金屬有機(jī)骨架化合物催化合成不對(duì)稱有機(jī)碳酸酯
周印羲 梁曙光 宋金良 吳天斌 胡素琴劉會(huì)貞 姜 濤 韓布興
(中國科學(xué)院化學(xué)研究所,分子科學(xué)中心,北京分子科學(xué)國家實(shí)驗(yàn)室,北京 100190)
金屬有機(jī)骨架化合物催化合成不對(duì)稱有機(jī)碳酸酯
周印羲 梁曙光 宋金良 吳天斌 胡素琴劉會(huì)貞 姜 濤*韓布興*
(中國科學(xué)院化學(xué)研究所,分子科學(xué)中心,北京分子科學(xué)國家實(shí)驗(yàn)室,北京 100190)
金屬有機(jī)骨架化合物(MOFs)是通過過渡金屬和有機(jī)配體的自組裝形成的一類新型材料,具有高表面積、多孔性、孔尺寸可調(diào)等優(yōu)點(diǎn),在催化、分離和氣體儲(chǔ)存等方面得到廣泛應(yīng)用.用三種不同方法制備了金屬有機(jī)骨架化合物,并用掃描電鏡(SEM)、X射線衍射(XRD)和紅外(IR)光譜等方法進(jìn)行了表征.結(jié)果表明,用不同方法制備的MOFs表現(xiàn)出不同的結(jié)構(gòu)和形貌,用直接混合法制備的MOFs是有效的催化碳酸二乙酯與醇酯交換制備有機(jī)碳酸酯的多相催化劑.系統(tǒng)地考察了反應(yīng)時(shí)間、反應(yīng)溫度、催化劑用量和底物摩爾比對(duì)反應(yīng)的影響,結(jié)果表明,碳酸二乙酯與芳香醇、脂環(huán)醇、脂肪醇和雜環(huán)醇均能以100%選擇性高產(chǎn)率地合成有機(jī)碳酸酯,固體催化劑經(jīng)簡(jiǎn)單離心分離可重復(fù)使用至少2次.
金屬有機(jī)骨架化合物;配位聚合物;酯交換;碳酸二乙酯;不對(duì)稱碳酸酯
Organic carbonates[1]are an important class of compounds in chemistry because they have many applications as intermediates for the synthesis of fine chemicals[2]and pharmaceuticals[3],and have also been utilized as plasticizers,lubricants[4],and solvents[5]. Because high toxic and harmful reagents,such as phosgene or halo formates,were involved,most procedures for the synthesis of these compounds are not environmentally acceptable[6-7].Alternative synthesis methods for organic carbonates have been developed in recent years,such as the coupling of alcohol,CO2, and alkyl halidesin the presence ofCs2CO3[8-9].However,these reactions produce stoichiometric amounts of salts or use stoichiometric amounts of base as the catalysts and reagents,reducing the atomic economy of the reactions.The synthesis of dimethyl carbonate(DMC)and diethyl carbonate(DEC)[10-12]has attracted considerable attention,as they are environmentally friendly substitutes to phosgene.Recently,the synthesis of unsymmetrical organic carbonates by transesterification of DEC with alcohols has been successfully achieved by solid base catalysts,such as MCM-41-TBD (TBD,1,5,7-triazabicyclo[4.4.0]dec-5-ene), Mg/La metal oxide,CsF/α-Al2O3,and nanocrystalline MgO[13-16]. But the activity of some of the solid catalysts was not high enough and long reaction time was required to reach high yield.
Metal-organic frameworks(MOFs)are a new class of crystalline porous coordination polymers that are formed by copolymerization of multidentate organic ligands with transition metal ions or metal ion clusters,leading in most cases to three-dimensional(3D)extended networks with channels and cavities of molecular dimensions[17-21].Due to their zeolite-like properties,such as high surface areas,microporosity,well-defined structures,and the ability to tune pore size on 0.1 nm scale,many applications have recently been developed in separation[22],catalysis[23],magnetism[24],and storage of gases[25-26].Among these applications, the catalytic property of MOFs is most fascinating.Many reactions have been reported using MOFs as the catalysts or the supports of catalysts[27-33].For example,MOFs and quaternary ammonium salts has excellent synergetic effect in promoting the cycloaddition of CO2with epoxides to produce five-membered cyclic carbonates under mild reaction conditions[34].
