基于席夫堿配體和兩種苯甲酸鹽構筑的兩例Dy4配合物的合成、結構及單分子磁體性質
李大偉 胡 瑞 師素云 涉葉葉 張夏梅 李亞紅*, 張義權*, 姚金雷
(1蘇州大學材料與化學化工學部,蘇州 215123)
(2南京師范大學物理科學與技術學院,江蘇省NSLSCS重點實驗室,南京 210023)
(3河南交通職業技術學院,鄭州 451460)
(4蘇州科技大學物理科學與技術學院,江蘇省微納熱流技術與能源應用重點實驗室,蘇州 215009)
0 Introduction
Single-molecule magnets (SMMs) are molecular entities that can store magnetic information at the molecular scale[1-3].SMMs have shown a diverse number of utilizations in information storage, spintronics devices[4], magnetic refrigeration[5], and quantum computing[6].Research on SMMs has been moving toward lanthanide-based SMMs since the first rare earth-based SMM was reported in 2003[7-9].Lanthanides have the potential to achieve a high anisotropic energy barrier(Ueff) due to their considerable unquenched orbital angular momentum and magnetic anisotropy[10-11].Benefiting from the unparalleled single-ion anisotropy and the Kramers ground state of6H15/2[12-13],the Dy(Ⅲ)ion has a very strong potential for the development of SMMs.
Many SMMs based on Dy(Ⅲ)ions have been reported.Tetranuclear Dy-SMMs continue to attract intensive attention because this type of SMMs exhibits aesthetically pleasing structures including linear-shaped[14-15],[2×2] square grid[16-18], triangle-based pyramid[19], rhombus-shaped (or butterfly-shaped)[20-22], and Y-shaped[23]topologies.It was found that a tetranuclear DyⅢ4complex with a defect-dicubane geometry behaved as an SMM with an anisotropic barrier of 170 K[24].
In our previous work, we found that the reactions of Dy(NO3)3·6H2O with a Schiff base ligand 2-(((2-hydroxy-3-methoxybenzyl)imino)methyl)-4-methoxyphenol (H2L) in three different solvents afforded three Dy2SMMs with different anisotropy barriers and variable relaxation mechanisms[25].We anticipated that the inclusion of different benzoic acids in the reaction systems may generate a series of tetranuclear DyⅢ4SMMs,in which the Dy2basic units were connected by benzoic acids, and the formation of DyⅢ4SMMs may provide a chance for us to understand the magneto-structural relationships.
Based on these considerations, we conducted the reactions of Dy(NO3)3·6H2O, H2L, and PhCO2H and 2-NO2-PhCO2H, respectively.Two linear tetranuclear dysprosium complexes of formulae [Dy4(L)4(PhCO2)2(NO3)2(EtOH)2] (1) and [Dy4(L)4(2-NO2-PhCO2)2(NO3)2(EtOH)2] (2) were prepared.Complexes 1 and 2 exhibit SMM properties.The complete-active-space selfconsistent field (CASSCF) calculations were carried out to analyze the magnetic properties of 1 and 2.Herein, we report the syntheses, structures, and SMM properties of 1 and 2.
1 Experimental
1.1 Reagents
All chemicals were obtained commercially and used directly.The intermediate compound 3-methoxysalicylamine that was used for synthesizing the H2L ligand was prepared by following the literaturereported procedure[25].
1.2 Preparation of ligand H2L
The synthesis process and related characterization of ligand H2L can be found in the literature previously reported by our research group[26].
1.3 Preparation of complex 1
Dy(NO3)3·6H2O (0.05 mmol), PhCO2H (0.05 mmol), H2L (0.05 mmol), MeCN (0.5 mL), EtOH (1.5 mL),and Et3N (0.15 mmol)were loaded in a Pyrex tube(10 mL).The tube was sealed and heated for 48 h at 70 ℃.The dark-yellow stripe crystals of complex 1 were formed.The yield was 0.015 g (48% based on Dy).Elemental Anal.Calcd.for C82H82Dy4N6O28(%): C,43.78; H, 3.67; N, 3.74.Found(%): C, 43.89; H, 3.44;N,3.76.IR data(KBr,cm-1):3 321(w),1 629(s),1 475(s),1 391(s),1 279(s),1 154(s),846(m),819(s),712(s),624(w).
