoyl chloride
The amount of oleoyl chloride had small effect of the DS and yield of OAE, while other variables were set as follows: 5 mL formic acid, reaction temperature 50 ℃, and reaction time 20 min (Fig.1b). In consideration of the price of oleoyl chloride, the minimum and maximum amount was chosen as 8 mL and 20 mL, respectively.
2.1.3Effect of the reaction temperature
The effect of the temperature was shown in Fig.1c, when other experimental conditions were as follows: 5 mL formic acid, 8 mL oleoyl chloride, and reaction time 20 min. As the amount of reaction temperature increased, there was an increase in the DS of OAE. But the yield of OAE decreased, especially beyond the temperature of 50 ℃. Therefore, 30 ℃and 50 ℃ were chosen as the reaction time range.
2.1.4Effect of the reaction time
Fig.1d shows the effect of the time on the DS and yield of OAE while other experimental conditions were as follows: 5 mL formic acid, 8 mL oleoyl chloride, and reaction temperature 50 ℃. The yield of OAE decreased slowly from 86.3% to 78.34% as the reaction temperature increased. But the DS of OAE was extremely low at reaction time of 10 min, and the maximum DS of 4.99 was observed at reaction time of 40 min. Therefore, we adopted reaction time 20-40 min.
2.2Predicted model and statistical analysis

Table1 Box-Behnken design matrix and the responses of the DS and yield

Table2 Analysis of variance (ANOVA) for the quadratic model of DS
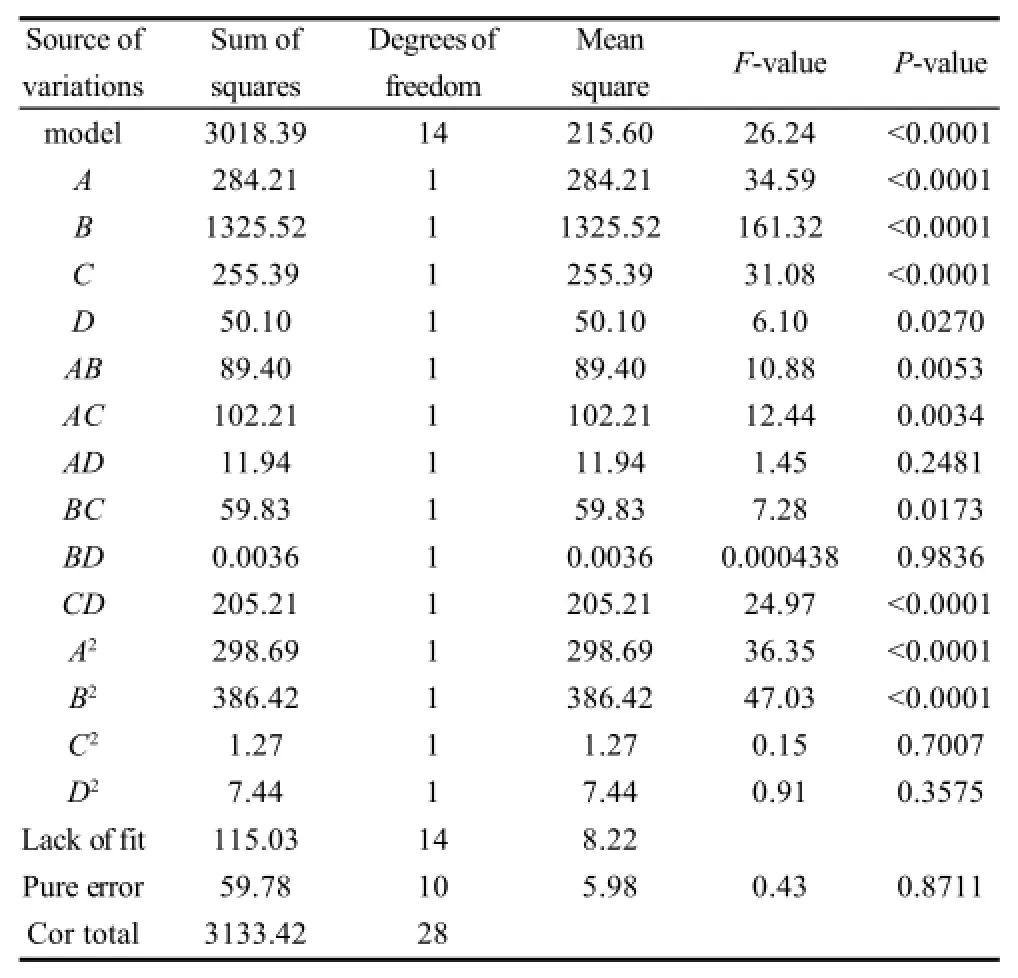
Table3 Analysis of variance (ANOVA) for the quadratic model of yield
The design matrix and the corresponding results of RSM experiments to determine the effects of the four independent variables including amount of formic acid (A), amount of oleoyl chloride (B), reaction time (C) and reaction temperature (D) were shown in Table 1. The application of RSM yielded the following second-order polynomial function:
