鹽脅迫下歐李葉片葉綠素代謝與超微弱發(fā)光的關系
孫聰 郭金麗
摘 ? ?要:【目的】解析鹽脅迫下歐李葉片葉綠素代謝與超微弱發(fā)光(ultraweak luminescence, UWL)的變化規(guī)律及二者之間的關系。【方法】以蒙原金秋歐李[Cerasus humilis (Bge.) Sok.]盆栽苗為試材,采用濃度為400 mmol·L-1、800 mmol·L-1的NaCl分別進行輕度和重度鹽脅迫處理,測定UWL強度和葉綠素代謝相關指標的變化規(guī)律并進行相關性分析。【結果】(1)不同程度鹽脅迫下,與對照相比,輕度和重度鹽脅迫下歐李葉片7種葉綠素合成前體物質(ALA、PBG、UroⅢ、CopⅢ、ProtoⅨ、Mg-protoⅨ、Pchl)含量均下降,主要合成過程酶(ALAD、MgCH)及葉綠素酶(Chlase)含量均表現(xiàn)為上升,葉綠素(Chla、Chlb、Chla+b)含量均下降;同時葉片UWL強度也持續(xù)下降。(2)2種鹽脅迫下,重度脅迫導致葉綠素代謝各指標及UWL強度的下降或上升幅度均較輕度脅迫更大。(3)相關分析顯示,2種鹽脅迫下,葉片UWL強度均與葉綠素合成前體物質含量及葉綠素含量呈顯著正相關,與葉綠素酶含量呈顯著負相關。【結論】歐李葉片UWL與葉綠素代謝密切相關:鹽脅迫下,隨著葉片葉綠素合成前體物質含量下降及葉綠素酶含量的上升,葉綠素合成代謝減弱而降解代謝加強,引起葉綠素含量下降;以上葉綠素代謝變化導致葉片UWL強度降低。
關鍵詞:蒙原金秋歐李;鹽脅迫;葉片;超微弱發(fā)光;葉綠素代謝
中圖分類號:S661.2 文獻標志碼:A 文章編號:1009-9980(2023)07-1411-10
The relationship between chlorophyll metabolism and ultraweak luminescence of leaves under salt stress in Cerasus humilis
SUN Cong, GUO Jinli*
(College of Horticulture and Plant Protection, Inner Mongolia Agricultural University, Hohhot 010010, Inner Mongolia, China)
Abstract: 【Objective】 Ultraweak luminescence (UWL) is a natural luminescence phenomenon in all living organisms. However, the understanding of the mechanism of luminescence is still limited. In order to analyze the excitation mechanism of UWL in plants, this study investigated the changes in UWL intensity, chlorophyll metabolism, and chlorophyll content in leaves of Cerasus humilis under salt stress, and carried out correlation analysis. The purpose was to reveal the relationship between plant physiological status and UWL, with focus on chlorophyll metabolism so as to provide understandings related to physiology of UWL emission in plants. 【Methods】 The potted seedlings of biennial C. humilis were taken as the materials in this study. The seedlings were subjected to mild and severe stress treatments with 400 mmol·L-1 and 800 mmol·L-1 NaCl, respectively. Each potted seedling was irrigated with 400 mL of salt solution at different concentrations, and irrigation with the same amount of water was taken as the control. The UWL intensity, main precursor substances of chlorophyll (ALA, PBG, UroⅢ, CopⅢ, ProtoⅨ, Mg-protoⅨ, and Pchl), main chlorophyll synthetases (ALAD and MgCH), enzymes related to chlorophyll degradation, and chlorophyll contents (Chla, Chlb, and Chla+b) in the leaves of C. humilis were measured every 2 days. The correlation between the indexes of chlorophyll metabolism and UWL was analyzed. About 10-20 mature leaves were selected from the base of the branches for measurement of the UWL intensity using an UWL test system (BPCL-2-SH, Beijing). Take 5 leaves from each treatment, and take samples from the top, middle, and bottom three parts of each leaf to measure UWL. Then, take a sufficient amount of leaves that were washed with distilled water and dried. After removal of the main leaf veins, they were quickly frozen with liquid nitrogen and stored at -80 ℃ to measure chlorophyll metabolism and contents. The tests were repeated three times, each with three biological repeats. 【Results】 (1) With the extension of stress time, the UWL intensity of C. humilis leaves under different levels of salt stress showed a decreasing trend, which was 40.80% and 83.26% lower in mild and severe stresses than that before the stress, respectively. During the whole stress period, UWL in severe stress treatment decreased more rapidly compared with that in the mild stress treatment, and at the end of the experiment, it decreased by 35.93% and 81.88%, compared with the control respectively. (2) With the extension of stress time, the contents of seven chlorophyll synthesis precursors (ALA, PBG, Uro Ⅲ, Cop Ⅲ, Proto Ⅸ, Mg-proto Ⅸ, and Pchl) in C. humilis leaves under different salt stress treatments showed a decreasing trend. Among them, Cop Ⅲ decreased fastest under mild stress with a reduction of 57.97% compared with that before stress, and Pchl decreased fastest under severe stress with a reduction of 67.17% compared with that before stress. The contents of the main synthetase (ALAD and MgCH) and the degrading enzymes chlorophyllase (Chlase) showed an increasing trend. Under mild and severe stress treatments, ALAD increased the fastest and was 1.59 times and 1.27 times higher than before stress, respectively. Chlorophyll (Chla, Chlb, and Chla+b) contents showed a decreasing trend, among which Chlb decreased fastest under mild stress with a reduction of 39.47% compared with that before stress, and Chla decreased fastest under severe stress with a reduction of 76.66%. At the same time, the severe stress led to greater changes in chlorophyll metabolism indicators than mild stress. (3) Correlation analysis showed that under the two salt stress treatments, the UWL intensity of leaves was significantly positively correlated with the contents of chlorophylls and their precursors, and was significantly negatively correlated with the content of chlorophyll metabolism enzymes. Under mild stress, the intensity of UWL was significantly positively correlated with the chlorophyll precursors, ALA, PBG, and Mg-proto Ⅸ, as well as Chla and Chla+b, but significantly negatively correlated with metabolism-related enzymes, ALAD and Chlase. Under severe stress, UWL intensity was significantly positively correlated with chlorophyll precursor PBG, Uro Ⅲ, and Cop Ⅲ, as well as Chla, Chlb and Chla+b, but negatively correlated with metabolic enzyme Chlase. 【Conclusion】 Salt stress blocked the chlorophyll synthesis process of leaves in C. humilis, enhanced the degradation enzymes, and decreased the chlorophyll content. The UWL intensity decreased with the decrease in chlorophyll synthesis precursors, chlorophyll enzyme activity, and chlorophyll content. The above changes in chlorophyll metabolism may lead to changes in leaf UWL intensity. Therefore, the UWL of leaves in C. humilis is closely related to chlorophyll metabolism. Salt stress led to the decrease in UWL emission, and the decrease becomes faster under more severe salt stresses.