In general,transesterification is catalyzed by acids or bases,in either homogeneous or heterogeneous systems[35].However,acid catalysts were less successfully applied in the reaction.Recent research on MOFs showed that the dissociation or removal of the terminal coordinated molecules from metal ions could yield more empty frameworks and leave Lewis acid sites on the surface,which haspotentialapplication in catalysis[36-37].In this work, we utilize MOFs as an acid catalyst for the transesterification reaction of DEC with alcohols(Scheme 1).Good yield of asymmetrical organic carbonates with high selectivity were achieved under moderate conditions.As a heterogeneous catalyst,MOFs could be recovered simply and reused at least two times without obvious loss of its catalytic activity.

Scheme 1 Synthesis of organic carbonates from alcohols and diethyl carbonate
1 Experimental
1.1 Chemicals and instruments
Diethyl carbonate(>99%)was purchased from Alfa Aesar. Other reagents,such as alcohols,Zn(NO3)2·6H2O,triethylamine, and 1,4-benzenedicarboxylic acid(H2BDC),were analytical grade and purchased from Beijing Chemical Reagents Company.All chemicals were used without further purification.
1.2 Catalyst preparation
MOFs were prepared by three different methods.(A)Liquidliquid diffusion method:Zn(NO3)2·6H2O(1.6 mmol)and H2BDC (0.8 mmol)were dissolved in 16 mL of N,N-dimethylformamide (DMF)and triethylamine(6.4 mmol)was dissolved in 10 mL of ethanol.The vial full of the former solution was loaded in a big vial full of the latter solution,and sealed.With the diffusion of triethylamine solution,MOFs appeared and grew slowly.After three days,the precipitate was collected by centrifugation and washed with 10 mL of DMF and 10 mL of ethyl ether in turn for 2 times.The solids were dried at 90℃ in vacuum and then stored in a vacuum desiccator to avoid moisture adsorption.(B) Solvothermal method:the procedures are similar to those developed by Zhao et al.[38].Zn(NO3)2·6H2O(1.6 mmol)and H2BDC (0.8 mmol)were dissolved in 16 mL of DMF under stirring to form a gel solution.The solution was sealed in an autoclave,and kept at 160℃for three days.The precipitate was collected by centrifugation and the followed steps were the same as those in method A.(C)“Direct mixing”method[39]:Zn(NO3)2·6H2O(4 mmol),and H2BDC(2 mmol)were dissolved in 40 mL of DMF and MOFs was precipitated with the addition of triethylamine (16 mmol)under vigorous stirring.After 2 h,the precipitate was collected by filtration and other steps were the same as those in method A.The catalysts prepared using the three methods were denoted as MOFs-A,MOFs-B,and MOFs-C,respectively.
1.3 Catalyst characterization
X-ray diffraction(XRD)measurements were conducted on an X-ray diffractometer(D/MAX-RC,Japan)operated at 40 kV and 200 mA with Cu Kα(λ=0.154 nm)radiation.FT-IR spectra were recorded on a spectrometer(Bruker Tensor 27,Germany)and the sample was prepared by the KBr pellet method.Scanning electron microscopy(SEM)images were taken using an instrument (Hitachi S-4300,Japan)operated at 15 kV.Thermogravimetric (TG)analysis of MOFs was performed on a thermogravimetric analysis system(Netzsch STA 409 PC/PG,Germany)in N2atmosphere at a heating rate of 20℃·min-1.The BET surface area measurement and pore analysis were carried out by N2adsorption at 77 K with use of Micromeritics ASAP 2020 V3.00 H (USA)surface area analyser.The sample was degassed at 250℃for 12 h under vacuum before the measurement.
