人參皂苷Rg1拮抗亞砷酸鈉誘導C57BL/6小鼠腎毒性研究
楊淵 宋爽 陳容 劉永蓮 劉春燕


摘要:目的 探討人參皂苷Rg1對亞砷酸鈉(SA)誘導小鼠腎臟毒性的干預效應。方法 20只雄性健康C57BL/6小鼠采用隨機數字表法均分為對照組(給予去離子水灌胃)、SA染毒組(10.0 μg/g SA進行灌胃)、人參皂苷Rg1+SA染毒組(20.0 μg/g人參皂苷Rg1在SA染毒前8 h腹腔注射+10.0 μg/g SA灌胃)、人參皂苷Rg1對照組(20.0 μg/g人參皂苷Rg1腹腔注射)。以上各組均隔天給予相應處理1次,持續14 d。HE染色觀察腎組織病理改變并進行腎小管損傷(TI)評分;酶聯免疫吸附試驗(ELISA)檢測血清肌酐(Scr)和腎組織谷胱甘肽(GSH)、血紅素加氧酶-1(HO-1)、丙二醛(MDA)含量;免疫印跡試驗檢測腎組織HO-1、磷酸化哺乳動物雷帕霉素靶蛋白(p-mTOR)、泛素結合蛋白P62(SQSTM1/p62)、unc-51樣激酶-1(ULK1)和微管相關蛋白輕鏈3B(LC3-B)表達水平;免疫熒光染色檢測LC3-B水平。結果 與對照組相比,SA染毒組小鼠TI評分、Scr和腎組織MDA、ULK1和LC3-B表達水平升高,腎組織GSH和HO-1、p-mTOR和SQSTM1/p62表達水平降低(P<0.05),呈紅色斑點的LC3-B染色強度增強、增多;與SA染毒組相比,人參皂苷Rg1+SA染毒組TI評分、Scr和腎組織MDA、ULK1和LC3-B表達水平降低,而GSH、HO-1、p-mTOR和SQSTM1/p62表達水平升高(P<0.05),LC3-B免疫熒光染色強度減弱、減少。結論 人參皂苷Rg1拮抗SA誘導的小鼠腎毒性,可能與HO-1信號激活和細胞自噬抑制有關。
關鍵詞:人參皂苷Rg1;砷中毒;血紅素加氧酶-1;自噬;腎毒性
中圖分類號:R114文獻標志碼:ADOI:10.11958/20221834
Study of ginsenoside Rg1 antagonizes sodium arsenite-induced
nephrotoxicity in C57BL/6 mice
YANG Yuan SONG Shuang CHEN Rong LIU Yonglian LIU Chunyan
1 Department of Toxicology, School of Public Health, Guizhou Medical University, Guiyang 550025, China;
2 Ethnic Medicine Research Center, Hunan University of Medicine
Abstract: Objective To investigate the intervention effect of ginsenoside Rg1 (Rg1) against sodium arsenite (SA) induced nephrotoxicity in mice. Methods Twenty healthy male C57BL/6 mice were randomly divided into the control group (given deionized water by gavage), the SA exposure group (10.0 μg/g SA by gavage), the Rg1 intervention+SA exposure group (20.0 μg/g Rg1 was injected intraperitoneally 8 hours before SA exposure+10.0 μg/g SA gavage) and the Rg1 control group (20.0 μg/g Rg1 intraperitoneal injection). All of groups were given corresponding treatment once every other day for 14 days. HE staining was performed to observe pathological changes of renal tissue and renal tubular injury (TI) score. Serum creatinine (Scr) and renal glutathione (GSH), heme oxygenase-1 (HO-1) and malondialdehyde (MDA) were detected by enzyme-linked immunosorbent assay (ELISA). The expression levels of HO-1, phosphorylated mammalian target of rapamycin (p-mTOR), ubiquitin-binding protein P62 (SQSTM1/p62), unc-51-like kinase-1 (ULK1) and microtubule-associated protein light chain 3B (LC3-B) in renal tissue were detected by Western blot assay. LC3-B levels were detected by immunofluorescence staining. Results Compared with the control group, the TI score, Scr and expression levels of MDA, ULK1 and LC3-B in renal tissue were increased in the SA group, while expression levels of GSH and HO-1, p-mTOR and SQSTM1/p62 in renal tissue were decreased (P<0.05). The staining intensity of red spot LC3-B was enhanced and increased. Compared with the SA group, TI score, Scr and expression levels of MDA, ULK1 and LC3-B in renal tissue were decreased in the Rg1 +SA group, while expression levels of GSH, HO-1, p-mTOR and SQSTM1/p62 were increased (P<0.05). The immunofluorescence staining intensity of LC3-B was weakened and decreased. Conclusion Rg1 antagonizes SA-induced nephrotoxicity in mice, which may be associated with the activation of HO-1 signal and the inhibition of autophagy.
