黃連素通過(guò)抑制氧化應(yīng)激和內(nèi)質(zhì)網(wǎng)應(yīng)激減輕非酒精性脂肪性肝炎小鼠肝臟炎癥
李銳楷?王鵬?李毓琪?尉秀清

【摘要】 目的 從氧化應(yīng)激和內(nèi)質(zhì)網(wǎng)應(yīng)激的角度探討黃連素(BBR)對(duì)非酒精性脂肪性肝炎(NASH)小鼠肝臟炎癥的減輕作用。方法 將24只C57BL/6J小鼠隨機(jī)分為4組,NASH和NASH+BBR組小鼠飼喂高脂高果糖高膽固醇飼料28周以誘導(dǎo)NASH疾病模型,正常維持飼料(CD)和CD+BBR組小鼠給予正常維持飼料,在造模的第25周開(kāi)始給予CD+BBR組和NASH+BBR組每日1次200 mg/(kg·d)的BBR灌胃,治療共持續(xù)4周。記錄小鼠每周體質(zhì)量;給藥第3周時(shí)進(jìn)行葡萄糖耐量實(shí)驗(yàn)和胰島素抵抗實(shí)驗(yàn);治療結(jié)束后檢測(cè)血清ALT、AST、甘油三酯、總膽固醇、LDL-C、HDL-C含量,檢測(cè)肝臟甘油三酯及總膽固醇含量;對(duì)肝臟進(jìn)行HE、油紅O和Masson染色;qPCR法檢測(cè)肝臟組織炎癥因子TNF-α和IL-1β mRNA表達(dá)水平;比色法檢測(cè)肝臟組織氧化應(yīng)激指標(biāo)丙二醛、總超氧化物歧化酶活性和總抗氧化能力水平;蛋白免疫印跡法檢測(cè)肝臟組織內(nèi)質(zhì)網(wǎng)應(yīng)激相關(guān)通路蛋白表達(dá)水平。結(jié)果 成功構(gòu)建NASH小鼠疾病模型。與NASH組小鼠比較,經(jīng)過(guò)BBR治療,抑制NASH+BBR組小鼠體質(zhì)量增加,改善糖耐量異常情況,增加胰島素敏感性,改善肝功能及高脂血癥(P均< 0.05);肝臟病理切片可見(jiàn)脂肪變性減輕,炎性細(xì)胞浸潤(rùn)減少,但纖維化無(wú)改善作用;肝臟炎癥因子mRNA表達(dá)水平下降(P均< 0.05);肝臟組織氧化應(yīng)激因子丙二醛水平下降,抗氧化因子總超氧化物歧化酶活性和總抗氧化能力水平升高(P均< 0.05);內(nèi)質(zhì)網(wǎng)應(yīng)激標(biāo)志物GRP78蛋白表達(dá)量及PERK/eIF2α/ATF4/CHOP內(nèi)質(zhì)網(wǎng)應(yīng)激信號(hào)通路下調(diào)(P均< 0.05)。結(jié)論 BBR治療能夠改善NASH小鼠的超重、糖脂代謝紊亂和肝功能異常問(wèn)題,能夠減輕肝臟脂肪變性及炎癥情況,但對(duì)纖維化無(wú)改善。BBR減輕NASH炎癥反應(yīng)的可能機(jī)制是抑制肝臟的氧化應(yīng)激和內(nèi)質(zhì)網(wǎng)應(yīng)激。
【關(guān)鍵詞】 黃連素;非酒精性脂肪性肝炎;炎癥;氧化應(yīng)激;內(nèi)質(zhì)網(wǎng)應(yīng)激
Berberine alleviates hepatic inflammation of nonalcoholic steatohepatitis mice by inhibiting oxidative stress and endoplasmic reticulum stress Li Ruikai△, Wang Peng, Li Yuqi, Wei Xiuqing.△Department of Gastroenterology, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China
Corresponding author, Wei Xiuqing, E-mail: weixq@mail.sysu.edu.cn