Key words: Cerasus humilis; Salt stress; Leaves; Ultraweak luminescence; Chlorophyll metabol
任何生物組織或細胞在生命活動的代謝過程中,都自發(fā)地輻射出一種超弱電子流,其強度僅為在1 s內1 cm2上幾個至幾千個光子(100~103 hv·s-1·cm-2),波長范圍為180~800 nm[1],稱為生物超微弱發(fā)光(ultraweak luminescence,UWL;ultraweak photon emission,UPE)。UWL是一種來自細胞內的本源信號,檢測這種信號并破譯其所攜帶的與生命活動相關的信息,可以了解各種生命過程的真實現(xiàn)象;未來UWL可能是研究植物信號識別、信息傳遞、細胞衰老等基本生命過程的重要工具[2]。1923年蘇聯(lián)細胞生物學家最早在“洋蔥實驗”中發(fā)現(xiàn)了UWL現(xiàn)象[3],一直到20世紀80年代,隨著超高靈敏度的弱光圖像探測器的發(fā)展,UWL的研究進入到一個新的階段,開始在生命科學、醫(yī)學、食品等領域開展研究[4-5]。自20世紀90年代UWL進入到農業(yè)領域開始,多數研究集中在UWL與環(huán)境因素及植物抗逆性的關系,如董家倫等[6]研究沙生植物的UWL,發(fā)現(xiàn)其與樹木品種之間的抗旱性有關;在低溫和高溫條件下,抗性強的品種具有更高的UWL強度[7],同時種子的發(fā)光強度也隨著溫度的升高而增強[8];鹽脅迫會導致植物UWL強度的降低,與抗性弱的品種相比,抗性強的品種種子的發(fā)芽率和UWL強度較高[9];因此,UWL也有望作為抗性品種鑒定和評價的有力工具。另外,有少數研究初步探索了UWL與植物部分生長發(fā)育進程的關系[10-12],但植物整個生命周期與UWL的關系如何?以及UWL產生的來源和機制均不夠清楚完整,仍有待于更多的、大量的試驗來研究驗證。
關于UWL產生機制,生物化學的觀點認為,UWL有可能來源于能級躍遷、活性氧發(fā)光、DNA發(fā)光和能量轉換發(fā)光等方面,對于以上假設觀點,已進行部分研究加以驗證。張新華等[13]對植物體外線粒體UWL的初步探索發(fā)現(xiàn),線粒體提取液的UWL強度與線粒體濃度呈正相關。前人[14-19]對草莓(Fragaria × ananassa Duch.)果實采后衰老過程中線粒體及其呼吸作用中能量代謝及活性氧與UWL的關系進行了研究,發(fā)現(xiàn)UWL強度可以反映果實的衰老程度;線粒體呼吸代謝的能量水平和生成效率與UWL的強度呈顯著正相關,活性氧主要通過影響線粒體功能而影響UWL強度,活性氧爆發(fā)導致線粒體功能下降,從而導致UWL強度下降。以上研究進一步驗證了線粒體是UWL產生的來源之一,能量代謝和活性氧水平與UWL激發(fā)有關。
那么,作為植物細胞中進行光合作用和能量轉換主要細胞器的葉綠體,從其承擔的作用與功能來看,應與植物中UWL的產生來源有關。針對該假設,筆者團隊前期以歐李[Cerasus humilis (Bge.) Sok.]和德景天(Sedum hybridus L.)作為材料研究發(fā)現(xiàn),干旱脅迫下兩者葉片的凈光合速率、蒸騰速率、胞間二氧化碳濃度、氣孔導度均與UWL強度顯著相關[20-21],初步說明植物光合作用與UWL有關,而葉綠體主要光能吸收色素—葉綠素及其代謝在其中扮演怎樣的角色仍未可知。另外,光合作用為植物生長發(fā)育提供能量和物質,是對鹽脅迫最敏感的生理過程之一;同時,歐李具有耐鹽堿的特點,但有關鹽脅迫下歐李葉片葉綠素代謝與UWL激發(fā)關系的研究還鮮見報道。故筆者在本研究中以歐李為試驗材料,在前期初步探索的基礎上,進一步對鹽脅迫下歐李葉片葉綠素代謝及UWL的變化規(guī)律進行研究,解析植物葉綠素代謝與UWL發(fā)生的關系,為揭示逆境脅迫下植物光合作用與UWL的關系及植物中UWL產生的來源提供理論依據。