Key words: Ginsenoside Rg1; arsenic poisoning; heme oxygenase-1; autophagy; nephrotoxicity
砷在環境中通常以三價化合物形式存在,可污染土壤、飲用水和農作物,在一定條件下暴露于機體,可導致腎小球或腎小管組織病理學損傷、腎功能障礙[1-2]。既往研究發現,砷暴露可誘導骨肉瘤細胞或雞睪丸組織細胞凋亡和自噬,與細胞內蛋白激酶B(protein kinase B,Akt/PKB)、哺乳動物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信號通路抑制有關,而活性氧(ROS)清除劑可明顯拮抗砷的毒性效應[3-4]。中藥人參(Panax ginseng C.A.Mey.)屬于五加科的多年生草本植物,其主要生物活性成分是人參多糖和人參皂苷。研究發現,人參多糖提取物具有抗氧化作用,能有效減輕順鉑誘導的小鼠急性腎損傷[5]。人參皂苷Rg1是人參主要藥物活性成分提取物,目前市售最為常見。其除增強機體的抗氧化活性外[6],還具有激活mTOR信號通路[7]、抗炎、抑制細胞凋亡和自噬的作用[8-9]。目前,人參皂苷Rg1對砷誘導腎毒性的干預效應尚不清楚。本研究旨在探討人參皂苷Rg1對亞砷酸鈉(sodium arsenite,SA)誘導小鼠腎毒性的拮抗效應及機制。
1 材料與方法
1.1 實驗動物
20只雄性SPF級C57BL/6小鼠購自湖南長沙斯萊克實驗動物有限公司,動物生產許可證號:SCXK(湘)2019-0004,使用許可證號:SYXK(湘)2019-0017。小鼠體質量24.1~26.8 g,平均(25.2±1.1)g,9~10周齡。在標準飼養條件(12 h光照/黑暗、溫度20~22 ℃和相對濕度60%~70%)適應性喂養1周。
1.2 主要試劑及儀器
亞砷酸鈉(NaAsO2)購自美國Sigma-Aldrich公司;人參皂苷Rg1購自上海篤瑪生物科技有限公司;血清肌酐(Scr)酶聯免疫吸附測定(ELISA)試劑盒購自上海篤瑪生物技術有限公司;丙二醛(malondialdehyde,MDA)、谷胱甘肽(glutathione,GSH)、血紅素加氧酶1(heme oxygenase 1,HO-1)酶聯免疫吸附試驗(ELISA)試劑盒均購自上海酶聯生物科技有限公司;RIPA裂解緩沖液購自美國BioVision公司;Bradford試劑購自美國Sigma-Aldrich公司;兔單克隆HO-1抗體、兔單克隆mTOR抗體與磷酸化mTOR抗體(p-mTOR)、兔多克隆酵母自噬基因Atg1同源物unc-51樣激酶-1(unc-51-like kinase-1,ULK1)抗體、泛素結合蛋白p62(sequestosome 1,SQSTM1/p62)抗體、微管相關蛋白輕鏈3B(microtubule associated protein light chain 3B,LC3-B)抗體均購自英國Abcam公司;兔抗辣根過氧化物酶(HRP)標記IgG二抗購自美國LSBio公司;化學發光試劑盒購自上海碧云天生物公司;酶標儀購自美國BioTek公司;Motic光學顯微鏡購自麥克奧迪公司。
1.3 分組
采用隨機數字表法將小鼠分為4組,每組5只。對照組:給予去離子水灌胃,隔天1次,持續14 d;SA染毒組:NaAsO2溶于去離子水,參考文獻[10-11]及大小鼠等效劑量換算比值,以10.0 μg/g劑量進行灌胃,隔天1次,持續14 d;人參皂苷Rg1+SA染毒組:參考文獻[12-13],以人參皂苷Rg1 20.0 μg/g,在SA染毒前8 h進行腹腔注射干預,然后給予SA10.0 μg/g灌胃處理;人參皂苷Rg1對照組:人參皂苷Rg1用去離子水稀釋,以20.0 μg/g劑量腹腔注射,隔天1次,持續14 d。實驗結束時,腹腔注射0.9%戊巴比妥鈉麻醉小鼠并實施安樂死,立即收集血液和腎組織進行分析。本動物研究方案經貴州醫科大學倫理委員會批準(批準號:1900222),根據動物實驗:報告體內實驗(Animal Research:Reporting of in vivo Experiments,ARRIVE)指南的原則執行本實驗方案。