【Abstract】 Objective To evaluate the alleviating effect of berberine (BBR) on hepatic inflammation in nonalcoholic steatohepatitis (NASH) mice from the perspectives of oxidative stress and endoplasmic reticulum stress. Methods A total of 24 C57BL/6J mice were randomly divided into 4 groups. Mice in the NASH and NASH+BBR groups were fed with high-fat, high-fructose, and high-cholesterol diets for 28 weeks to induce the NASH disease models, whereas mice in the CD and CD+BBR groups were given with normal diets, and mice in the CD+BBR and NASH+BBR groups were given with 200 mg/(kg·d) of BBR by gavage once a day starting from the 25th week for 4 consecutive weeks. The body weight of mice was recorded weekly. Glucose tolerance test and insulin resistance test were performed at the 3rd week of administration. Serum alanine aminotransferase, aspartate aminotransferase, triglyceride, total cholesterol, LDL cholesterol, and HDL cholesterol levels, and hepatic triglyceride and total cholesterol levels were measured at the end of treatment. Liver samples were treated with HE, Oil-Red O and Masson staining. The expression levels of liver inflammatory factors of TNF-α and IL-1β mRNA were detected by qPCR. The levels of malondialdehyde, total superoxide dismutase activity and total antioxidant capacity, indicators of oxidative stress in liver tissues were assessed by colorimetric assay. The expression levels of proteins in the endoplasmic reticulum stress-related pathways in liver tissues were detected by Western blot. Results NASH mouse models were successfully established. Compared with the mice in the NASH group, weight gain was inhibited, glucose tolerance abnormality was mitigated, insulin sensitivity was increased and liver function and hyperlipidemia were improved after treatment with BBR in the NASH+BBR group (all P < 0.05). Hepatic pathological slices showed a reduction in steatosis , and a decrease in inflammatory cell infiltration, but there was no significant alleviation in fibrosis. The mRNA expression levels of liver inflammatory factors were down-regulated (all P < 0.05). The levels