1.4 Preparation of complex 2
H2L (0.05 mmol), Dy(NO3)3·6H2O (0.05 mmol), 2-NO2-PhCO2H (0.05 mmol), Et3N (0.15 mmol), MeCN (1 mL), and EtOH (2.5 mL) were loaded in a Pyrex tube.The tube was sealed and heated for 48 h at 70 ℃.Yellow stripe crystals of complex 2 were formed.The yield of 2 was 0.026 g (72% based on Dy).Elemental Anal.Calcd.for C82H80Dy4N8O32(%): C, 42.10; H, 3.45;N, 4.79.Found(%): C, 42.29; H, 3.41; N, 4.27.IR data(KBr, cm-1): 3 324 (w), 1 628 (s), 1 602 (s), 1 473 (s),1 383 (s), 1 272 (s), 1 219 (s), 1 153 (s), 1 071 (s), 841(s),786(s),747(s),712(s).
1.5 Physical measurements
IR spectra were determined by a Bruker VERTEX 70 FTIR spectrophotometer in the 600-4 000 cm-1range.The elemental analyses for carbon,nitrogen,and hydrogen were obtained from a Perkin-Elmer 2400 analyzer.Magnetic data were measured on a Quantum Design Dynacool-9 in the 2-300 K range.Powder X-ray diffraction (PXRD) patterns were measured on a Rigaku D/Max-2500 diffractometer with CuKαradiation (λ=0.154 06 nm) at 40 kV and 100 mA with a scanning range of 5°-50°.The structures of complexes 1 and 2 were determined from a Bruker SMART APEXⅡCCD diffractometer with MoKα(λ=0.071 073 nm)and theφ-ωscan.The structures were solved using the Olex2 and SHELXTL packages[27-28].Complex 2 contains highly disordered solvents that could not be satisfactorily refined.The SQUEEZE routine of PLATON was used in the treatment of the crystallographic data.Although it is difficult to determine the free solvents in the crystal lattice, the composition and connectivity of complex 2 are definite.The structure parameters for 1 and 2 are listed in Table 1.The selected bond lengths and angles are summarized in Table S1 (Supporting information).
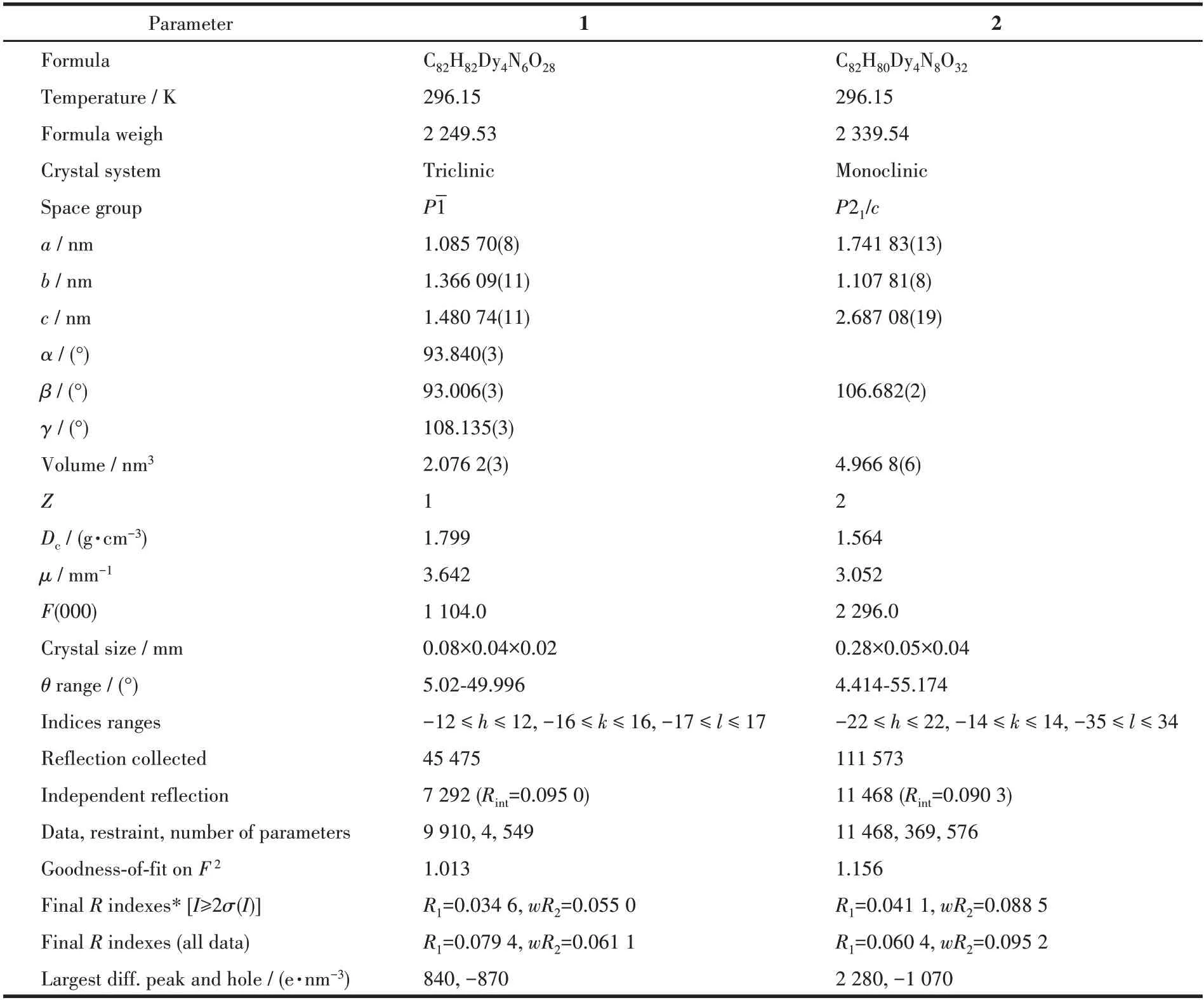
Table 1 Crystallographic data and structure refinement of complexes 1 and 2
CCDC:2047006,1;2047008,2.
2 Results and discussion
2.1 Synthesis of ligand H2L and complexes 1 and 2
The H2L ligand was synthesized based on the synthetic route of Scheme 1 and characterized by1H and13C NMR spectroscopy (Fig.S1), IR spectroscopy (Fig.S3), and elemental analysis.The potential coordination mode for the H2L ligand is displayed in Scheme 1.Complexes 1 and 2 were obtained from the 1∶1∶1∶3 reactions of H2L, Dy(NO3)3·6H2O PhCO2H/2-NO2-PhCO2H,and triethylamine in EtOH/MeCN(Scheme 2).