1.4 腎功能和組織病理學評價
取0.5 mL血后以4 ℃、400 r/min離心5 min,根據ELISA試劑盒操作說明檢測上清液Scr含量。腎組織經4%多聚甲醛固定72 h后,用石蠟包埋以制備5 μm病理切片,然后在顯微鏡下行蘇木精-伊紅(HE)染色,并進行腎小管損傷(TI)評分[14]。TI定義為:腎小管擴張,管狀上皮腫脹,細胞呈空泡樣或顆粒樣變性、壞死,刷狀緣缺失或呈管型結構。通過顯微鏡觀察腎組織切片中腎小管病變區域,評估每個高倍視野下病變區域的損傷面積百分率(%),每張切片計算10個高倍視野下TI評分平均值。
1.5 ELISA檢測腎組織HO-1、GSH和MDA含量
腎組織分離并稱質量,按1∶9質量體積比比例加入磷酸鹽緩沖液(PBS)配制成0.1 g/mL,使用勻漿器進行勻漿,然后加入RPMI培養基(含有0.05%Ⅱ型膠原酶,0.002%DNase Ⅰ和0.6%胎牛血清),37 ℃下孵育30 min后,進行3次凍融循環以裂解腎組織細胞。最后,組織勻漿液4 ℃冷凍離心10 min(1 500 r/min),收集上清液,參照ELISA試劑盒操作說明書,使用酶標儀在450 nm波長下測量光密度(OD)值,并根據標準曲線將OD值轉換為腎組織MDA、GSH和HO-1含量。
1.6 免疫印跡試驗檢測腎組織HO-1、p-mTOR、ULK1、SQSTM1/p62和LC3-B表達
腎組織分離后加入RIPA裂解緩沖液以裂解細胞提取總蛋白,使用Bradford試劑測定蛋白質濃度,腎組織樣品通過十二烷基硫酸鈉-聚丙烯酰胺凝膠電泳并轉移到聚乙烯亞胺(PVD)膜上。隨后使用5%脫脂奶粉室溫搖床封閉2 h以阻斷非特異性結合位點后,將膜與HO-1(1∶1 500)、p-mTOR(1∶1 000)、mTOR(1∶1 500)、ULK1(1∶500)、SQSTM1/p62(1∶800)、LC3-B抗體(1∶400)于4 ℃孵育16 h。TBS-T緩沖液洗膜3次后,加入二抗HRP IgG 室溫孵育2 h,然后加入化學發光顯影試劑成像。通過目標靶蛋白與抗β-肌動蛋白內參抗體的吸光度比值來評估靶蛋白表達相對水平。
1.7 免疫熒光染色檢測LC3-B表達
腎組織分離后加入4%多聚甲醛溶液,4 ℃過夜固定,制備5 μm切片,切片經脫蠟、水化、洗滌后,進行高壓抗原修復和山羊血清封閉,然后與LC3-B一抗在4 ℃孵育過夜。PBS緩沖液洗滌3次后,與HRP標記IgG二抗在37 ℃孵育40 min,PBS再次洗滌3次,然后在室溫下用4',6-二脒基-2-苯基吲哚(DAPI)復染5 min,再次洗滌切片后,通過共聚焦顯微鏡(LeicaTCS-SP5)進行熒光成像觀察(Alexa Fluor? 555發射波長為535 nm,DAPI發射波長為340 nm)。
1.8 統計學方法
采用SPSS 23.0進行數據分析,計量資料以均數±標準差(x±s)表示,多組間比較采用單因素方差分析,組間多重比較采用LSD-t檢驗,P<0.05為差異有統計學意義。
2 結果
2.1 各組腎臟組織病理損傷比較
對照組腎組織形態正常,腎小管上皮細胞核膜光滑,細胞質染色均勻,核質比正常,細胞間距正常;與對照組相比,SA染毒組腎小球球囊增大,毛細血管充血,腎小管管腔不規則,小管上皮細胞水腫,部分核消失,Scr和TI評分明顯升高(P<0.05)。與SA染毒組相比,人參皂苷Rg1+SA染毒組腎小球毛細血管充血、腎小管上皮細胞和腎間質水腫改善,Scr和TI評分下調(P<0.05)。人參皂苷Rg1對照組腎組織形態正常,與對照組類似。見圖1、表1。
2.2 各組腎組織氧化應激指標比較
與對照組相比,SA染毒組小鼠腎組織GSH和HO-1表達水平降低,MDA表達水平升高(P<0.05);與SA染毒組相比,人參皂苷Rg1+SA染毒組小鼠腎組織GSH和HO-1細胞水平升高,而MDA細胞水平降低(P<0.05),見表1。