of hepatic tissue oxidative stress factor of malondialdehyde were decreased, whereas those of antioxidant factors of total superoxide dismutase activity and total antioxidant capacity were increased (all P < 0.05). The expression levels of endoplasmic reticulum stress marker of GRP78 protein and PERK/eIF2α/ATF4/CHOP endoplasmic reticulum stress signaling pathway were significantly down-regulated (all P < 0.05). Conclusions BBR can alleviate overweight, glycolipid metabolism disorder and liver function abnormality in NASH mice. Besides, it can also mitigate hepatic steatosis and inflammation, but it has no significant effect on the alleviation of fibrosis. BBR mitigates inflammatory response in NASH mice probably by suppressing oxidative stress and endoplasmic reticulum stress in the liver.
【Key words】 Berberine; Nonalcoholic steatohepatitis; Inflammation; Oxidative stress; Endoplasmic reticulum stress
代謝相關(guān)脂肪性肝病(MAFLD)曾用名為非酒精性脂肪性肝病(NAFLD),是指在無(wú)過(guò)量飲酒史及明確的肝損傷因素條件下,以肝臟脂肪變性為主要特征的一組慢性疾病,與胰島素抵抗和遺傳易感性密切相關(guān),常與肥胖、2型糖尿病以及代謝綜合征等疾病并存。MAFLD疾病譜包括非酒精性脂肪肝(NAFL)、非酒精性脂肪性肝炎(NASH)及其相關(guān)肝硬化和肝細(xì)胞癌[1-3]。雖然其發(fā)生機(jī)制至今未完全明確,但在目前廣泛接受的“多重打擊”學(xué)說(shuō)發(fā)病機(jī)制中,過(guò)度的氧化應(yīng)激和內(nèi)質(zhì)網(wǎng)應(yīng)激被認(rèn)為是參與NASH發(fā)生發(fā)展的重要機(jī)制[4-6]。
本研究首先通過(guò)飼喂高脂高果糖高膽固醇飼料成功誘導(dǎo)NASH疾病小鼠模型;其次驗(yàn)證了黃連素(BBR)對(duì)NASH小鼠的治療效果;最后從氧化應(yīng)激和內(nèi)質(zhì)網(wǎng)應(yīng)激的角度探討B(tài)BR對(duì)NASH小鼠肝臟炎癥減輕的可能機(jī)制。
材料與方法
一、材 料
1.實(shí)驗(yàn)試劑
高脂高果糖高膽固醇飼料(Research Diets,美國(guó)),BBR(Sigma,美國(guó)),甘油三酯、總膽固醇、丙二醛、總超氧化物歧化酶活性(T-SOD)和總抗氧化能力(T-AOC)測(cè)定試劑盒(Elabscience,武漢),GRP78 BiP Rabbit mAb、Phospho-PERK(Thr982)
Rabbit pAb、PERK Rabbit mAb、Phospho-eIF2α(Ser51)Rabbit mAb、eIF2α Mouse mAb、ATF4 Rabbit pAb、DDIT3/CHOP Rabbit mAb(zen-bio,成都),Anti-beta Actin Rabbit pAb(Servicebio,武漢)。
2.實(shí)驗(yàn)動(dòng)物