Scheme 1 Synthetic route of the H2L ligand and its two coordination pockets

Scheme 2 Syntheses of complexes 1 and 2
2.2 Structure descriptions of complexes 1 and 2
X-ray crystallographic analysis results indicated that complexes 1 and 2 possess similar structures.The space groups of 1 and 2 are triclinicP1 and monoclinicP21/c.We choose 1 as an example to present its structural features in detail (Fig.1).Complex 1 is crystallographically centrosymmetric.It is mainly comprised of four Dy(Ⅲ)ions, four doubly deprotonated H2L ligands(L2-),two benzoate ions,two nitrate ions,and two EtOH molecules.The central Dy1 ions in the Dy4chain are seven-coordinated by one N atom (N1) from an L2-ligand, five O atoms (O2, O2A, O3, O7, and O8) from three L2-ligands, and one O atom (O9) of the PhCO2-ion (Fig.1a).The Dy2 ions adopt the NO7coordination mode.The two ligands exhibit theμ3∶η2∶η1∶η2∶η1coordination fashion,and the other two ligands show theμ2∶η1∶η1∶η2∶η1mode.The carboxyl groups in the two complexes display the monodentate coordination mode.Complex 1 shows a linear topological structure, in which the [Dy4O6] core is made by the fusion of two[Dy2O2] units bridged via two phenolic oxygen atoms(Fig.S5).The Dy (Ⅲ)ions exhibit capped pentagonal bipyramid and triangular dodecahedron geometries(Fig.1b), which are proven by the SHAPE 2.0 software calculation (Table S2).The distances of Dy1…Dy2 and Dy1…Dy1A are 0.380 18(4) and 0.377 21(5) nm,respectively.The bond angles of Dy2—Dy1—Dy1A and Dy1—Dy1A—Dy2A are both 139.835(11)°.The bond lengths of Dy—O are within the range of 0.217 1(3)-0.246 8(4) nm.The bond distances of Dy—N vary from 0.245 0(4) to 0.284 2(5) nm.The bond angles of O—Dy—O are within a range of 52.37(14)°-164.53(12)°.The O—Dy—O angles and Dy—O distances are comparable to some of the Dy4clusters in the literature[24,29].Theπ…πand hydrogen-bonding interactions held the individual molecules to form a supramolecular network(Fig.S6-S7).