2.3 各組腎組織中HO-1、p-mTOR、SQSTM1/p62、ULK1和LC3-B水平比較
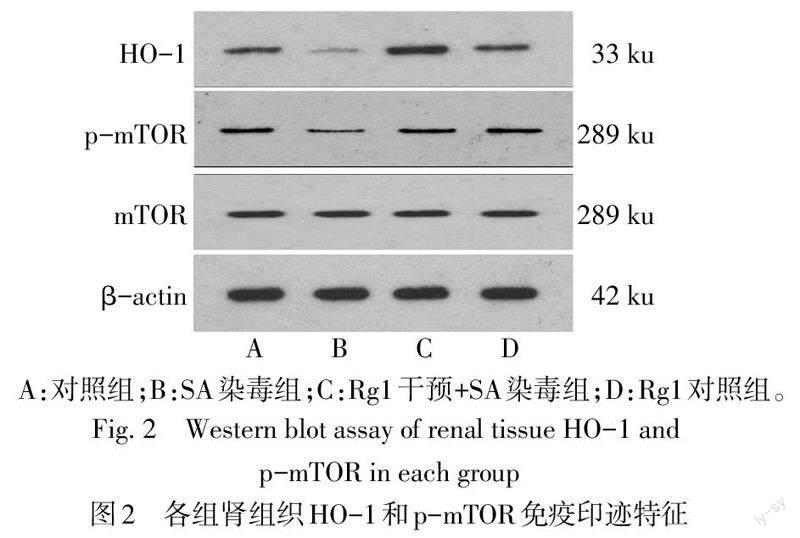
與對照組相比,SA染毒組小鼠腎組織中HO-1、p-mTOR和SQSTM1/p62表達水平降低,ULK1和LC3-B表達水平升高(P<0.05)。與SA染毒組相比,人參皂苷Rg1+SA染毒組HO-1、p-mTOR和SQSTM1/p62水平上調,ULK1和LC3-B水平降低(P<0.05),見圖2、3,表2。腎組織免疫熒光染色顯示,與對照組相比,SA染毒組小鼠腎組織呈紅色斑點的LC3-B染色強度增強、增多。與SA染毒組相比,人參皂苷Rg1+SA染毒組顯示LC3-B免疫熒光染色強度減弱、減少,見圖4。
3 討論
砷是一種環境中廣泛存在的高毒性致癌物,通常由于含砷礦開采冶煉、含砷農藥生產使用等活動導致環境砷污染,通過直接接觸或食物鏈攝入過量砷可導致急慢性砷中毒發生,產生肝、腎、皮膚、神經系統等多器官損害效應[15]。本研究發現,急性砷暴露可導致小鼠腎臟組織病理損傷,表現為Scr、腎小管損傷評分和氧化應激產物MDA水平升高,而腎組織抗氧化劑GSH和HO-1含量降低,提示氧化應激在砷致腎毒性中扮演著重要的角色。人參作為一種廣泛使用的傳統中藥,其生物活性提取物富含與多糖結合的甾體皂苷,其用于中樞神經系統、代謝、感染和腫瘤等疾病治療已有約5 000年歷史[16]。研究發現,人參皂苷Rg1具有上調GSH和下調MDA的抗氧化應激效應[17],具有抑制小鼠NOD樣受體熱蛋白結構域相關蛋白3(NOD-like receptor thermal protein domain associated protein 3,NLRP3)炎癥信號激活及改善腎臟衰老期腎小球纖維化效應[18]。Fan等[19]發現,人參皂苷Rg1可拮抗D-半乳糖誘導的小鼠亞急性腎組織損傷。Guo等[20]發現人參皂苷Rg1可通過抑制腎小管上皮細胞鐵死亡而改善膿毒癥誘導的急性腎損傷。本研究中,與SA染毒組相比,人參皂苷Rg1干預+SA染毒組小鼠腎組織損傷程度明顯減輕,Scr、腎小管損傷評分和腎組織MDA水平降低,HO-1和GSH水平升高,而自噬標志物ULK1和LC3-B水平降低以及自噬反應底物SQSTM1/p62蛋白水平升高,提示HO-1激活、自噬抑制與人參皂苷Rg1拮抗砷誘導小鼠腎毒性效應有關。
HO-1是機體內廣泛分布的一種重要的抗氧化酶,與細胞內絲氨酸或蘇氨酸激酶家族mTOR信號激活有關。乙醇可誘導人食管鱗癌細胞HO-1水平上調,伴隨著p38絲裂原活化蛋白激酶(p38MAPK)和mTOR信號激活[21];HO-1可與甾醇異構酶相互作用,從而激活mTOR信號通路以減輕膽固醇誘導的心肌細胞缺氧[22]。mTOR信號通路激活后,可磷酸化ULK1第757位絲氨酸并阻止ULK1激活,破壞ULK1與腺苷酸活化蛋白激酶的相互作用,從而抑制細胞自噬[23]。反之,mTOR信號抑制可導致下游靶p70核糖體蛋白S6激酶和真核起始因子4E結合蛋白1磷酸化抑制,導致細胞內基因-蛋白質翻譯減少、細胞生長周期及細胞增殖抑制,從而激活細胞自噬[24]。在青蒿琥酯染毒的類風濕性關節炎小鼠模型中,抑制mTOR信號通路可加速軟骨細胞自噬[25]。本研究發現,與SA染毒組相比,人參皂苷Rg1+SA染毒組腎組織p-mTOR水平升高,因此推測人參皂苷Rg1可誘導HO-1介導的mTOR信號激活,促進細胞生長及增殖、抑制腎組織細胞自噬以改善SA誘導的腎組織損傷。
綜上,本研究發現人參皂苷Rg1通過激活HO-1信號介導抗氧化應激,并激活mTOR信號介導的細胞自噬抑制效應減輕SA誘導小鼠腎臟病理損傷。目前,人參皂苷Rg1拮抗砷誘導的腎毒性作用機制尚未完全清楚,人參皂苷Rg1激活HO-1和mTOR信號通路以及抑制細胞自噬的分子調控機制需要進一步探索。
參考文獻
[1] ROBLES-OSORIO M L,SABATH-SILVA E,SABATH E. Arsenic-mediated nephrotoxicity[J]. Ren Fail,2015,37(4):542-547. doi:10.3109/0886022X.2015.1013419.