1 材料和方法
1.1 試驗材料
試驗以內蒙古農業(yè)大學歐李科研基地的2年生蒙原金秋歐李(C. humilis)盆栽苗為材料。
1.2 試驗方法
選擇生長正常、長勢一致的歐李盆栽苗進行鹽脅迫處理。根據預試驗結果,采用NaCl濃度為400 mmol·L-1、800 mmol·L-1分別進行輕度和重度鹽脅迫。兩種鹽脅迫均以澆灌法進行處理,每盆一次性澆400 mL不同濃度鹽溶液,重復將澆灌后流出的鹽溶液倒回盆內直至達到完全吸收;對照(Control)澆等量清水。各處理材料按完全隨機排列,每處理每重復各10盆,5次重復。分別于脅迫0、2、4、6、8、10、12 d取樣,取樣時選取歐李植株當年基生枝由基部向上10~20枚之間的成熟葉片,用于UWL測定的葉片用冰盒帶回,進行葉綠素代謝相關指標測定的葉片以蒸餾水洗凈擦干去除主葉脈后,液氮速凍帶回。
1.3 試驗指標及測定方法
1.3.1 ? ?UWL的測定 ? ?使用超微弱發(fā)光測試系統(tǒng)(BPCL-2-SH,北京)進行測定。開機后調制高壓1100 V,預熱30 min,用打孔器(10 mm)對所取歐李葉片進行打孔,迅速將打孔部分葉片平鋪于測量杯中,打開光窗立即測定。每個處理每次取5枚葉片,每片葉取樣3次。以15次減去本底值的最大值的平均值表示UWL強度。
1.3.2 ? ?葉綠素代謝試驗指標的測定 ? ?葉綠素合成前體物質的測定:δ-氨基乙酰丙酸(ALA)含量測定參照金鑫[22]的方法;膽色素原(PBG)、尿卟啉原Ⅲ(UroⅢ)和糞卟啉原Ⅲ(CopⅢ)含量的測定按照Bogorad[23]的方法;原卟啉Ⅸ(ProtoⅨ)、Mg-原卟啉Ⅸ(Mg-ProtoⅨ)和原葉綠素酸酯(Pchl)含量的測定參照Liu等[24]的方法。
葉綠素代謝酶含量的測定:用購自睿信生物科技有限公司(泉州)的Elasa試劑盒測定δ-氨基酮戊酸脫水酶(ALAD)、鎂螯合酶(MgCH)、葉綠素酶(Chlase)含量。具體方法按操作說明進行。
葉綠素含量的測定參照李合生[25]的方法。
以上指標測定均重復3次試驗,每次試驗3個生物重復。
1.4 數據處理與方法
采用Excel統(tǒng)計軟件進行數據處理,采用Origin軟件繪圖,采用SPSS軟件進行相關性分析。
2 結果與分析
2.1 鹽脅迫下歐李葉片超微弱發(fā)光的變化
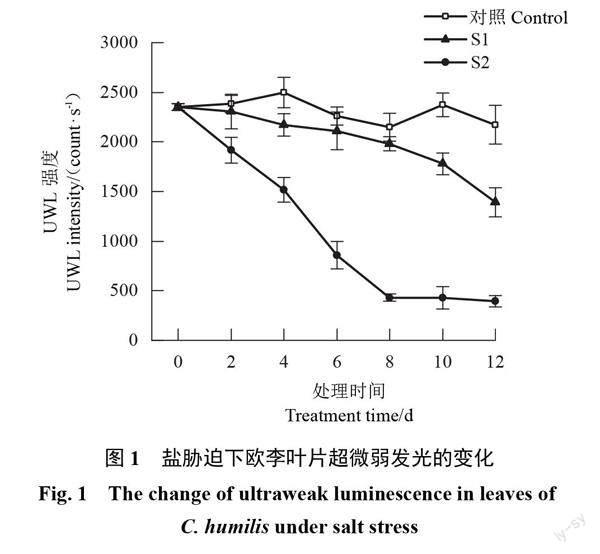
隨著脅迫時間的延長,對照葉片的UWL強度基本保持不變,2種鹽脅迫下葉片的UWL強度均整體呈下降趨勢。輕度脅迫下UWL強度緩慢下降,脅迫結束時UWL強度為1 392.47 count·s-1,比脅迫前降低了40.80%;重度脅迫下UWL強度于前8 d快速下降,之后基本不變,脅迫結束時UWL強度為393.81 count·s-1,比脅迫前降低了83.26%。整個脅迫期間,2種鹽脅迫下UWL強度均明顯低于對照,且重度脅迫的UWL強度明顯低于輕度脅迫;脅迫結束時輕度脅迫下比對照降低了35.93%,重度脅迫下比對照降低了81.88%(圖1)。可見鹽脅迫會導致歐李葉片UWL強度下降,鹽濃度越大UWL強度下降幅度越大。
2.2 鹽脅迫下歐李葉片葉綠素代謝及葉綠素含量的變化
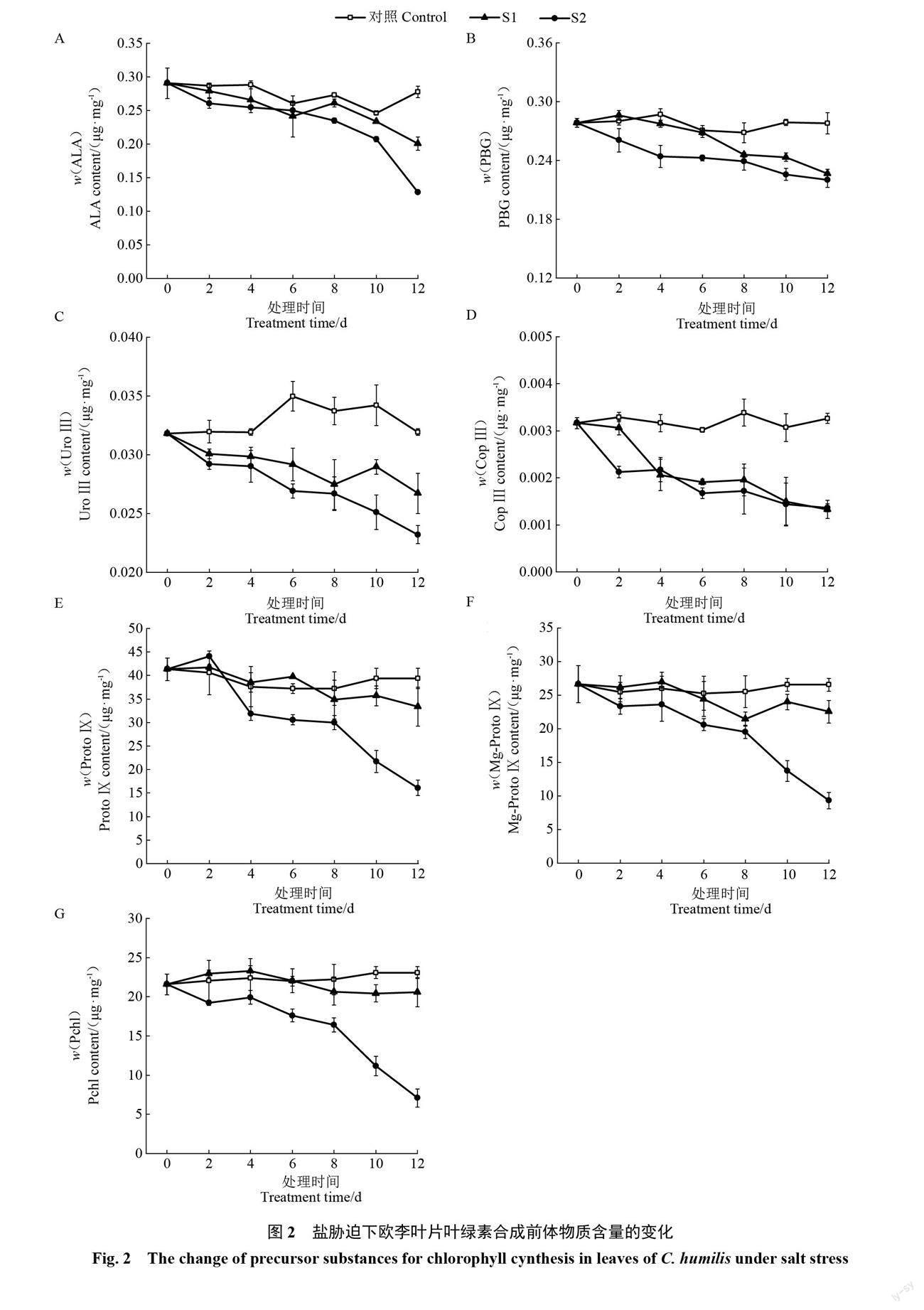