24只6~8周齡SPF級(jí)雄性C57BL/6J小鼠,體質(zhì)量20~25 g,購(gòu)自北京維通利華實(shí)驗(yàn)動(dòng)物技術(shù)有限公司,實(shí)驗(yàn)動(dòng)物許可證號(hào):SCXK(浙)2019-0001。該研究通過(guò)大學(xué)動(dòng)物倫理委員會(huì)的批準(zhǔn)(批件號(hào):IACUC-20210112-03)。
二、方 法
1.分組、造模與給藥
小鼠適應(yīng)性喂養(yǎng)7 d后隨機(jī)分為4組,每組6只,分別為正常維持飼料(CD)、CD+BBR、NASH和NASH+BBR組。NASH組和NASH+BBR組小鼠給予28周的高脂高果糖高膽固醇飼料,CD組和CD+BBR組小鼠給予正常維持飼料,在第25周開(kāi)始給予CD+BBR組和NASH+BBR組每日1次
200 mg/(kg·d)的BBR灌胃,CD組和NASH組給予同等體積的體積分?jǐn)?shù)為0.5%羧甲基纖維素鈉混懸液灌胃,治療共持續(xù)4周。記錄小鼠每周體質(zhì)量。
2.葡萄糖耐量實(shí)驗(yàn)(IPGTT)和胰島素抵抗實(shí)驗(yàn)(IPITT)
在給予BBR治療第3周進(jìn)行IPGTT和IPITT以評(píng)估小鼠血糖代謝能力。禁食禁水12 h后,按照體質(zhì)量2 g/kg給予小鼠腹腔注射葡萄糖溶液,從小鼠尾部采血檢測(cè)注射后0、15、30、60、120 min的血糖水平[7]。間隔3 d進(jìn)行IPITT實(shí)驗(yàn),禁食禁水12 h后,按照體質(zhì)量0.8 U/kg給予小鼠腹腔注射短效諾和靈R胰島素,檢測(cè)注射后0、15、30、60、120 min血糖值,并根據(jù)血糖-時(shí)間圖計(jì)算曲線下面積(AUC),單位以毫摩爾/升×分鐘(mmol/L·min)表示[8]。
3.血清和肝臟指標(biāo)測(cè)定
經(jīng)小鼠眼眶后靜脈叢采集全血,室溫靜置1 h后,4 ℃ 1 000×g離心15 min取上層血清,使用全自動(dòng)生化分析儀檢測(cè)ALT、AST、甘油三酯、總膽固醇、LDL-C和HDL-C水平;冰上迅速分離小鼠肝臟進(jìn)行稱重,按照檢測(cè)試劑盒說(shuō)明書(shū)檢測(cè)其甘油三酯、總膽固醇、丙二醛、T-SOD和T-AOC水平。
4.肝臟組織病理學(xué)評(píng)價(jià)
對(duì)小鼠肝臟組織進(jìn)行HE、油紅O和Masson染色,由專業(yè)病理科醫(yī)師閱片,參照2010年《非酒精性脂肪性肝病診療指南》進(jìn)行NAFLD活動(dòng)度積分(NAS)和肝纖維化評(píng)分[9]。肝臟系數(shù)(%)= 肝臟重量(g)/小鼠體質(zhì)量(g)。
5.組織RNA提取和qPCR檢測(cè)
使用TRIzol試劑提取小鼠肝臟組織總RNA,以總RNA為模板反轉(zhuǎn)錄生成cDNA,采用實(shí)時(shí)熒光定量PCR檢測(cè)儀進(jìn)行qPCR。反應(yīng)體系配制及反應(yīng)程序設(shè)置均按照說(shuō)明書(shū)進(jìn)行。目的基因的相對(duì)表達(dá)量結(jié)果使用2-??Ct方法進(jìn)行計(jì)算。
6.總蛋白提取及蛋白免疫印跡
使用總蛋白裂解液提取肝臟總蛋白,BCA蛋白定量法測(cè)量濃度,進(jìn)行聚丙烯酰胺凝膠電泳,應(yīng)用轉(zhuǎn)膜儀將蛋白印跡轉(zhuǎn)移至PVDF膜上,室溫封閉2 h,一抗4 ℃孵育過(guò)夜,對(duì)應(yīng)的二抗室溫孵育2 h,利用化學(xué)發(fā)光法顯像,應(yīng)用Image J圖像分析軟件進(jìn)行灰度分析。
三、統(tǒng)計(jì)學(xué)處理
采用SPSS 26.0統(tǒng)計(jì)軟件進(jìn)行數(shù)據(jù)處理,計(jì)量資料用描述。符合正態(tài)分布的多組間比較采用單因素方差分析,兩兩比較采用LSD法(方差齊時(shí))或Tamhane’T2法(方差不齊時(shí))。P < 0.05為差異有統(tǒng)計(jì)學(xué)意義。
結(jié) 果
一、小鼠體質(zhì)量變化
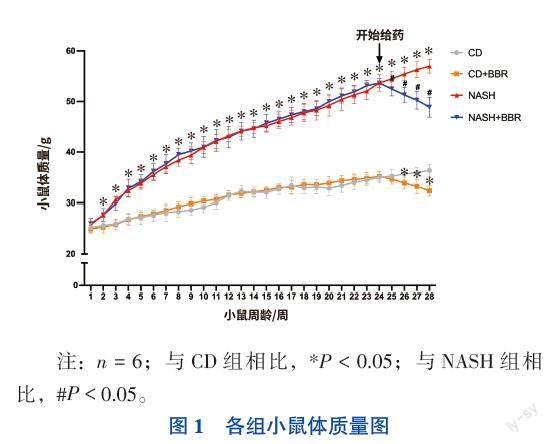