1.4 Catalytic reactions
Typical reaction procedures for the reactions of diethyl car-bonate(DEC)with alcohols were as follows:2 mmol of alcohol, 33 mmol(4 mL)of DEC,and 0.25%(w)(based on total mass of reactants)of catalyst were charged into a flask of 10 mL equipped with a magnetic stirring bar and a reflux condenser.The mixture was heated up to 130℃and stirred until the completion of the reaction.After the reaction,the reaction mixture was centrifuged to separate the catalyst.The products were analyzed by a gas chromatography(GC,Agilent 6820,USA)equipped with a flame-ionized detector(FID)and a capillary column(PEG-20M, 0.25 mm in diameter,30 m in length).The structure of the products was identified by GC-MS(Shimadzu QP2010,Japan).
n-Pentanol was employed as the internal standard for quantitive calculation.The conversion of alcohol and yield of asymmetrical carbonate were calculated using the following equations:

where c is the conversion,y is the yield of asymmetrical carbonate,misis the mass of internal standard,Miis the molecular weight of component i,Aiis the peak area of component i,Aisis the peak area of internal standard,fiis the response factor of component i,and n0is the initial moles of alcohol.The selectivity to asymmetrical carbonate can be obtained from the ratio of yield to conversion.
2 Results and discussion
2.1 Catalyst screening
MOFs prepared via different methods were used to catalyze the transesterification reaction of DEC with benzyl alcohol to produce benzyl ethyl carbonate(BEC).The results are summarized in Table 1.The reaction could not proceed in the absence of a catalyst(Entry 1).When the precursors for the MOFs or ZnO were used as the catalysts for the reaction,poor yield of the objective product was achieved or no reaction occurred(Entries 2-4).The activity of MOFs prepared by different methods was compared.The reactions did not occur in the presence of MOFs-A or MOFs-B(Entries 5,6),while high yield(95.1%)was achieved when MOFs-C was used(Entry 7)as the catalyst.
The effect of the amount of MOFs-C on the yield of BEC wasalso studied.As shown in Table 1(Entries 7-11),high yield of BEC was also achieved,even if a very small amount of MOFs-C was used.When the amount of MOFs-C was 0.25%(w),the conversion of benzyl alcohol exhibited a maximum and did not change significantly with the amount of MOFs-C.Moreover, MOFs-C showed high selectivity and no byproduct was detected.These results indicated that MOFs prepared via“direct mixing”method were highly active and selective for the synthesis of BEC from DEC and benzyl alcohol.