DS=9.76856-2.14400A+0.77435B-0.18850C-0.22483D-0.093667AB+0.015000AC+0.053600AD-0.001875BC-0.005625BD+0.00370CD+0.12373A2-0.00327546B2-0.00121667C2+0.000145833D2
Yield=-89.98787+26.45800A+3.80148B+4.55758C+2.68450D+0.63033AB-0.4044AC+0.13820AD -0.064458BC+0.0005BD-0.071625CD-4.34293A2-0.21440B2+0.00441667C2-0.010708D2
The analysis of variance (ANOVA) for the quadratic model of DS and yield are shown in Table 2 and 3, respectively. The significance of each coefficient was determined by F-test and P-values. The larger the magnitude of the F-value and smaller the P-value, the more significant is the corresponding coefficient[13-14]. The F-value with a very low P-value of DS and yield demonstrate a high significance for the regression models. For the DS, B, C, D, AB, AD, BD, CD and A2are significant model terms. For the yield, A, B, C, D, AC, BC, CD, A2, B2are significant model terms.
2.3Process analysis

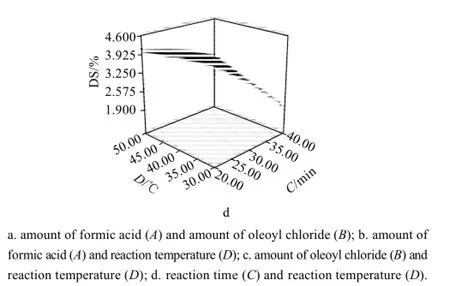
Fig.2 Response surface plots showing the effect of combined variables on the DS
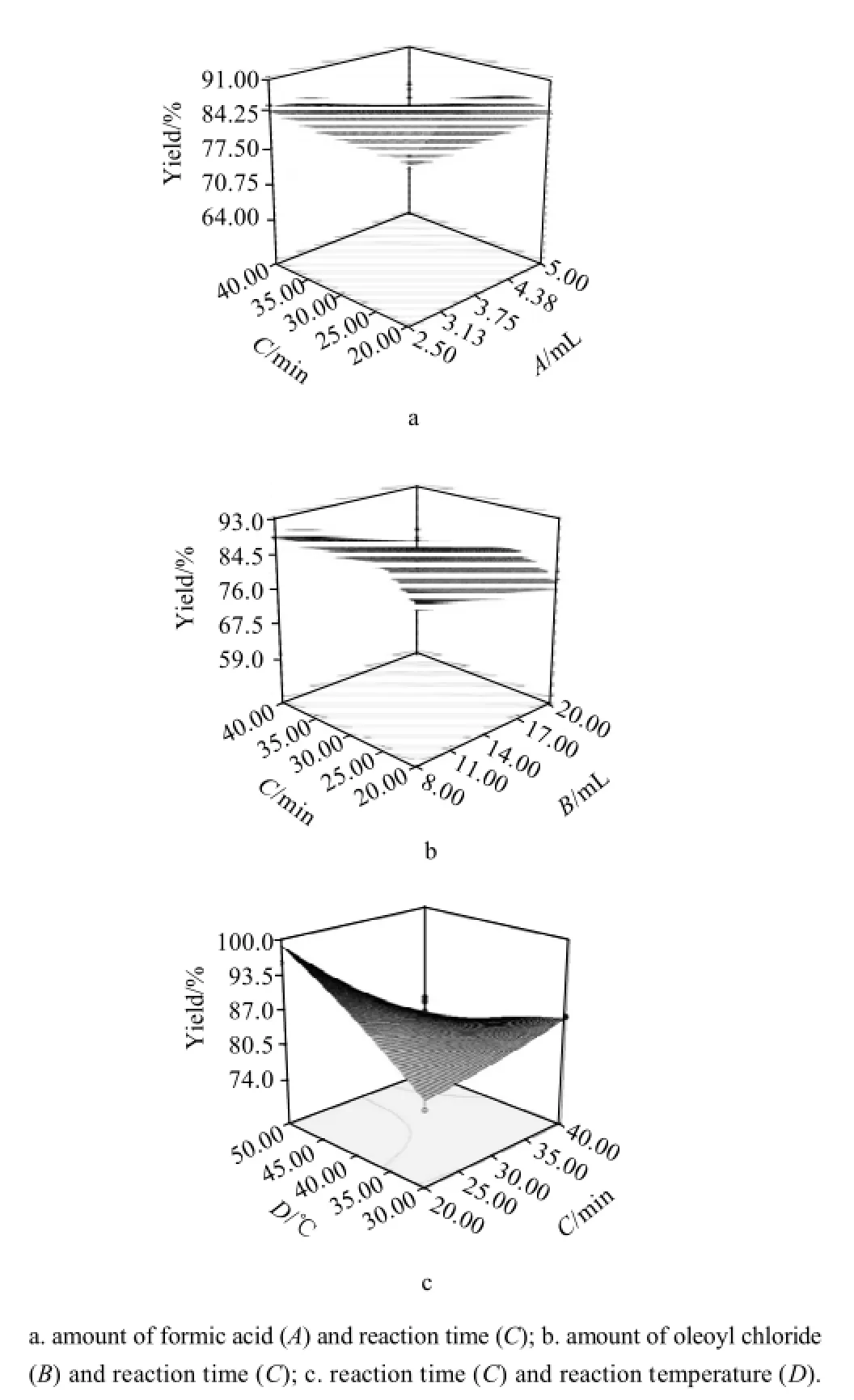
Fig.3 Response surface plots showing the effect of combined variables on the yield
The response surface plot of significant model terms[15]for DS is shown in Fig.2(a, b, c and d), when other variables are fixed at 0 level. In Fig.2a, at the lowest the amount of formic acid, an increase in the amount of oleoyl chloride enhances the DS of OAE. At highest the amount of oleoyl chloride, a decrease in the amount of oleoyl chloride led to an increase in the DS. The highest DS is obtained at the highest the amount of formic acid and the highest reaction temperature in Fig.2b. The DS increases with the amount of oleoyl chloride reaction time and reaction temperature in Fig. 2c and 2d.