飼喂高脂高果糖高膽固醇飼料的小鼠體質(zhì)量呈上升趨勢(shì),在第2周與CD組小鼠體質(zhì)量相比,NASH組小鼠體質(zhì)量增加具有統(tǒng)計(jì)學(xué)意義(F=9.712,P < 0.05),差異持續(xù)至實(shí)驗(yàn)終點(diǎn)。NASH+BBR組小鼠在經(jīng)過(guò)BBR治療1周后,與NASH組小鼠相比體質(zhì)量下降,差異有統(tǒng)計(jì)學(xué)意義(F=398.797,P < 0.05),CD+BBR組小鼠在治療2周后,體質(zhì)量較CD組相比下降,差異有統(tǒng)計(jì)學(xué)意義(F=427.005,P < 0.05)。見(jiàn)圖1。
二、小鼠糖耐量及胰島素抵抗情況
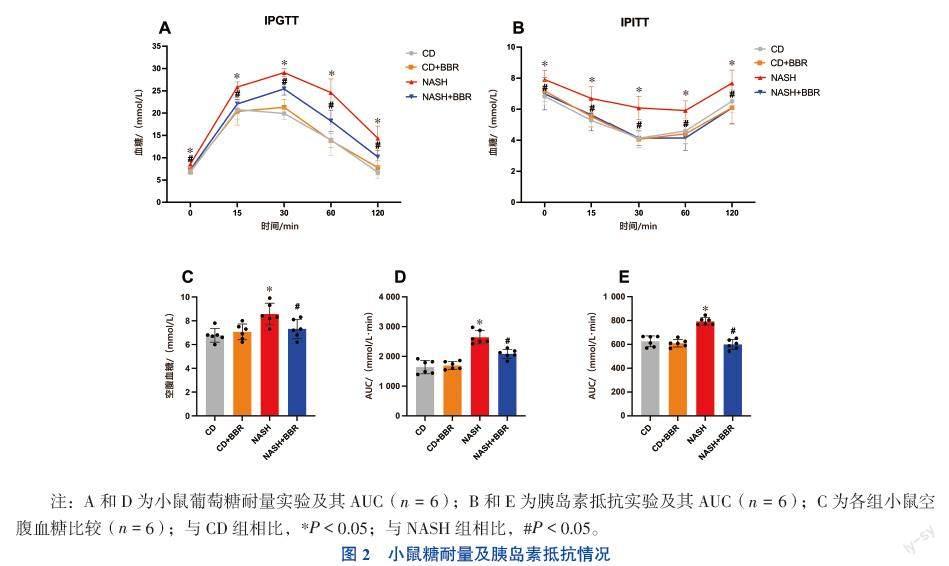
NASH組小鼠空腹血糖水平較CD組升高[(8.57±0.91) mmol/L vs. (6.78±0.58)mmol/L,F(xiàn)=6.647,P < 0.05)],見(jiàn)圖2C。NASH組小鼠在給予葡萄糖溶液注射后,血糖水平峰值及AUC值均高于CD組小鼠(P均< 0.05),而 NASH+BBR組小鼠血糖情況改善(P均< 0.05),見(jiàn)圖2A、D。NASH組小鼠在葡萄糖耐量受損的情況下同時(shí)出現(xiàn)了胰島素抵抗(P均< 0.05),給予BBR治療后,小鼠對(duì)胰島素的敏感性得到改善(P均< 0.05),見(jiàn)圖2B、E。
三、小鼠血清和肝臟生化參數(shù)
與CD組小鼠對(duì)比, NASH組小鼠血清ALT、AST、甘油三酯、總膽固醇和LDL-C水平增加,HDL-C水平降低(P均< 0.05)。與NASH組小鼠對(duì)比,經(jīng)過(guò)4周BBR治療的NASH+BBR組小鼠,血清ALT、AST、甘油三酯、總膽固醇、LDL-C水平下降,血清HDL-C水平上升(P均< 0.05)。見(jiàn)表1。
四、小鼠肝臟組織病理學(xué)評(píng)價(jià)
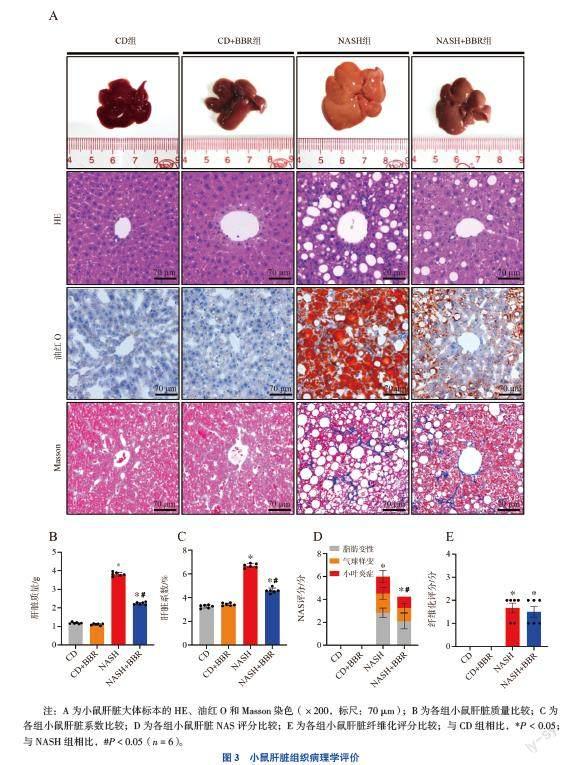
NASH組小鼠肝臟體積增大,呈淡黃色,有油膩感,邊緣圓鈍。鏡下可見(jiàn)NASH特征性病變:大小不等的脂肪空泡和脂滴;部分肝細(xì)胞氣球樣變;小葉內(nèi)混合性炎癥細(xì)胞浸潤(rùn);膠原纖維沉積。而NASH+BBR組小鼠肝臟除膠原纖維沉積情況外,其他病理情況均有所改善。見(jiàn)圖3A。
NASH+BBR組小鼠肝臟質(zhì)量及肝臟系數(shù)較NASH組下降[(2.23±0.08)g vs.(3.61±0.14)g,P < 0.05;(6.17±0.17)% vs.(8.25±0.34)%,P < 0.05)]。見(jiàn)圖3B、C。NASH+BBR組小鼠NAS評(píng)分降低(6.00±0.89 vs. 4.25±0.76,P < 0.05),但間質(zhì)纖維組織增生情況沒(méi)有改善,纖維化評(píng)分未見(jiàn)統(tǒng)計(jì)學(xué)差異(1.67±0.52 vs. 1.50±0.55,P > 0.05)。見(jiàn)圖3D、E。
五、小鼠肝臟組織炎癥因子指標(biāo)
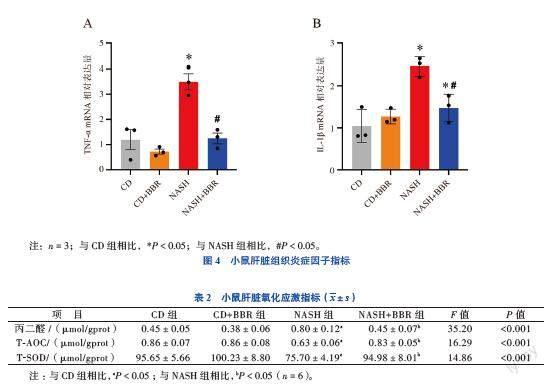
與CD組小鼠比較,NASH組小鼠肝臟組織中炎癥因子TNF-α和IL-1β的mRNA表達(dá)水平均升高(FIL-1β=13.508,F(xiàn)TNF-α=19.458,P均< 0.05);NASH+
BBR組小鼠肝臟組織中炎癥因子TNF-α和IL-1β的mRNA表達(dá)水平均降低(P均< 0.05)。見(jiàn)圖4。
六、小鼠肝臟組織氧化應(yīng)激指標(biāo)
與CD組小鼠比較,NASH組小鼠肝臟組織中丙二醛水平升高(P < 0.05),T-AOC水平和T-SOD活性降低(P均< 0.05);而與NASH組比較,NASH+BBR組小鼠肝臟組織中丙二醛水平降低(P < 0.05),T-SOD活性和T-AOC水平升高(P均< 0.05)。見(jiàn)表2。
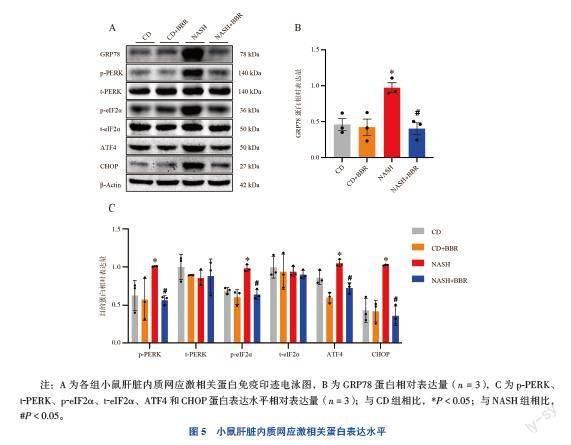
七、小鼠肝臟內(nèi)質(zhì)網(wǎng)應(yīng)激相關(guān)蛋白表達(dá)水平