[2] JALILI C,KAZEMI M,CHENG H,et al. Associations between exposure to heavy metals and the risk of chronic kidney disease: a systematic review and meta-analysis[J]. Crit Rev Toxicol,2021,51(2):165-182. doi:10.1080/10408444.2021.1891196.
[3] WANG G,ZHANG T,SUN W,et al. Arsenic sulfide induces apoptosis and autophagy through the activation of ROS/JNK and suppression of Akt/mTOR signaling pathways in osteosarcoma[J]. Free Radic Biol Med,2017,106:24-37. doi:10.1016/j.freeradbiomed.2017.02.015.
[4] SHAO Y Z,ZHAO H J,WANG Y,et al. The apoptosis in arsenic-induced oxidative stress is associated with autophagy in the testis tissues of chicken[J]. Poult Sci,2018,97(9):3248-3257. doi:10.3382/ps/pey156.
[5] WEI X M,JIANG S,LI S S,et al. Endoplasmic reticulum stress-activated PERK-eIF2α-ATF4 signaling pathway is involved in the ameliorative effects of Ginseng polysaccharides against cisplatin-induced nephrotoxicity in mice[J]. ACS Omega,2021,6(13):8958-8966. doi:10.1021/acsomega.0c06339.
[6] XIE W,ZHOU P,SUN Y,et al. Protective effects and target network analysis of Ginsenoside Rg1 in cerebral ischemia and reperfusion injury:a comprehensive overview of experimental studies[J]. Cells,2018,7(12):270. doi:10.3390/cells7120270.
[7] XU X,QU Z,QIAN H,et al. Ginsenoside Rg1 ameliorates reproductive function injury in C57BL/6J mice induced by di-N-butyl-phthalate[J]. Environ Toxicol,2021,36(5):789-799. doi:10.1002/tox.23081.
[8] NI X J,XU Z Q,JIN H,et al. Ginsenoside Rg1 protects human renal tubular epithelial cells from lipopolysaccharide-induced apoptosis and inflammation damage[J]. Braz J Med Biol Res,2017,51(2):e6611. doi:10.1590/1414-431X20176611.