2.2.1 ? ?葉綠素代謝合成前體物質含量的變化 ? ?隨著脅迫時間的延長,對照葉片的ALA、PBG、UroⅢ、CopⅢ、ProtoⅨ、Mg-ProtoⅨ及Pchl含量均基本保持不變,2種鹽脅迫下葉片的以上7項指標值均整體呈下降趨勢。輕度脅迫下葉片7項指標均下降較為緩慢,重度脅迫下7項指標的下降幅度均不同程度地大于輕度脅迫。整個脅迫期間,2種鹽脅迫下葉片的7項指標均低于對照,且重度脅迫下7項指標均低于輕度脅迫;其中,2種鹽脅迫下葉片的UroⅢ和CopⅢ含量均明顯低于對照,重度脅迫下葉片的ProtoⅨ、Mg-ProtoⅨ及Pchl含量均明顯低于對照(圖2)。以上不同程度鹽脅迫下7項指標的變化表明,鹽脅迫會導致歐李葉片葉綠素代謝過程中主要合成前體物質含量下降,鹽濃度越大合成前體物質含量下降幅度越大。
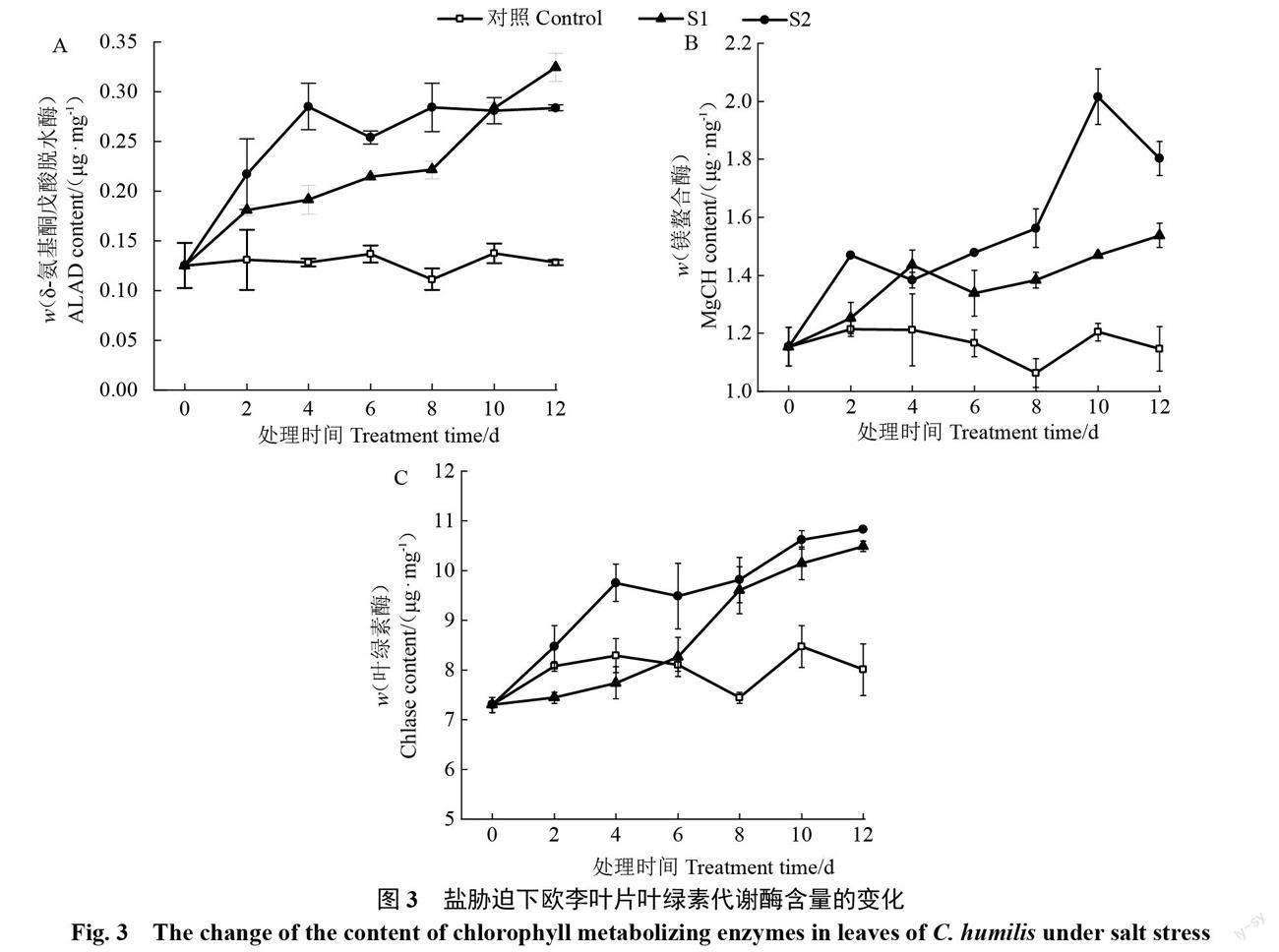
2.2.2 ? ?葉綠素代謝過程中代謝相關酶含量的變化 隨著脅迫時間的延長,對照葉片的葉綠素代謝合成過程酶ALAD、MgCH及Chlase含量均基本保持穩(wěn)定,2種鹽脅迫下葉片的以上3種酶含量均整體呈上升趨勢。輕度脅迫下葉片3種酶含量均上升較為緩慢,重度脅迫下3種酶含量的上升幅度均不同程度地大于輕度脅迫。整個脅迫期間,2種鹽脅迫下葉片的3種酶含量均明顯高于對照,且重度脅迫下3種酶含量均不同程度地高于輕度脅迫(圖3)。以上不同程度鹽脅迫下葉綠素代謝合成過程酶及葉綠素酶含量的變化表明,鹽脅迫下歐李葉片葉綠素代謝2種合成過程酶及葉綠素降解酶的含量均表現(xiàn)為增加趨勢,鹽濃度越大以上各種酶的含量上升幅度越大。
2.2.3 葉綠素含量的變化 隨著脅迫時間的延長,對照葉片的Chla、Chlb及Chla+b含量的變化小有起伏,整體上保持平穩(wěn);2種鹽脅迫下葉片的3種葉綠素含量均整體呈下降趨勢。輕度脅迫下葉片3種葉綠素含量均下降較為緩慢,脅迫結束時Chla、Chlb及Chla+b含量比脅迫前分別降低了26.38%、39.47%、29.73%;重度脅迫下3種葉綠素含量的下降幅度均不同程度地大于輕度脅迫,比脅迫前分別降低了76.66%、72.16%、75.51%。整個脅迫期間,2種鹽脅迫下3種葉綠素含量均低于對照,且重度脅迫下3種葉綠素含量均低于輕度脅迫;脅迫結束時輕度脅迫下Chla、Chlb及Chla+b含量比對照分別降低了34.73%、39.84%、35.93%,重度脅迫下比對照分別降低了79.31%、72.33%、77.67%(圖4)。以上不同程度鹽脅迫下葉綠素含量的變化表明,鹽脅迫會導致歐李葉片葉綠素含量下降,鹽濃度越大葉綠素含量下降幅度越大。
2.3 鹽脅迫下歐李葉片葉綠素代謝與UWL的關系
鹽脅迫下,與對照相比,不同程度鹽脅迫歐李葉片葉綠素代謝過程中的7種合成前體物質含量均下降,2種合成過程酶及葉綠素酶含量均表現(xiàn)為上升,Chla、Chlb及Chla+b含量均下降;同時葉片UWL強度也下降。且鹽濃度越高,以上葉綠素代謝指標值及UWL強度的下降或上升的幅度越明顯。葉片葉綠素代謝與UWL的相關性分析顯示,對照葉片的UWL強度與合成前體物質PBG含量及合成過程酶MgCH含量呈顯著正相關。輕度脅迫下,UWL強度與合成前體物質ALA、PBG、Mg-protoⅨ含量呈極顯著正相關,與UroⅢ、CopⅢ含量呈顯著正相關;與代謝相關酶ALAD、Chlase含量極顯著負相關,與MgCH含量呈顯著負相關;與Chla及Chla+b含量呈極顯著正相關,與Chlb含量呈顯著正相關。重度脅迫下,UWL強度與合成前體物質PBG、UroⅢ、CopⅢ含量呈極顯著正相關,與ProtoⅨ、Mg-protoⅨ、Pchl含量呈顯著正相關;與代謝酶Chlase含量呈極顯著負相關,ALAD與MgCH含量呈顯著負相關;與Chla、Chlb和Chla+b含量均呈顯著正相關(表1)。以上鹽脅迫下葉綠素代謝與UWL強度的相關性分析表明,葉片UWL強度與葉綠素合成前體物質、相關代謝酶及葉綠素含量密切相關。