與CD組比較,NASH組小鼠肝臟內(nèi)質(zhì)網(wǎng)應(yīng)激反應(yīng)標(biāo)志物GRP78蛋白和內(nèi)質(zhì)網(wǎng)應(yīng)激相關(guān)PERK信號(hào)通路蛋白p-PERK、p-eIF2α、ATF4和CHOP蛋白表達(dá)水平均升高(FGRP78=9.800,F(xiàn)p-PERK=4.596,F(xiàn)p-eIF2α=
20.558,F(xiàn)ATF4=22.760,F(xiàn)CHOP=19.191,P均< 0.05);NASH+BBR組小鼠肝臟組織中GRP78、p-PERK、p-eIF2α、ATF4和CHOP蛋白表達(dá)水平相較NASH組小鼠均降低(P均< 0.05)。見(jiàn)圖5。
討 論
MAFLD作為全球慢性肝病的主要原因,與肝細(xì)胞癌和死亡等不良預(yù)后相關(guān),亞洲的總體患病率已經(jīng)超過(guò)29%,其臨床負(fù)擔(dān)日益增加[10-11]。從NAFL到NASH的轉(zhuǎn)變是肝臟損傷進(jìn)展的重要一步,在如今被廣泛認(rèn)可的“多重打擊”學(xué)說(shuō)中,認(rèn)為脂毒性、氧化應(yīng)激、內(nèi)質(zhì)網(wǎng)應(yīng)激、炎性細(xì)胞因子等多重打擊因素,可使肝脂肪變性向脂肪性肝炎發(fā)展。 所以抗氧化應(yīng)激和抗內(nèi)質(zhì)網(wǎng)應(yīng)激也成為NASH治療的可能策略[12-14]。
BBR亦稱為小檗堿,是最初從中草藥黃連分離出來(lái)的一種季銨型異喹啉類(lèi)生物堿,多年來(lái)的臨床研究已證明BBR對(duì)MAFLD具有積極的治療作用,但其機(jī)制暫未完全明確[15-18]。因此,本研究首先驗(yàn)證了BBR對(duì)于NASH小鼠的治療作用,結(jié)果表明給予BBR治療,可以減輕NASH小鼠的體質(zhì)量、改善其糖耐量受損和胰島素抵抗問(wèn)題,還可以降低NASH小鼠的高脂血癥,改善肝功能和減輕肝臟脂肪變性。這一結(jié)果與先前他人所報(bào)道的研究結(jié)果相符[19-21]。
其次我們通過(guò)qPCR檢測(cè)和NAS評(píng)分,證實(shí)BBR不僅可以減少肝臟炎癥因子TNF-α和IL-1β mRNA的表達(dá)水平,而且可以減少肝臟組織中小葉內(nèi)炎癥細(xì)胞的浸潤(rùn),但對(duì)NASH纖維化并無(wú)改善作用。而B(niǎo)BR如何減輕肝臟的炎癥反應(yīng)具體機(jī)制需進(jìn)一步深入研究。
氧化應(yīng)激及內(nèi)質(zhì)網(wǎng)應(yīng)激作為機(jī)體的保護(hù)性應(yīng)激反應(yīng),與NF-κB途徑、NLRP3炎癥小體和TLR4途徑等炎癥反應(yīng)信號(hào)通路相偶聯(lián),通過(guò)引發(fā)炎癥反應(yīng)廣泛參與炎癥性疾病的病理過(guò)程,而炎癥反應(yīng)又會(huì)使氧化應(yīng)激及內(nèi)質(zhì)網(wǎng)應(yīng)激增強(qiáng)[22-26]。本研究從氧化應(yīng)激和內(nèi)質(zhì)網(wǎng)應(yīng)激的角度對(duì)BBR減輕肝臟炎癥反應(yīng)的可能機(jī)制進(jìn)行探討。
肝細(xì)胞過(guò)度蓄積的游離脂肪酸會(huì)使線粒體氧化反應(yīng)增加,當(dāng)氧化反應(yīng)產(chǎn)生的活性氧物質(zhì)超過(guò)細(xì)胞抗氧化能力時(shí),便會(huì)引起氧化應(yīng)激。我們通過(guò)測(cè)量氧化應(yīng)激因子丙二醛水平和體內(nèi)清除自由基的主要抗氧化因子T-SOD和T-AOC水平,發(fā)現(xiàn) NASH小鼠肝臟組織細(xì)胞的氧化應(yīng)激水平上升,而B(niǎo)BR治療可以減輕NASH小鼠肝臟組織細(xì)胞的氧化應(yīng)激水平。