Fig.1 Molecular structure(a)and the coordination polyhedrons of Dy(Ⅲ)ions(b)in complex 1
Complexes 1 and 2 join a large family of Dy4complexes (several hundred Dy4complexes were found in the CCDC database).However, Dy4complexes with linear structures are relatively rare[14,30-36].
2.3 Thermal properties
The TGA of complexes 1 and 2 (Fig.S18) were studied to investigate the stability of the crystal frameworks and the existence of the guest molecules.It was found that the first weight loss of 1.96% of complex 1 occurred between 120 and 180 ℃, which is consistent with the loss of one ethanol molecule (2.05%).The further weight loss indicates the decomposition of the crystal frameworks.For complex 2, the first weight loss of 2.02% between 65 and 130 ℃was determined,which almost agrees with the loss of one ethanol guest molecule (2.06%).It began to break down upon further heating.
2.4 Magnetic properties of complexes 1 and 2
Under an applied magnetic field of 1 000 Oe, the DC magnetic susceptibility data of complexes 1 and 2 were measured.As displayed in Fig.2, the determinedχMTvalues of 1 and 2 at room temperature were 56.60 and 57.05 cm3·mol-1·K.These values were close to the expected values of 56.68 cm3·mol-1·K for four uncoupled Dy(Ⅲ)ions(6H15/2,S=5/2,L=5,J=15/2,g=4/3)for 1 and 2.
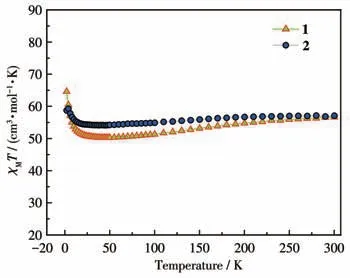
Fig.2 χMT values under 1 000 Oe from 2 to 300 K for complexes 1 and 2
TheχMTvalues of 1 and 2 declined slightly from 300 to 2 K.TheχMTvalues reached the lowest points of 50.25 and 54.14 cm3·mol-1·K at 46 K.This trend is possibly induced by depopulation of the excited Stark sublevels[37-38].When the temperature was dropped further, theχMTvalues increased rapidly, reaching 64.61 and 58.71 cm3·mol-1·K at 2 K.
At a zero-DC field,the AC magnetic susceptibility data for 1 and 2 were determined to explore the dynamics of magnetizations.The AC magnetic susceptibility data were examined at 32, 100, 320, 666, 780, and 1 000 Hz from 2 to 40 K (30 K) for 1 and 2 (Fig.3).Both complexes presented single-molecule magnet behavior indicated by the appearance of significant frequency dependence in theχ′andχ″signals(Fig.S9).