綜合鹽脅迫下葉綠素代謝和UWL的變化規(guī)律及兩者之間的相關性關系,表明歐李葉片UWL強度與葉綠素代謝密切相關。鹽脅迫下,隨著葉片葉綠素合成前體物質含量下降、葉綠素酶含量的上升,葉綠素合成代謝減弱而降解代謝占據優(yōu)勢,從而引起葉綠素含量下降;以上葉綠素代謝變化導致了葉片UWL強度降低。
3 討 論
植物在逆境脅迫中表現(xiàn)出不同的UWL現(xiàn)象已有一些報道。如接玉玲等[26]研究干旱脅迫下湖北海棠[Malus hupehensis (Pamp.) Rehder]幼苗UWL的變化發(fā)現(xiàn),隨著脅迫程度的加深,葉片UWL強度逐漸降低。卜令豪等[27]研究發(fā)現(xiàn),鹽脅迫下羅布麻(Apocynum venetum L.)葉片UWL強度隨脅迫時間的延長而降低,且脅迫程度越重,UWL強度下降幅度越大。類似地,筆者在研究中發(fā)現(xiàn),輕度和重度鹽脅迫下歐李葉片的UWL強度均低于未脅迫處理,隨著脅迫時間延長,UWL強度持續(xù)下降;且重度脅迫下UWL強度的下降幅度更大。以上研究結果顯示,植物受到逆境脅迫時會導致UWL強度下降,脅迫程度越重UWL強度下降幅度越大,UWL強度能夠反映植物受到的逆境脅迫的程度。
李德紅等[28]早期對白菜(Brassica rapa var. glabra Regel)體外葉綠體的UWL進行了研究,發(fā)現(xiàn)葉綠體的UWL不能簡單地歸因于該過程的自由基,植物的UWL應與植物光形態(tài)建成和光合作用過程相關。但具體與植物光合作用過程中哪些因素有關的研究還未見報道。葉綠素作為葉綠體光合作用中的聚光色素和反應中心色素,承擔著光能的吸收和傳遞、并通過光化學反應將光能轉變?yōu)殡娔艿穆毮埽_啟了光合作用的第一步。閆妮等[29]發(fā)現(xiàn)鹽脅迫會導致番茄(Solanum lycopersicum L.)葉綠素含量下降,進而使光合作用效率降低。葉綠素代謝反映了葉綠體功能及其光合作用的效能[30],其過程分為葉綠素合成與降解兩部分,合成與降解的動態(tài)平衡決定了葉綠素的含量。葉綠素的生物合成主要是通過谷氨酸(Glu)→ALA→PBG→UroⅢ→CopⅢ→ProtⅨ→Mg-ProtoⅨ→Pchl→Chla→Chlb途徑完成[31]。其中,ALA的合成和Mg離子插入ProtⅨ是葉綠素合成的2個主要控制點;合成途徑中任何一步發(fā)生異常都會使葉綠素合成受阻,導致葉綠素含量下降[32]。Chlase是葉綠素降解的關鍵酶,催化Chla轉化為Chlidea,由此開啟了葉綠素的降解。毛晶晶等[33]研究發(fā)現(xiàn),低溫脅迫下葉綠素合成途徑中ALA和Mg-Proto Ⅸ的積累量顯著上升,PBG、Urogen Ⅲ、CoprogenⅢ、Proto Ⅸ、Mpe、Mpde、Pchlide的積累量低于常溫,推測ALA向PBG的轉化及Mg-Proto Ⅸ向Mpe的轉化過程受到低溫脅迫抑制,從而導致玉米(Zea mays L.)葉綠素含量下降,抑制了玉米的轉綠過程。王穎等[34]研究發(fā)現(xiàn),鹽脅迫使菠菜(Spinacia oleracea L.)葉片ALA和PBG含量升高,而UroⅢ、ProtoⅨ、Mg-ProtoⅨ、Pchl、Chla、Chlb及Chla+b含量均降低,說明鹽脅迫下葉片葉綠素合成受阻位點在PBG向UroⅢ的轉化過程中。上述研究表明不同環(huán)境因素對葉綠素合成途徑的影響有所差異,這種差異可能由植物種類及環(huán)境因子不同所致。筆者在本試驗中,不同程度鹽脅迫下,與對照相比,歐李葉片7種葉綠素合成前體物質ALA、PBG、UroⅢ、CopⅢ、ProtoⅨ、Mg-protoⅨ、Pchl的含量均下降;主要合成過程酶ALAD、MgCH的含量均表現(xiàn)為上升,Chlase含量大幅度上升;同時Chla、Chlb、Chla+b的含量均下降。