肝細(xì)胞內(nèi)過(guò)多的游離脂肪酸還會(huì)通過(guò)破壞內(nèi)質(zhì)網(wǎng)結(jié)構(gòu)及鈣離子穩(wěn)態(tài),使內(nèi)質(zhì)網(wǎng)激活未折疊蛋白反應(yīng)(UPR),從而誘發(fā)內(nèi)質(zhì)網(wǎng)應(yīng)激以減少細(xì)胞內(nèi)異常蛋白的聚集,在UPR中PERK信號(hào)通路起到重要的作用[27-29]。我們的研究結(jié)果表明,NASH組小鼠肝臟組織中內(nèi)質(zhì)網(wǎng)應(yīng)激標(biāo)志蛋白GRP78蛋白水平表達(dá)升高,提示NASH小鼠肝臟組織細(xì)胞內(nèi)質(zhì)網(wǎng)處于應(yīng)激狀態(tài);而NASH+BBR組小鼠肝臟GRP78蛋白水平表達(dá)下降,表明BBR治療可以減輕內(nèi)質(zhì)網(wǎng)應(yīng)激,隨后我們檢測(cè)PERK信號(hào)通路的蛋白表達(dá),發(fā)現(xiàn)BBR治療可通過(guò)下調(diào)
PERK/eIF2α/ATF4/CHOP信號(hào)通路的蛋白表達(dá),從而減輕NASH小鼠肝臟的內(nèi)質(zhì)網(wǎng)應(yīng)激。
綜上所述,BBR治療有益于NASH小鼠體質(zhì)量控制、改善糖脂代謝紊亂、減輕肝功能異常,緩解肝臟脂肪變性及炎癥情況,但對(duì)纖維化無(wú)改善。緩解肝臟炎癥情況的可能機(jī)制是抑制氧化應(yīng)激和內(nèi)質(zhì)網(wǎng)應(yīng)激PERK/eIF2α/ATF4/CHOP信號(hào)通路,從而起到保護(hù)肝臟的積極作用。
參 考 文 獻(xiàn)
[1] Bessone F, Razori M V, Roma M G. Molecular pathways of nonalcoholic fatty liver disease development and progression[J]. Cell Mol Life Sci, 2019, 76(1): 99-128.
[2] Kumar S, Duan Q, Wu R, et al. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis[J]. Adv Drug Deliv Rev, 2021, 176: 113869.
[3] Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans[J]. Mol Metab, 2021, 50: 101122.
[4] Farrell G C, Haczeyni F, Chitturi S. Pathogenesis of NASH: how metabolic complications of overnutrition favour lipotoxicity and pro-inflammatory fatty liver disease[J]. Adv Exp Med Biol, 2018, 1061: 19-44.
[5] Gabbia D, Cannella L, De Martin S. The role of oxidative stress in NAFLD-NASH-HCC transition-focus on NADPH oxidases[J]. Biomedicines, 2021, 9(6): 687.
[6] Lebeaupin C, Vallée D, Hazari Y, et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease[J]. J Hepatol, 2018, 69(4): 927-947.
[7] Andrikopoulos S, Blair A R, Deluca N, et al. Evaluating the glucose tolerance test in mice[J]. Am J Physiol Endocrinol Metab, 2008, 295(6): E1323-E1332.
[8] Tang H, Zeng Q, Tang T, et al. Kaempferide improves glycolipid metabolism disorder by activating PPARγ in high-fat-diet-fed mice[J]. Life Sci, 2021, 270: 119133.