The response surface plots of significant model terms for yield are shown in Fig.3(a, b and c), which indicates maximal yield is obtained at about middle the amount of formic acid, low the amount of oleoyl chloride, long reaction time and high reaction temperature. Compared to other independent variables, time has less effect on the yield.
2.4Process optimization
When DS weights 4 and yield weights 2, maximum DS and yield were 5% and 93.99%, respectively, for the following condition: 4.95 mL formic acid, 11.27 mL oleoyl chloride, reaction time 20 min and reaction temperature 50 ℃. In the optimal conditions, the experimental values of DS and yield were 4.93% and 92.27%, respectively, which agreed with the predicted value. Therefore, the results indicated suitability of the model employed and the success of RSM in optimizing the extraction conditions[16].
3 Conclusion
Optimization of the synthesis of OAE using RSM along with Box-Behnken design was performed. The regression models of DS and yield had high significance. For the DS, B, C, D, AB, AD, BD, CD and A2are significant model terms. For the yield, A, B, C, D, AC, BC, CD, A2, B2are significant model terms. The optimal conditions were 4.95 mL formic acid, 11.27 mL oleoyl chloride, reaction temperature 50 ℃ and reaction time 20 min, when the amount of alginate was 0.5 g. Maximum DS and yield were 4.93% and 92.27%, respectively, which agreed with the predicted value.
References:
[1]LERTSUTTHIWONG P, NOOMUN K, JONGAROONNGAMSANG N, et al. Preparation of alginate nanocapsules containing turmeric oil[J]. Carbohydr Polym, 2008, 74(2): 209-214.