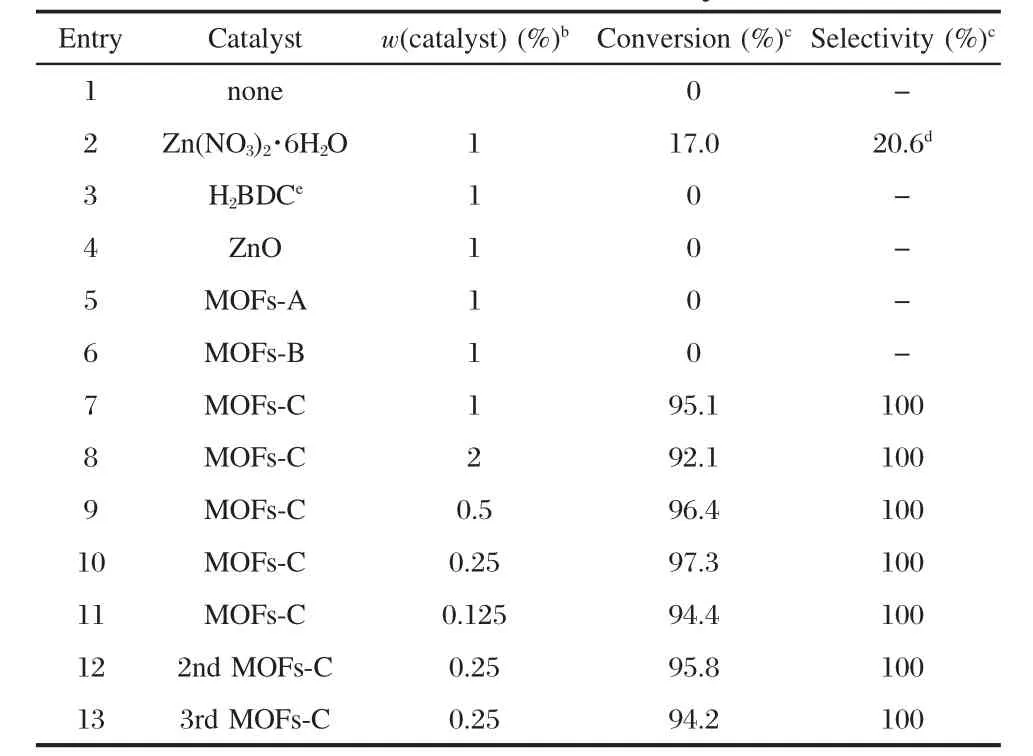
Table 1 Reaction of diethyl carbonate(DEC)and benzyl alcohol with different catalystsa

Fig.1 SEM images of MOFs prepared via different methods(A)diffusion method;(B)solvothermal method;(C)“direct mixing”method
As discussed above,the MOFs were synthesized by different methods,including diffusion method,solvothermal synthesis, and“direct mixing”method.The obtained samples were characterized with SEM,XRD,and IR techniques.MOFs prepared via the three methods are completely different in morphology and microstructure.SEM images of MOFs are shown in Fig.1.As shown in image C,uniform nanocrystals were synthesized by“direct mixing”method,which is in good agreement with the resultsin literature[39].However,MOFs-A and MOFs-B were composed of large sheetlike structures.Although MOFs prepared via the three methods were completely different in morphology and microstructure,similar crystalline structures were obtained,as demonstrated by XRD patterns in Fig.2.IR spectra in Fig.3 indi-cated that all MOFs showed strong characteristic absorptions for the symmetric and asymmetric vibrations of BDC(1610-1550 cm-1and 1420-1335 cm-1).Unlike MOFs-A and MOFs-B,MOFs-C did not show absorption of protonated BDC(1715-1680 cm-1), confirming the complete deprotonation of H2BDC by NEt3.The BET surface areas of the three catalysts were 63,607,and 698 m2·g-1for MOFs-A,MOFs-B,and MOFs-C,respectively.The pore volume/average pore diameter were 0.03 cm3·g-1/28 nm, 0.03 cm3·g-1/4 nm,and 0.3 cm3·g-1/12 nm for MOFs-A,MOFs-B,and MOFs-C,respectively.From the procedures to prepare MOFs,the“direct mixing”method produced more ligands instantly,which is a suitable method for fast synthesis of MOFs. Therefore,the high activity of MOFs-C should be attributed to the small size and high surface of MOFs-C.
The catalytic activity of MOFs-C in the transesterification reaction of benzyl alcohol with DEC was compared with those of MCM-41-TBD,Mg/La mixed oxides,supported fluorides,and nanocrystalline MgO and the results are listed in Table 2.It can be known from the data in the table that MOFs-C had very high activity compared with other catalysts.
