Tissue Clearing for Three-dimensional Visualization of The Neurovascular Network in The Whole Mount Auricular Skin*
ZHANG Kai-Wen, GUO Ya-Ting, SU Yu-Xin, WANG Yu-Qing, CUI Jing-Jing,ZHANG Jian-Liang, WANG Jia, BAI Wan-Zhu
(Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing100700,China)
Abstract Objective To study the spatial correlation of the nerve fibers and blood vessels in the auricular skin by using the combination of histological staining technique and tissue clearing strategy. Methods The anterior and posterior auricular skins were carefully peeled away from the intervening cartilage and the auricular nerve fibers and blood vessels were directly immunofluorescent stained with protein gene product 9.5 (PGP 9.5) and phalloidin followed with tissue clearing treatment. After that, these auricular skins were mounted on the microscope slide in the whole mount pattern and examined under an epifluorescence microscope and a laser scanning confocal microscope. Results It was shown that the PGP 9.5+ nerve fibers aligning with phalloidin-labeled blood vessels ran from the base part of auricle to its peripheral region, forming the neurovascular network in the auricular skin. Beyond the conventional immunofluorescence staining, the additional tissue clearing treatment demonstrated the auricular nerve fibers and blood vessels better in morphological detail. Conclusion From the perspective of methodology, tissue clearing technique enhance the visualization of the immunofluorescence labeling within the auricular skin, which should be an effective approach for insight into the auricular neurovascular network in a three-dimensional view under the physiological and pathological conditions.
Key words tissue clearing, auricular skin, nerve fibers, blood vessels, neurovascular network
Histological techniques have been the standard procedure for investigating morphological and molecular aspects in tissues through the cut sections for several decades, however, conventional histological sectioning restricted the available information to two dimensions, providing only a part of spatial information in tissue architecture[1-3]. Recent revolutionized advances in tissue clearing techniques started a new era of histology for visualizing the fluorescent labeling in the thicker tissue sections,intact organs, and even entire organisms on the cellular and sub-cellular levels in a three-dimensional pattern, providing more opportunity to analyze the spatial correlation of the different structural components comprehensively in the target structure[4-7].
In line with these studies, we developed a new protocol that combining the immunofluorescence technique with the solvent-based tissue clearing strategy and tested it on the auricular skin of mouse[8-9]. Since auricular skin is readily accessible for dissection, and also is a highly informative model system for neurovascular network[8-9], it is benefit to assess the neural and vascular labeling in the whole mount style. Here, the auricular nerve fibers and blood vessels were labeled with protein gene product 9.5 (PGP 9.5) and phalloidin, respectively[1-2,10-12]. In order to clarify whether the tissue clearing could enhance the visualization of the auricular neural and vascular labeling, based on the conventional immunofluorescence staining, the stained auricular skin was further treated with tissue clearing reagent for microscopic morphological examination.
By this study, we expected to describe how we adapted and applied tissue clearing technique to exhibit the neural and vascular labeling from the superficial layer to the deep layer of auricular skin in a three-dimensional pattern, which may help us to better understand the auricular neurovascular network.
1 Materials and methods
1.1 Animal subjects and experiments
Four adult male mice (8-10 weeks, weight (20±2) g) were used in this study. Animals (license number SCXK (JING) 2016-002) were provided by the National Institutes for Food and Drug Control. All animals were allowed free access to food and water and housed in a 12 h light/dark cycle with controlled temperature and humidity. The handling and care of experimental animals described here were approved by the ethics committee at Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences.
1.2 Perfusion and tissue preparation
The mice were anesthetized with an overdose of sodium pentobarbital (40 mg/kg) to induce euthanasia and immediately removed auricular hairs with hair removal cream (Veet, Reckitt Benckiser, UK), and then perfused transcardially with 20 ml of 0.9%physiological saline followed by 20 ml 4%paraformaldehyde in 0.1 mol/L phosphate buffer (PB,pH 7.4). After that, the anterior and posterior auricular skins were carefully peeled away from the intervening cartilage, and the attached adipose tissues and connective tissues on the auricular skin were removed under the stereo microscope, and then collected orderly in the twelve-hole Petri dish in 30% sucrose in 0.1 mol/L PB (pH 7.4) at 4°C.
1.3 Immunofluorescence staining and tissue clearing treatment
In this study, the auricular skin was directly used for immunofluorescence staining and tissue clearing treatment without sectioning.