其中,葉綠素合成首要前體ALA含量降低,因而造成后續(xù)葉綠素合成原料不足,應該是鹽脅迫下葉綠素合成代謝受阻的主要原因;兼之Chlase含量明顯上升,加快了葉綠素的分解進程。以上兩方面綜合作用引起鹽脅迫下歐李葉片葉綠素含量下降。同時,試驗中鹽脅迫下歐李葉片葉綠素合成主要酶ALAD、MgCH的含量均上升,且上升幅度均為重度脅迫大于輕度脅迫,猜測該結果應該是鹽脅迫下歐李本身的一種應激反應,但仍有待于進一步研究驗證。鹽脅迫下,在以上葉綠素代謝變化的過程中,葉片UWL強度也持續(xù)下降;相關分析結果顯示,不同程度鹽脅迫下,UWL強度均與葉綠素合成前體物質含量及葉綠素含量呈顯著正相關,與葉綠素酶含量呈顯著負相關。上述結果表明歐李葉片葉綠素代謝與UWL密切相關。
4 結 論
鹽脅迫下,隨著葉片葉綠素合成前體物質含量下降及葉綠素酶含量的上升,葉綠素合成代謝減弱而降解代謝加強,從而引起葉綠素含量下降,以上葉綠素代謝變化導致葉片UWL發(fā)生受阻,發(fā)光強度降低,表明鹽脅迫下歐李葉片葉綠素代謝與UWL激發(fā)密切相關。
參考文獻References:
[1] POPP F A,LI K H,GU Q. Recent advances in biophoton research and its applications[M]. Singapore:World Scientific Publishing Conpany,1992:1-46.
[2] 程海鵬,王君暉,池浩超,朱睦元. 豌豆種子萌發(fā)過程中超微弱發(fā)光的研究[J]. 浙江大學學報(理學版),2001,28(6):682-685.
CHENG Haipeng,WANG Junhui,CHI Haochao,ZHU Muyuan. Study on ultraweak luminescence of Pisumsativumseeds at the stage of germination[J]. Journal of Zhejiang University (Sciences Edition),2001,28(6):682-685.
[3] GURWITSCH A. Die natur des spezifischen erregers der zellteilung[J]. Archiv FüR Mikroskopische Anatomie und Entwicklungsmechanik,1923,100:11-40.
[4] 王暢,蔣禮林,王樂新,朱文霞. 奶牛血清微弱發(fā)光的分析與應用研究[J]. 江蘇農業(yè)科學,2013,41(2):187-189.
WANG Chang,JIANG Lilin,WANG Lexin,ZHU Wenxia. Analysis and application of weak luminescence in dairyserum[J]. Jiangsu Agricultural Sciences,2013,41(2):187-189.
[5] 岳霞麗,劉永紅,胡先文,董元彥. 水華魚腥藻的超弱發(fā)光研究[J]. 光譜實驗室,2008,25(4):673-676.
YUE Xiali,LIU Yonghong,HU Xianwen,DONG Yuanyan. The study of ultraweak luminescence of Anabaena flos-aquae[J]. Chinese Journal of Spectroscopy Laboratory,2008,25(4):673-676.
[6] 董家倫,李樹真,劉生龍. 一些沙生植物苗期超弱發(fā)光特征研究[J]. 中國沙漠,1990,10(2):29-34.