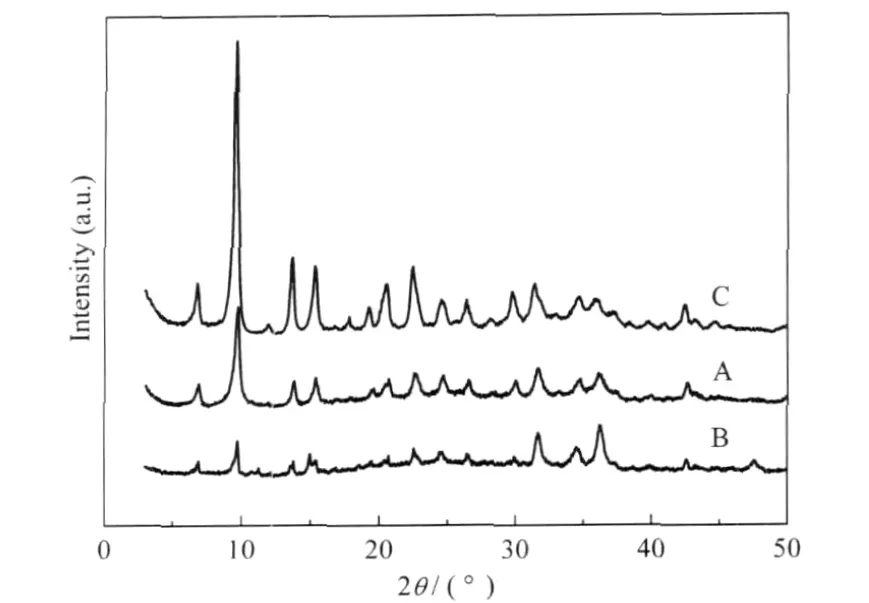
Fig.2 XRD patterns of MOFs prepared via different methods(A)diffusion method,(B)solvothermal method,(C)“direct mixing”method

Fig.3 IR spectra of MOFs prepared via different methods(A)diffusion method,(B)solvothermal method,(C)“direct mixing”method
2.2 Effects of reaction conditions
Based on the above results,the effects of reaction conditions on the transesterification reaction of DEC with benzyl alcohol were investigated using MOFs-C as the catalyst.Fig.4 shows the effect of reaction time on the yield of BEC.The yield of BEC increased rapidly within 30 min,and then remained unchanged. Nearly complete conversion could be achieved with 100%selectivity within 60 min.The selectivity obviously remained unchanged even if the reaction time was prolonged.
The effect of reaction temperature on the yield and selectivity of BEC is shown in Fig.5.The yield of BEC increased rapidly with increasing the temperature from 110 to 130℃.At 130℃, the yield of BEC reached a maximum of 97.3%.However,further increase in temperature was not favorable to the formationof BEC,which was possible due to heat decomposition of the product or the escape of DEC from the reactor because the boiling point of DEC was only 126.8℃.The selectivity decreased by about 3%when temperature was increased from 130 to 140℃because of the formation of byproducts.

Table 2 Comparison of MOFs-C with other heterogeneous catalysts for catalyzing the reaction between benzyl alcohol and DECa
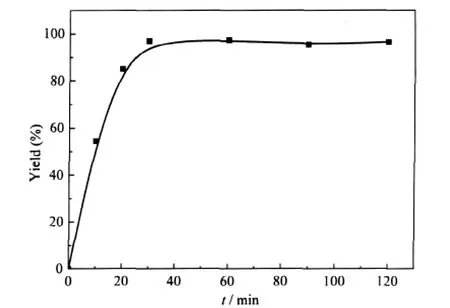
Fig.4 Effect of reaction time on the yield of benzyl ethyl carbonate(BEC)over MOFs-Creaction conditions:benzyl alcohol,2 mmol;DEC,4 mL(33 mmol); MOFs-C,0.25%(w);temperature,130℃

Fig.5 Effect of reaction temperature on the yield and selectivity of BEC over MOFs-Creaction conditions:benzyl alcohol,2 mmol;DEC,4 mL(33 mmol); MOFs-C,0.25%(w);reaction time,60 min

Fig.6 Kinetic plots of BEC formation from transesterification of DEC with benzyl alcohol
2.3 Catalyst leaching test

Fig.7 XRD patterns of fresh and reused(2nd)MOFs-C
A leaching experiment was performed to figure out weather the reaction takes place homogeneously or heterogeneously.The kinetic plot of the reaction of DEC with benzyl alcohol over MOFs-C at 130℃was compared with that of another reaction where the reaction was stopped after 10 min,and then continued after filtering out the solid catalyst.The results are shown in Fig. 6.There was no further increase in the BEC yield after the solid catalyst was separated out,demonstrating that MOFs-C was a heterogeneous catalyst for the transesterification reaction.Furthermore,only 3.5%yield of BEC was obtained at 130℃for 60 min when Zn(NO3)2·6H2O was used as a homogenous catalyst (Table 1,Entry 2).This confirmed indirectly that the active site in MOFs-C is not soluble in the reaction mixture.