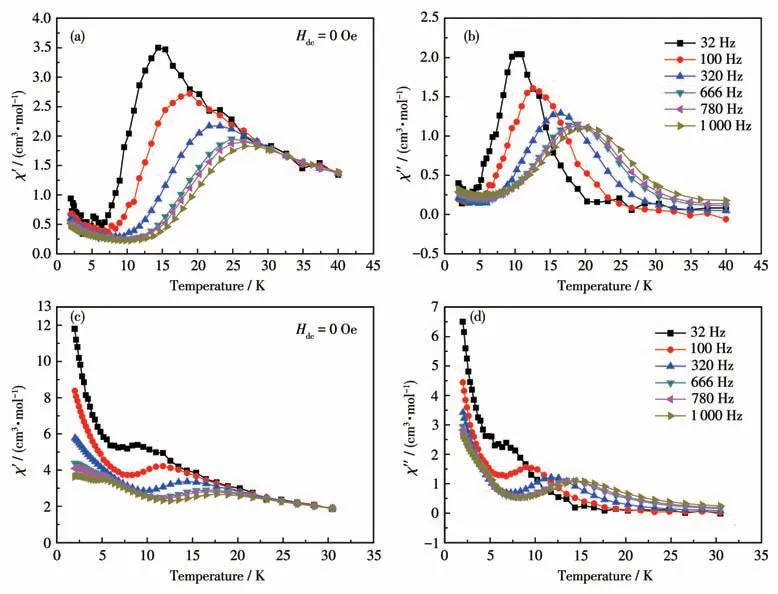
Fig.3 Temperature dependence of the in-phase(χ′)and out-of-phase(χ″)susceptibilities for 1(a,b)and 2(c,d)under a zero DC field
The peaks of AC signals of 2 appeared between 8 and 16 K at the six frequencies (Fig.3).When the temperature was decreased, a remarkable increase in theχ′ andχ″ signals, such as a “tail”, was present.The QTM (quantum tunneling of the magnetization) found in many reported lanthanide - based SMMs[39-40]may appear.
The effective barriers of magnetic relaxation were analyzed by Cole-Cole plots.Fig.4 and S10 show the best fits for complexes 1 and 2,respectively.The relaxation time (τ=(2πν)-1) in the high-temperature range obeys the Arrhenius equation:τ=τ0exp[Ueff/(kBT)] (1).TheUeff(effective energy barrier) values of 110 and 108 K, andτ0values of 1.03×10-6and 7.22×10-8s for complexes 1 and 2, respectively, were derived.These data were comparable to some Dy4SMMs reported in the literature[41-45].

Fig.4 Cole-Cole plots of complex 1(12 to 22 K)under a zero DC field
At low temperatures, the relaxation times were not linear, indicating the presence of other processes, such as Raman and QTM, in addition to the thermally activated Orbach process.Thus, the magnetic susceptibility data of complex 1 were fitted by employing the equation:τ-1=τ0-1exp[-Ueff/(kBT)]+τQTM-1(2).The best fit of complex 1 produced the parameters ofUeff/kB=104.06 K,τ0=1.50×10-6s, andτQTM=7.29×10-3s.The Orbach and QTM processes may exist for the possible relaxation processes in complex 1.For complex 2, we fitted the magnetic data with Eq.2, where the relaxations of both the Orbach and QTM were taken into account.The best fit of Eq.2 produced the parameters ofUeff/kB=103.24 K,τ0=1.07×10-7s, andτQTM=7.03×10-3s for complex 2, showing proximity to the values fitted only by the Arrhenius equation(Fig.S12).
2.5 Theoretical calculations
Using the X-ray-determined geometries, CASSCF computations on each Dy (Ⅲ)ion of complexes 1 (Dy1,Dy2) and 2 (Dy1, Dy2) were performed with the programs MOLCAS 8.4[46]and SINGLE_ANISO[47](the details are presented in the Supporting information).The mainmJdata of the lowest eight KDs (Kramers doublets) of each Dy (Ⅲ)ion, the energy levels (cm-1),andg(gx,gy,gz) tensors are listed in Table S8.ThemJdata for the lowest two KDs of each Dy(Ⅲ)ion are collected in Table S9.The ground KDs of Dy1(2) consist of severalmJstates and the others mainly consist of a singlemJ=±15/2 state.For 1 and 2, the first excited states both consist of severalmJstates.For each Dy(Ⅲ)ion, the magnetic blocking barriers are presented in Fig.S14.The transversal magnetic moments for Dy1(1), Dy2 (1), and Dy2 (2) in the ground KDs were all close to 0.1μB; therefore, the QTM could be restrained in their ground KDs at low temperatures.In the first excited KDs of these ions, the transversal magnetic moments were close to 0.1μB; thus, a fast QTM in their first excited KDs is possible[48].However, in the ground KDs of Dy1 (2), the transverse magnetic moments were 0.11μB, thus permitting a fast QTM in their ground KDs.While the magnetic anisotropies of 1 and 2 originate from a single Dy(Ⅲ)ion, the interactions between two Dy(Ⅲ)ions influence their slow relaxation of magnetization.