[2]YANG Liqun, ZHANG Bifang, WEN Liqun, et al. Amphiphilic cholesteryl grafted sodium alginate derivative: Synthesis and self-assembly in aqueous solution[J]. Carbohydr Polym, 2007, 68(2): 218-225.
[3]YAO Bolong, NI Caihua, XIONG Cheng. Hydrophobic modification of sodium alginate and its application in drug controlled release[J]. Bioprocess Biosyst Eng, 2010, 33(4): 457-463.
[4]LI Qian, LIU Chenguang, XUE Fangfang, et al. Preparation and charac-terization of nanoparticles based on oleoyl-alginate as oral vehicles for vitamin D3[J]. J Agric Food Chem, 2011, 59(5): 1962-1967.
[5]SHAW S, SHAW D S, ROSSOL M. Overexposure: health hazards in photography[M]. New York: Allworth Press, 1991.
[6]LADOU J. Current occupational & environmental medicine[M]. New York: The McGraw-Hill Companies, 2003.
[7]ELIBOL M, OZER D. Response surface analysis of lipase production by freely suspended Rhizopus arrhizus[J]. Process Biochem, 2002, 38(3): 367-372.
[8]LIN J F, CHOU C C. The response surface method and the analysis of mild oxidational wear[J]. Tribol Int, 2002, 35(11): 771-785.
[9]GUO Xia, ZOU Xiang, SUN Min. Optimization of extraction process by response surface methodology and preliminary characterization of polysaccharides from Phellinus igniarius[J]. Carbohydr Polym, 2010, 80(2): 344-349.
[10]KHOO L P, CHEN C H. Integration of response surface methodology with genetic algorithms[J]. Cirp Ann-Manuf Techn, 2001, 18(7): 483-489.
[11]ELOMAA M, ASPLUND T, SOININEN P, et al. Determination of the degree of substitution of acetylated starch by hydrolysis,1H NMR and TGA/IR[J]. Carbohydr Polym, 2004, 57(3): 261-267.
[12]YUE Wu, YAO Pingjia, WEI Yuanan, et al. An innovative method for preparation of acid-free-water-soluble low-molecular-weight chitosan (AFWSLMWC)[J]. Food Chem, 2008, 108(3): 1082-1087.
[13]ADINARAYANA K, ELLAIAH P, SRINIVASULU B, et al. Response surface methodological approach to optimize the nutritional parameters for neomycin production by Streptomyces marinensis under solid-state fermentation[J]. Process Biochem, 2003, 38(11): 1565-1572.
[14]WU Shijin, YU Xiang, HU Zhihang, et al. Optimizing aerobic biodegradation of dichloromethane using response surface methodology[J]. J Environ Sci, 2009, 21(9): 1276-1283.
[15]YE Ling. WU Yinglong. Optimization of bleached pulverized konjac flour preparation process by response surface method[J]. Food Science, 2008, 29(6): 151-155.
[16]CHO I H, ZOH K D. Photocatalytic degradation of azo dye (Reactive Red 120) in TiO2/UV system: Optimization and modeling using a response surface methodology (RSM) based on the central composite design[J]. Dyes Pigments, 2007, 75(3): 533-543.
E-mail:liucg@ouc.edu.cn
中圖分類號:TS236.9
文獻標識碼:A
文章編號:1002-6630(2011)10-0006-06
收稿日期:2010-06-30
基金項目:國家“863”計劃項目(2007AA10Z349)
作者簡介:李倩(1984—),女,博士研究生,研究方向為納米技術在食品中的應用。E-mail:kikyoqq@163.com
*通信作者:劉晨光(1968—),男,教授,博士,研究方向為納米技術在生物、醫藥與食品中的應用。
響應面法優化油酰海藻酸酯的合成工藝
摘 要:采用響應面法分析不同因素對油酰海藻酸酯合成工藝的影響。選擇甲酸用量、油酰氯用量、反應溫度和反應時間4個因素,采用響應面分析法,根據Box-Behnken組合設計原理設計試驗。結果表明:最適條件為海藻酸量0.5g、4.95mL甲酸、11.27mL油酰氯、反應溫度50℃、反應時間20min。在此條件下,取代度可達4.93%、產率可達92.27%,與方程的預測值相符。
關鍵詞:油酰海藻酸酯;響應面法;合成工藝