2.4 Catalyst recycling
Apart from the catalytic activity,the stability of the catalyst isanother important aspect.After the completion of the reaction, the catalyst was centrifugated,washed with ethyl ether,and dried under vacuum.The transesterification reaction over the recovered catalyst gave similar result as that of the fresh catalyst (Table 1,Entries 10,12,13).The slight decrease in the conversion was attributed to the small loss of the catalyst during the recovery.

Table 3 Reactions of DEC with different alcohols using MOFs-C as the catalysta
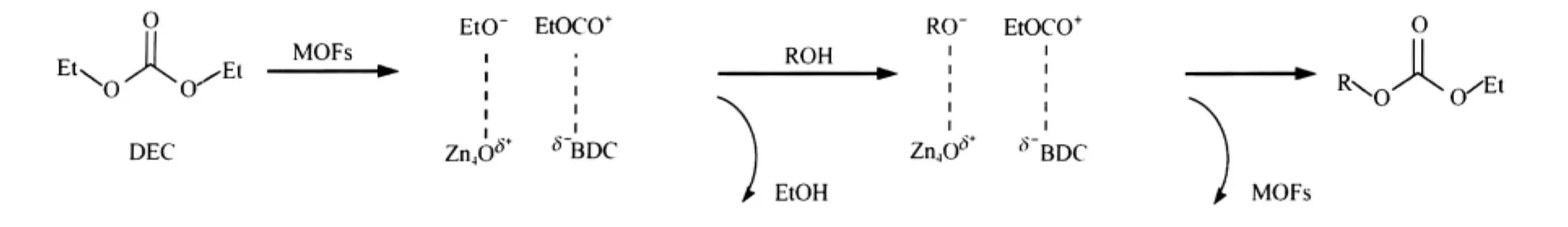
Scheme 2 Possible mechanism of transesterification of DEC with alcohol
The XRD pattern of the recovered catalyst,as shown in Fig.7, agreed with that of the fresh one.Although the intensity of the diffraction peaks weakened,2θ angle of diffraction peaks did not change significantly,indicating that the catalyst remained its crystalline structure after being used.
2.5 Other substrates
The above results indicated that BEC can be obtained in high yield by the transesterification reaction of DEC and benzyl alcohol in the presence of MOFs-C.The catalyst was also used for the transesterification reactions of DEC with other alcohols under the optimized conditions.The results are summarized in Table 3.MOFs-C were applicable to a variety of alcohols including aromatic,cyclic,heterocyclic,and aliphatic compounds, to produce corresponding asymmetrical organic carbonates in moderate to high yields with 100%selectivity.Because of microporosity of MOFs-C,the steric hindrance to the reactive hydroxyl group is a limiting factor.Longer time was required to obtain reasonable yield of the objective product.Phenolic OH has not been carboxylated under similar reaction conditions. Phenol was transformed to phenyl ethyl ether.
2.6 Mechanism
A possible mechanism was proposed based on a typical transesterification process,where the more nucleophilic reagent displaces the less nucleophilic one,or the less volatile compound displaces the more volatile one when both of the reagents have similar nucleophilicity.In the present process,the nucleophilic displacement of the ethoxy by a second molecule of the alcoholic reagent probably led to the corresponding unsymmetrical carbonate.As shown in Scheme 2,DEC was chemisorbed on Lewis acid site in MOFs-C to yield EtO-and EtOCO+ion species firstly.Subsequent attack of EtO-on the alcohol gave the RO-species,which combined with EtOCO+to yield the product and restored the catalyst.
3 Conclusions
In conclusion,MOFs prepared with“direct mixing”method is a very effective heterogeneous catalyst for the transesterification reaction of diethyl carbonate with alcohols.Asymmetrical organic carbonates can be produced with moderate to high yields and 100%selectivity when diethyl carbonate reacts with aromatic,cyclic,heterocyclic,or aliphatic alcohol.The solid catalyst can be recovered simply by centrifugation and reused for at least two cycles.