For 1 and 2, the computed ground gzdata of each Dy (Ⅲ)ion were all approximately 20, and the interactions between two Dy(Ⅲ)ions could be mostly treated as the Ising-type in the process for fitting.The POLY_ANISO program[48]was utilized to fit the DC data of 1 and 2 by employing the parameters listed in Table 2.

Table 2 Fitted Jexch,Jdip,and J values between Dy(Ⅲ)ions in complexes 1 and 2*
The parameters in Table 2 were computed consideringS=1/2 of the Dy (Ⅲ)ion.The schemes of the Dy(Ⅲ)…Dy(Ⅲ)interactions in 1 and 2 are displayed in Fig.S15.During the fitting, theJparameters (total for dipolar and exchange) were involved in the fit of the DC data.The computed and determinedχMTvsTcurves of 1 and 2 are presented in Fig.S16.At low temperatures, the fits agreed with the determined data[49].From Table 2, the Dy1…Dy2 and Dy1…Dy1′ interactions for both complexes are ferromagnetic using the Lines model[50].Somegzvalues for the lowest eight or two doublets, the exchange energies, and the energy difference between each exchange doubletΔtfor 1 and 2 are listed in Table S10.In the ground exchange states, thegzvalues were close to 70, revealing that the interactions between two Dy(Ⅲ)ions are ferromagnetic.Because thegzvalue of a single Dy(Ⅲ)ground state is close to 20, if four ferromagnetic particles are in parallel, it is close to 80, and if they are antiparallel, the result should be 0.Fig.S17 shows the magnetic axes for each Dy(Ⅲ)ion.The angles between Dy1…Dy2 and the magnetic axes were 70.6° and 62.1° for 1 and 2,respectively.
Compared with the previously reported Dy2complexes[26], 1 and 2 showed slightly higher energy barriers than those of the three Dy2complexes,which exhibited energy barriers of 104, 99, 76, and 46 K (two-step relaxation).It can be seen that the distances of Dy1…Dy2 were increased, and the values ofJtotalinteractions and the angles (θ) between the magnetic axis and the line connecting Dy1 and Dy2 ions were also increased,after the introduction of the different benzoates in 1 and 2.However, the energy barriers were not greatly altered.Thus, the geometry of each Dy (Ⅲ)ion may be the main factor determining the energy barriers, as the geometries of the Dy (Ⅲ)ions in 1 and 2 are different from those of the reported Dy2complexes.
3 Conclusions
Two linear tetranuclear complexes[Dy4(L)4(PhCO2)2(NO3)2(EtOH)2] (1) and [Dy4(L)4(2-NO2-PhCO2)2(NO3)2(EtOH)2](2)were prepared employing 2-(((2-hydroxy-3-methoxybenzyl)imino)methyl)-4-methoxyphenol (H2L)as the ligand and aromatic carboxylic acids PhCO2H and 2-NO2-PhCO2H as ancillary ligands.Complexes 1 and 2 are isostructural.The results for AC magnetic studies indicated that the two complexes exhibit SMM properties at zero DC field.Ueff/kBvalues of 110 and 108 K were determined for 1 and 2, respectively.By comparing the relevant structures and magnetic data with the previously reported Dy2complexes supported by the H2L ligand, we found that when the distances of Dy1…Dy2 were increased, the values ofJtotalinteractions and the angles (θ) between the magnetic axis and the lines connecting Dy1 and Dy2 ions were also increased in 1 and 2, after the introduction of different benzoates.Due to the introduction of adjacent nitro groups on the auxiliary ligand in 2, the electronwithdrawing ability is increased.Considering the increase in steric hindrance during the formation of the complex,the angle between Dy1…Dy2 and the magnetic axis in its structure of 2 (62.1°) was slightly smaller than that in 1 (70.6°), resulting in a slightly higher energy barrier of 1.The calculated results provided detailed information about the complexes.Thus, the combination of the experimental results with the calculated outcomes will help us to understand the magnetostructural relationships of 1 and 2 and guide us to develop new SMMs.
Supporting information is available at http://www.wjhxxb.cn