[9] 中華醫(yī)學(xué)會(huì)肝臟病學(xué)分會(huì)脂肪肝和酒精性肝病學(xué)組. 非酒精性脂肪性肝病診療指南[J]. 中國(guó)肝臟病雜志(電子版), 2010, 2(4): 43-48.
Fatty Liver and Alcoholic Liver Disease Group, Liver Disease Branch, Chinese Medical Association.Guidelines for diagnosis and treatment of nonalcoholic fatty liver disease[J]. Chin J Liver Dis Electron Version, 2010, 2(4): 43-48.
[10] Li J, Zou B, Yeo Y H, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2019, 4(5): 389-398.
[11] De Roza M A, Goh G B B. The increasing clinical burden of NAFLD in Asia[J]. Lancet Gastroenterol Hepatol, 2019, 4(5): 333-334.
[12] Wang Y, Zhou X, Zhao D, et al. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes[J]. PLoS One, 2020, 15(5): e0232630.
[13] Chen Z, Tian R, She Z, et al. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease[J]. Free Radic Biol Med, 2020, 152: 116-141.
[14] Mak K K, Zhang S, Chellian J, et al. Swietenine alleviates nonalcoholic fatty liver disease in diabetic mice via lipogenesis inhibition and antioxidant mechanisms[J]. Antioxidants, 2023, 12(3): 595.
[15] Zhou M, Deng Y, Liu M, et al. The pharmacological activity of berberine, a review for liver protection[J]. Eur J Pharmacol, 2021, 890: 173655.
[16] Suárez M, Boqué N, Del Bas J M, et al. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease[J]. Nutrients, 2017, 9(10): 1052.
[17] Bansod S, Saifi M A, Godugu C. Molecular updates on berberine in liver diseases: bench to bedside[J]. Phytother Res, 2021, 35(10): 5459-5476.
[18] 林峰, 吳潔, 王曉, 黃引平, 等. 黃連素改善妊娠期糖尿病大鼠胰島素抵抗及其機(jī)制研究[J]. 中華全科醫(yī)學(xué), 2019, 17(10): 1647-1651.
Lin F, Wu J, Wang X, et al. Experimental study of berberine improving insulin resistance in gestational diabetes mellitus rat model and its possible mechanisms[J]. Chin J Gen Pract, 2019, 17(10): 1647-1651.
[19] Wei X, Wang C, Hao S, et al. The therapeutic effect of berberine in the treatment of nonalcoholic fatty liver disease: a meta-analysis[J]. Evid Based Complementary Altern Med, 2016, 2016: 3593951.
[20] Cicero A F G, Colletti A, Bellentani S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients, 2018, 10(9): 1153.
[21] Sun Y, Xia M, Yan H, et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21[J]. Br J Pharmacol, 2018, 175(2): 374-387.
[22] Farzanegi P, Dana A, Ebrahimpoor Z, et al. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation[J]. Eur J Sport Sci, 2019, 19(7): 994-1003.
[23] Peng M L, Fu Y, Wu C W, et al. Signaling pathways related to oxidative stress in diabetic cardiomyopathy[J]. Front Endocrinol, 2022, 13: 907757.
[24] Deng J, Lu P D, Zhang Y, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2[J]. Mol Cell Biol, 2004, 24(23): 10161-10168.
[25] Liu C M, Zheng G H, Ming Q L, et al. Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway[J]. Free Radic Res, 2013, 47(3): 192-201.
[26] 雷一鳴, 楊逸冬, 譚嗣偉, 等. TNF-α通過(guò)內(nèi)質(zhì)網(wǎng)應(yīng)激信號(hào)通路誘導(dǎo)肝癌細(xì)胞自噬并促進(jìn)增殖的研究[J]. 新醫(yī)學(xué), 2017, 48(11): 770-774.
Lei Y M, Yang Y D, Tan S W, et al. TNF-α induces autophagy and promotes proliferation of liver cancer cells via ER stress signaling pathway[J]. J New Med, 2017, 48(11): 770-774.
[27] Wang J, He W, Tsai P J, et al. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease[J]. Lipids Health Dis, 2020, 19(1): 72.
[28] Li T, Chen Y, Tan P, et al. Dihydroartemisinin alleviates steatosis and inflammation in nonalcoholic steatohepatitis by decreasing endoplasmic reticulum stress and oxidative stress[J]. Bioorg Chem, 2022, 122: 105737.
[29] Kopp M C, Larburu N, Durairaj V, et al. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor[J]. Nat Struct Mol Biol, 2019, 26(11): 1053-1062.
(收稿日期:2023-09-25)
(本文編輯:楊江瑜)