1 Shaikh,A.A.G.;Sivaram,S.Chem.Rev.,1996,96:951
2 Ono,Y.Appl.Catal.A-Gen.,1997,155:133
3 Parrish,J.P.;Salvatore,R.N.;Jung,K.W.Tetrahedron,2000,56: 8207
4 Gryglewicz,S.;Oko,F.A.;Gryglewicz,G.Ind.Eng.Chem.Res., 2003,42:5007
5 Mizia,F.;Rivetti,F.Use of organic carbonates as solvents for the washing metal surface.US,6565663[P].2003-5-20
6 Burk,R.M.;Roof,M.B.Tetrahedron Lett.,1993,34:395
7 Bertolini,G.;Pavich,G.;Vergani,B.J.Org.Chem.,1998,63: 6031
8 Kim,S.I.;Chu,F.;Dueno,E.E.;Jung,K.W.J.Org.Chem.,1999, 64:4578
9 Salvatore,R.N.;Flanders,V.L.;Ha,D.;Jung,K.W.Org.Lett., 2000,2:2797
10 Ono,Y.Catal.Today,1997,35:15
11 Delledonne,D.;Rivetti,F.;Romano,U.Appl.Catal.A-Gen., 2001,221:241
12 Tundo,P.;Selva,M.Acc.Chem.Res.,2002,35:706
13 Carloni,S.;De Vos,D.E.;Jacobs,P.A.;Maggi,R.;Sartori,G.; Sartorio,R.J.Catal.,2002,205:199
14 Veldurthy,B.;Figueras,F.Chem.Commun.,2004:734
15 Veldurthy,B.;Clacens,J.M.;Figueras,F.Eur.J.Org.Chem., 2005,10:1972
16 Kantam,M.L.;Pal,U.;Sreedhar,B.;Choudary,B.M.Adv.Synth. Catal.,2007,349:1671
17 Rowsell,J.L.C.;Yaghi,O.M.Microporous Mesoporous Mat., 2004,73:3
18 Yaghi,O.M.;O′Keeffe,M.;Ockwig,N.W.;Chae,H.K.; Eddaoudi,M.;Kim,J.Nature,2003,423:705
19 Cheetham,A.K.;Ferey,G.;Loiseau,T.Angew.Chem.Int.Edit., 1999,38:3268
20 Ferey,G.;Mellot-Draznieks,C.;Serre,C.;Millange,F.Acc.Chem. Res.,2005,38:217
21 Lin,W.B.;Wang,Z.Y.;Ma,L.J.Am.Chem.Soc.,1999,121: 11249
22 Chen,B.;Liang,C.;Yang,J.;Contreras,D.S.;Clancy,Y.L.; Lobkovsky,E.B.;Yaghi,O.M.;Dai,S.Angew.Chem.Int.Edit., 2006,45:1390
23 Lor,B.G.;Puebla,G.;Iglesias,M.;Monge,M.A.;Valero,C.R.; Snejko,N.Chem.Mater.,2005,17:2568
24 Kahn,O.Acc.Chem.Res.,2000,33:647
25 Li,Y.;Yang,R.T.Langmuir,2007,23:12937
26 Morris,R.E.;Wheatley,P.S.Angew.Chem.Int.Edit.,2008,47: 4966
27 Hasegawa,S.;Horike,S.;Matsuda,R.;Furukawa,S.;Mochizuki, K.;Kinoshita,Y.;Kitagawa,S.J.Am.Chem.Soc.,2007,129: 2607
28 Horcajada,P.;Surblé,S.;Serre,C.;Hong,D.Y.;Seo,Y.K.; Chang,J.S.;Grenéche,J.M.;Margiolaki,I.;Férey,G.Chem. Commun.,2007:2820
29 Dewa,T.;Saiki,T.;Aoyama,Y.J.Am.Chem.Soc.,2001,123: 502
30 Cho,S.H.;Ma,B.;Nguyen,S.T.;Hupp,J.T.;Albrecht-Schmitt, T.E.Chem.Commum.,2006:2563
31 Sabo,M.;Henschel,A.;Fr?de,H.;Klemm,E.;Kaskel,S.J.Mater. Chem.,2007,17:3827
32 Seo,J.S.;Whang,D.;Lee,H.;Jun,S.I.;Oh,J.;Jeon,Y.J.;Kim, K.Nature,2000,404:982
33 Xamena,F.X.L.I.;Casanova,O.;Tailleur,R.G.;Garcia,H.; Corma,A.J.Catal.,2008,255:220
34 Song,J.L.;Zhang,Z.F.;Hu,S.Q.;Wu,T.B.;Jiang,T.;Han,B. X.Green Chem.,2009,11:1031
35 Meyer,U.;Hoelderichm,W.F.Appl.Catal.A-Gen.,1999,178: 159