DONG Jialun,LI Shuzhen,LIU Shenglong. A stuay on the characteristics of ultra-weak luminescence of some psammophytes during seedling stage[J]. Journal of Desert Research,1990,10(2):29-34.
[7] 楊起簡. 幾種作物籽粒萌發(fā)時超弱發(fā)光與其抗逆性關系[J]. 生物化學與生物物理進展,1993,20(4):315-317.
YANG Qijian. Relationship between ultra-weak luminescence and stress resistance of several crops during grain germination[J]. Progress in Biochemistry and Biophysics,1993,20(4):315-317.
[8] 習崗. 植物超弱發(fā)光及其在農業(yè)上的應用[J]. 物理,1994,23(9):548-552.
XI Gang. Ultra-weak luminescence of plants and its application in agriculture[J]. Physics,1994,23(9):548-552.
[9] 楊起簡,周禾,ПОГОСЯН C И,ЯКОВЛЕВ А Ф. 鹽脅迫下豌豆幼苗的超弱發(fā)光[J]. 激光生物學報,2001,10(4):265-268.
YANG Qijian,ZHOU He,ПОГОСЯН C И,ЯКОВЛЕВ А Ф. Study on superweak luminescence of pea seedlingunder the different Na-salt stress[J]. Acta Laser Biology Sinica,2001,10(4):265-268.
[10] 侯仙慧,廖祥儒,李穎,張曉晴,卜文娟,賈燕,李光. 莧菜種子萌發(fā)過程的超微弱發(fā)光及其機理研究[J]. 種子,2004,23(7):24-27.
HOU Xianhui,LIAO Xiangru,LI Ying,ZHANG Xiaoqing,BU Wenjuan,JIA Yan,LI Guang. Ultraweak biophoton emission and its mechanism during seed germination of Amaranthus hypochondriacus[J]. Seed,2004,23(7):24-27.
[11] 林桂玉,黃在范,張翠華,鄭成淑. 菊花花芽分化期超微弱發(fā)光及生理代謝的變化[J]. 園藝學報,2008,35(12):1819-1824.
LIN Guiyu,HUANG Zaifan,ZHANG Cuihua,ZHENG Chengshu. Changes in ultraweak luminescence intensity,respiration rate and physiological metabolism of Chrysanthemum during floral differentiation[J]. Acta Horticulturae Sinica,2008,35(12):1819-1824.
[12] 趙丹瑩,生吉萍,丁洋,申琳,范蓓,劉燦. 超微弱發(fā)光用于番茄果實冷害發(fā)生程度的無損監(jiān)測[J]. 光譜學與光譜分析,2010,30(9):2493-2495.
ZHAO Danying,SHENG Jiping,DING Yang,SHEN Lin,F(xiàn)AN Bei,LIU Can. Nondestructive examination of tomato chilling injury by Ultraweak Luminescence[J]. Spectroscopy and Spectral Analysis,2010,30(9):2493-2495.
[13] 張新華,楊洪強. 植物葉綠體和線粒體的超微弱發(fā)光[J]. 植物生理學通訊,2004,40(1):111-114.
ZHANG Xinhua,YANG Hongqiang. Ultraweak bioluminescence of chloroplast and mitochondria in plants[J]. Plant Physiology Communications,2004,40(1):111-114.
[14] 郭金麗,劉歡,梁爽,朱冠宇,白楊,李連國. 活性氧調控下草莓果實衰老過程中活性氧與超微弱發(fā)光的關系[J]. 果樹學報,2017,34(3):363-369.
GUO Jinli,LIU Huan,LIANG Shuang,ZHU Guanyu,BAI Yang,LI Lianguo. Relationship between reactive oxygen species and ultraweak luminescence in strawberry fruit during senescence under various reactive oxygen regulation treatments[J]. Journal of Fruit Science,2017,34(3):363-369.
[15] 劉歡,梁爽,閆宇彤,白楊,郭金麗. 活性氧和能量調控下草莓果實衰老與超微弱發(fā)光的關系[J]. 西北植物學報,2017,37(6):1182-1188.
LIU Huan,LIANG Shuang,YAN Yutong,BAI Yang,GUO Jinli. Relationship between senescence and ultraweak photon emission under controlling of reactive oxygen and energy in strawberry fruit[J]. Acta Botanica Boreali-Occidentalia Sinica,2017,37(6):1182-1188.
[16] GUO J L,ZHU G Y,LI L G,LIU H,LIANG S. Ultraweak photon emission in strawberry fruit during ripening and aging is related to energy level[J]. Open Life Sciences,2017,12(1):393-398.
[17] GUO J L,LIU H,BAI Y,YAN Y T,LI L G. Manipulation of cellular energy reveals the relationship between ultraweak luminescence and cellular energy during senescence of strawberry (Fragaria × ananassa) fruits[J]. Acta Physiologiae Plantarum,2018,40(7):134.
[18] 孫聰,白楊,李連國,郭金麗. 草莓果實線粒體呼吸代謝與超微弱發(fā)光的關系[J]. 西北植物學報,2019,39(10):1805-1811.
SUN Cong,BAI Yang,LI Lianguo,GUO Jinli. Relationship between mitochondrial respiratory metabolism and ultraweak luminescence in strawberry fruit[J]. Acta Botanica Boreali-Occidentalia Sinica,2019,39(10):1805-1811.
[19] SUN C,LIU J C,LIU H,GUO J L. Reactive oxygen species mediate the relationship between mitochondrial function and delayed luminescence during senescence of strawberry (Fragaria ananassa) fruits[J]. Acta Physiologiae Plantarum,2022,44(2):25.
[20] 郭金麗,梁爽,邵長芬,白楊,閆宇彤,李連國. 干旱脅迫下景天植物光合作用與超微弱發(fā)光的關系[J]. 西北植物學報,2017,37(9):1789-1796.
GUO Jinli,LIANG Shuang,SHAO Changfen,BAI Yang,YAN Yutong,LI Lianguo. Relationship between photosynthesis and ultraweak luminescence in Sedum hybridum under drought stress[J]. Acta Botanica Boreali-Occidentalia Sinica,2017,37(9):1789-1796.