[9] MAO N,TAN R Z,WANG S Q,et al. Ginsenoside Rg1 inhibits angiotensin Ⅱ-induced podocyte autophagy via AMPK/mTOR/PI3K pathway[J]. Cell Biol Int,2016,40(8):917-925. doi:10.1002/cbin.10634.
[10] TURK E,KANDEMIR F M,YILDIRIM S,et al. Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats[J]. Biol Trace Elem Res,2019,189(1):95-108. doi:10.1007/s12011-018-1443-6.
[11] YANG Y,SONG S,NIE Y,et al. Lentinan alleviates arsenic-induced hepatotoxicity in mice via downregulation of OX40/IL-17A and activation of Nrf2 signaling[J]. BMC Pharmacol Toxicol,2022,23(1):16. doi:10.1186/s40360-022-00557-7.
[12] QIN Q,LIN N,HUANG H,et al. Ginsenoside Rg1 ameliorates cardiac oxidative stress and inflammation in streptozotocin-induced diabetic rats[J]. Diabetes Metab Syndr Obes,2019,12:1091-1103. doi:10.2147/DMSO.S208989.
[13] GAO Y,LI J,CHU S,et al. Ginsenoside Rg1 protects mice against streptozotocin-induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways[J]. Eur J Pharmacol,2020,866:172801. doi:10.1016/j.ejphar.2019.172801.
[14] SONG M F,YANG Y,YI Z W,et al. Sema 3A as a biomarker of the activated mTOR pathway during hexavalent chromium-induced acute kidney injury[J]. Toxicol Lett,2018,299:226-235. doi:10.1016/j.toxlet.2018.09.005.
[15] GUO H,LI X,ZHANG Y,et al. Metabolic characteristics related to the hazardous effects of environmental arsenic on humans:a metabolomic review[J]. Ecotoxicol Environ Saf,2022,236:113459. doi:10.1016/j.ecoenv.2022.113459.
[16] MANCUSO C,SANTANGELO R. Panax ginseng and Panax quinquefolius:from pharmacology to toxicology[J]. Food Chem Toxicol,2017,107(Pt A):362-372. doi:10.1016/j.fct.2017.07.019.
[17] ZHANG G,ZHANG M,YU J,et al. Ginsenoside Rg1 prevents H2O2-induced lens opacity[J]. Curr Eye Res,2021,46(8):1159-1165. doi:10.1080/02713683.2020.1869266.
[18] SHEN X,DONG X,HAN Y,et al. Ginsenoside Rg1 ameliorates glomerular fibrosis during kidney aging by inhibiting NOX4 and NLRP3 inflammasome activation in SAMP8 mice[J]. Int Immunopharmacol,2020,82:106339. doi:10.1016/j.intimp.2020.106339.
[19] FAN Y,XIA J,JIA D,et al. Mechanism of ginsenoside Rg1 renal protection in a mouse model of d-galactose-induced subacute damage[J]. Pharm Biol,2016,54(9):1815-1821. doi:10.3109/13880209.2015.1129543.
[20] GUO J,WANG R,MIN F. Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells[J]. J Leukoc Biol,2022,112(5):1065-1077. doi:10.1002/JLB.1A0422-211R.
[21] HU J L,XIAO L,LI Z Y,et al. Upregulation of HO-1 is accompanied by activation of p38MAPK and mTOR in human oesophageal squamous carcinoma cells[J]. Cell Biol Int,2013,37(6):584-592. doi:10.1002/cbin.10075.
[22] JIN X,XU Z,CAO J,et al. HO-1/EBP interaction alleviates cholesterol-induced hypoxia through the activation of the AKT and Nrf2/mTOR pathways and inhibition of carbohydrate metabolism in cardiomyocytes[J]. Int J Mol Med,2017,39(6):1409-1420. doi:10.3892/ijmm.2017.2979.
[23] KIM J,KUNDU M,VIOLLET B,et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nat Cell Biol,2011,13(2):132-141. doi:10.1038/ncb2152.
[24] WANG Y,ZHANG H. Regulation of autophagy by mTOR signaling pathway[J]. Adv Exp Med Biol,2019,1206:67-83. doi:10.1007/978-981-15-0602-4_3.
[25] FENG F B,QIU H Y. Effects of Artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis[J]. Biomed Pharmacother,2018,102:1209-1220. doi:10.1016/j.biopha.2018.03.142.
(2022-11-07收稿 2023-03-01修回)
(本文編輯 李志蕓)