36 Reineke,T.M.;Eddaoudi,M.;O′Keeffe,M.;Yaghi,O.M.Angew. Chem.Int.Edit.,1999,38:2590
37 Reineke,T.M.;Eddaoudi,M.;Fehr,M.;Kelley,D.;Yaghi,O.M. J.Am.Chem.Soc.,1999,121:1651
38 Sun,J.Y.;Weng,L.H.;Zhou,Y.M.;Chen,J.X.;Chen,Z.X.;Liu, Z.C.;Zhao,D.Y.Angew.Chem.Int.Edit.,2002,41:4471
39 Huang,L.M.;Wang,H.T.;Chen,J.X.;Wang,Z.B.;Sun,J.Y.; Zhao,D.Y.;Yan,Y.S.Microporous Mesoporous Mat.,2003,58: 105
November 18,2009;Revised:December 24,2009;Published on Web:February 4,2010.
Synthesis of Asymmetrical Organic Carbonates Catalyzed by Metal Organic Frameworks
ZHOU Yin-Xi LIANG Shu-Guang SONG Jin-Liang WU Tian-Bin HU Su-Qin LIU Hui-Zhen JIANG Tao*HAN Bu-Xing*
(Beijing National Laboratory for Molecular Sciences,Centre for Molecular Science,Institute of Chemistry, Chinese Academy of Sciences,Beijing 100190,P.R.China)
Metal organic frameworks(MOFs)are a new class of materials that are formed by the copolymerization of organic ligands with transition metals.Because of their properties such as high surface areas,microporosity,and the ability to tune pore size,many applications have recently been developed in catalysis,separation,and gas storage.We prepared MOFs by three different methods and characterized them with scanning electron microscopy(SEM),X-ray diffraction(XRD),and infrared(IR)spectroscopy.MOFs prepared via the three methods are completely different in morphology and microstructure.We demonstrate that the MOFs prepared via the“direct mixing”method are very effective heterogeneous catalyst for the transesterification of diethyl carbonate with alcohols to prepare organic carbonates.We studied the effects of the amount of MOFs,the reaction time,and the reaction temperature on product yield.Asymmetric organic carbonates can be produced with moderate to high yields and 100%selectivity via the reaction of diethyl carbonate with aromatic,cyclic,heterocyclic,or aliphatic alcohols.The solid catalyst can be recovered simply by centrifugation and reused for at least two cycles.
Metal organic framework;Coordination polymer;Transesterification;Diethyl carbonate; Asymmetrical organic carbonate
*Corresponding authors.Email:Jiangt@iccas.ac.cn,Hanbx@iccas.ac.cn;Tel/Fax:+86-10-62562821.
The project was supported by the National Natural Science Foundation of China(20932002,20733010),National Key Basic Research Program of China(973)(2006CB202504),and Chinese Academy of Sciences Fund(KJCX2.YW.H16).
國家自然科學(xué)基金(20932002,20733010),國家重點(diǎn)基礎(chǔ)研究發(fā)展規(guī)劃項(xiàng)目(973)(2006CB202504)和中國科學(xué)院基金(KJCX2.YW.H16)資助韓布興,北京大學(xué)化學(xué)與分子工程學(xué)院兼職教授.
O643