[21] 孫聰,任鵬達,李連國,李曉艷,郭金麗. 干旱脅迫下歐李葉片葉綠體光合參數與超弱光子輻射的關系[J]. 北方園藝,2021(9):25-31.
SUN Cong,REN Pengda,LI Lianguo,LI Xiaoyan,GUO Jinli. Relationship between ultraweak photon emission and photosynthetic parameters of Cerasus humilis leaves chloroplast under drought stress[J]. Northern Horticulture,2021(9):25-31.
[22] 金鑫. 柑橘黃化脈明病毒對檸檬、柚和甜橙葉綠素代謝影響的研究[D]. 重慶:西南大學,2017.
JIN Xin. Effect on chlorophyll metabolism in lemon,pummelo and sweet orange by Citrus yellow vein clearingvirus[D]. Chongqing:Southwest University,2017.
[23] BOGORAD L. [122] Porphyrin synthesis[J]. Methods in Enzymology,1962,5:885-895.
[24] LIU J,WANG J Y,YAO X Y,ZHANG Y,LI J Q,WANG X X,XU Z J,CHEN W F. Characterization and fine mapping of thermo-sensitive chlorophyll deficit mutant1 in rice (Oryza sativa L.)[J]. Breeding Science,2015,65(2):161-169.
[25] 李合生. 植物生理生化實驗原理和技術[M]. 北京:高等教育出版社,2000.
LI Hesheng. Principles and techniques of plant physiological biochemical experiment[M]. Beijing:Higher Education Press,2000.
[26] 接玉玲,趙海洲,張偉,楊洪強,李德全,束懷瑞. 甜菜堿對干旱脅迫下湖北海棠超微弱發(fā)光及抗氧化能力的影響[J]. 應用生態(tài)學報,2006,17(12):2394-2398.
JIE Yuling,ZHAO Haizhou,ZHANG Wei,YANG Hongqiang,LI Dequan,SHU Huairui. Effects of glycinebetaine on ultraweak luminescence and anti-oxidative capability of Malus hupehensis under drought stress[J]. Chinese Journal of Applied Ecology,2006,17(12):2394-2398.
[27] 卜令豪,陳忠祥,劉志華.鹽脅迫對羅布麻生理生化指標及超微弱發(fā)光的影響[J/OL].分子植物育種,2022:1-15. http://kns.cnki.net/kcms/detail/46.1068.S.20220822.1657.004.html.
BU Linghao,CHEN Zhongxiang,LIU Zhihua. Effects of salt stress on physiological and biochemical lndices of rob hemp and ultra-faint Luminescence[J/OL]. Molecular Plant Breeding,2022:1-15. http://kns.cnki.net/kcms/detail/46.1068.S.20220822.1657.004.html.
[28] 李德紅,唐永紅,何永紅,邢達. 白菜葉綠體的超弱發(fā)光機理初探[J]. 激光生物學報,2002,11(1):64.
LI Dehong,TANG Yonghong,HE Yonghong,XING Da. Preliminary study on ultra-weak luminescence mechanism of chloroplast in Chinese cabbage[J]. Acta Laser Biology Sinica,2002,11(1):64.
[29] 閆妮,馮棣,楊鳳娟,張敬敏,桑茂鵬,祝海燕. γ-氨基丁酸浸種對鹽分脅迫下番茄出苗及幼苗生長的影響[J]. 中國瓜菜,2022,35(10):58-63.
YAN Ni,F(xiàn)ENG Di,YANG Fengjuan,ZHANG Jingmin,SANG Maopeng,ZHU Haiyan. GABA soaking affects tomato emergence and seedling growth under salt stress[J]. China Cucurbits and Vegetables,2022,35(10):58-63.
[30] 周振翔,李志康,陳穎,王志琴,楊建昌,顧駿飛. 葉綠素含量降低對水稻葉片光抑制與光合電子傳遞的影響[J]. 中國農業(yè)科學,2016,49(19):3709-3720.
ZHOU Zhenxiang,LI Zhikang,CHEN Ying,WANG Zhiqin,YANG Jianchang,GU Junfei. Effects of reduced chlorophyll content on photoinhibition and photosynthetic electron transport in rice leaves[J]. Scientia Agricultura Sinica,2016,49(19):3709-3720.
[31] 孫錦,賈永霞,郭世榮,李娟. 海水脅迫對菠菜(Spinacia olerancea L.)葉綠體活性氧和葉綠素代謝的影響[J]. 生態(tài)學報,2009,29(8):4361-4371.
SUN Jin,JIA Yongxia,GUO Shirong,LI Juan. Effects of seawater stress on metabolism of reactive oxygen species and chlorophyll in chloroplasts of spinach (Spinacia olerancea L.)[J]. Acta Ecologica Sinica,2009,29(8):4361-4371.
[32] 靳曉青. 外源γ-氨基丁酸調控活性氧和葉綠素代謝增強甜瓜幼苗鹽堿脅迫耐性[D]. 楊凌:西北農林科技大學,2019.
JIN Xiaoqing. Exogenous γ-aminobutyric acid regulates reactive oxygen species and chlorophyll metabolism to enchance salinity-alkalinity stress tolerance in muskmelon seedlings[D]. Yangling:Northwest A & F University,2019.
[33] 毛晶晶,李澤嬌,趙雨晴,袁澍,袁明. 低溫脅迫對玉米轉綠過程中葉綠素生物合成的影響[J]. 四川農業(yè)大學學報,2019,37(5):617-622.
MAO Jingjing,LI Zejiao,ZHAO Yuqing,YUAN Shu,YUAN Ming. The effects of low temperature on chlorophyll synthesis during greening of maize[J]. Journal of Sichuan Agricultural University,2019,37(5):617-622.
[34] 王穎,郭世榮,束勝,劉芳,劉濤,孫錦. 外源亞精胺對鹽脅迫下菠菜葉綠素合成前體含量的影響[J]. 西北植物學報,2015,35(10):2026-2034.
WANG Ying,GUO Shirong,SHU Sheng,LIU Fang,LIU Tao,SUN Jin. Effects of exogenous spermidine on chlorophyll precursors content of spinach plants under salt stress[J]. Acta Botanica Boreali-Occidentalia Sinica,2015,35(10):2026-2034.