On the first day, the auricular skin was incubated in solution of 2% Triton X-100 in 0.1 mol/L PB overnight at 4°C. On the next day, the auricular skin was changed into the blocking solution containing with 10% normal donkey serum, 1% Triton-X 100,and 0.2% sodium azide in 0.1 mol/L PB and rotated on the shaker for 24 h at 4°C. On the third day,the tissue was transferred into primary antibody solution containing with polyclonal rabbit anti-PGP 9.5 (1∶1 000; Abcam 27503; Thermo Fisher; Waltham,MA, USA) in dilution buffer (1% normal donkey serum, 0.2% Triton-X 100 and 0.2% sodium azide in 0.1 mol/L PB) and rotated on the shaker for 2 d at 4°C,after that, the auricular tissue was washed 3 times with washing buffer (0.2% Triton-X 100 in 0.1 mol/L PB, pH 7.4) at room temperature, then keep washing on the shaker overnight at 4°C. On the sixth day, the tissue was transferred into the solution with the secondary antibody of Alexa Fluor 488 donkey anti-rabbit IgG (1∶500; A21206; Thermo Fisher,Waltham, MA, USA) and mixed with Alexa Fluor 568 phalloidin (1∶1 000; A12380; Thermo Fisher,Waltham, MA, USA) and rotated on the shaker for 5 h at 4°C. After that, the auricular tissue was washed 3 times with washing buffer at room temperature, and then kept washing on the shaker overnight at 4°C. On the seventh day, the auricular specimens were divided into two groups: the first group with conventional immunofluorescence staining alone, and the second group with the combination of conventional immunofluorescence staining and tissue clearing. For the former, the auricular skin was mounted on a Superfrost Plus microscope slide (Thermo Fisher,Waltham, CA, USA), flattened with forceps, and coverslipped with the Tissue-Tek OCT compound(Sakura Finetek, Japan). For the latter, the auricular tissue was further incubated in 2 ml of the tissue clearing reagent (RapiClear?1.52 solution,RC152001, SunJin Lab Co., Taiwan, China) for 1 h at room temperature, the added reagent was about 5 times the volume of auricular skin. After that, the cleared specimen was also mounted on a Superfrost Plus microscope slide, circled with a spacer microchamber (SunJin Lab Co., Taiwan, China), and coverslipped with fresh RapiClear?1.52. In this procedure, it should keep the inside of the posterior and anterior auricular skins facing up on the slide.
1.4 Imaging
The outside views of the posterior and anterior auricular skins before and after tissue clearing treatment were taken with a fluorescence stereo microscope (MVX10, Olympus, Tokyo, Japan), and the auricular skin with immunofluorescence staining was scanned with the panoramic tissue slice scanner(VS120, Olympus, Japan), in which the representative regions were further imaged with a laser scanning confocal microscope (FV1200, Olympus, Japan). For the auricular skin (about 200 μm thickness), 40 images (Z-stacks) were captured in 5 μm frames and performed in a single in-focus image, which was further integrated with an image processing system for three-dimensional reconstruction. All figures were finally processed with Adobe Photoshop CS5 and Adobe Illustration CS5 (Adobe Systems, San Jose,CA, USA).
2 Results
The tissue transparency of whole mount auricular skin was compared before and after the tissue clearing treatment. With tissue clearing treatment, the auricular skin was turned more transparently than that of auricular skin without tissue clearing (Figure 1).
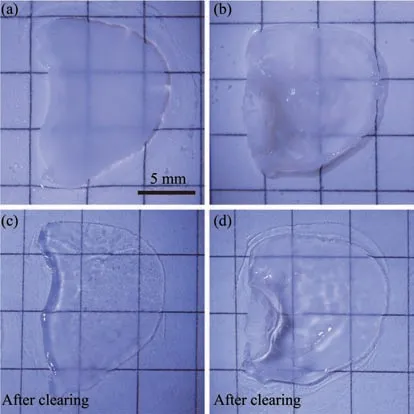
Fig. 1 The optical transparency comparison of the whole mount auricular skins before and after tissue clearing treatment

Fig. 2 Montage views of the distribution of the protein gene product 9.5 (PGP 9.5)- and phalloidin (Pha)-labeling in the auricular skins
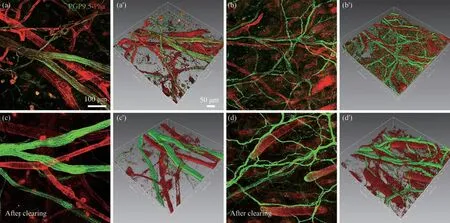
Fig. 3 Three-dimensional views of the protein gene product 9.5 (PGP 9.5)- and phalloidin (Pha)-labeling on the posterior auricular skin
By immunofluorescence staining, the nerve fibers and blood vessels were separately labeled with PGP 9.5 and phalloidin. It should be noted here that,as the cytoskeletal component in the smooth muscular and endothelial cells, phalloidin was expressed not only on the blood vessels, but also on the muscular and epidermal tissues[10-11](Figure 2).
In this study, the neural and vascular labeling was scanned with the panoramic tissue slice scanner equipped with epifluorescence microscope (Figure 2).It was shown that PGP 9.5+ nerve bundles aligning with phalloidin-labeled blood vessels ran from the base part of auricle to its peripheral region forming the auricular neurovascular network (Figure 2). On the basis of conventional immunofluorescence staining, the visualization of the neural and vascular labeling was enhanced by the tissue clearing treatment(Figure 2).
According to the distributional characteristic of nerve-vessel alignment in the auricular skin, we focused our observation on the posterior auricular skin. Here, the neural and vascular labeling was further assessed with the higher magnified view under a laser scanning confocal microscope (Figure 3). With conventional immunofluorescence staining, the labeled nerve fibers and blood vessels could be imaged to a depth up to 200 μm within the auricular skin. However, as a comparison, with the additional tissue clearing treatment, the labeled nerve fibers and blood vessels were obviously enhanced with their clarity and integrity and easily captured from the superficial layer to the deep layer of auricular skin,which was superior to that of the conventional immunofluorescence staining alone (Figure 3).
In addition, the spatial correlation of auricular nerve fibers and blood vessels was reconstructed in a three-dimensional pattern with the image processing system. Similarly, reconstructed image from the cleared specimen was also better than that of conventional one (Figure 3). Since the high resolution of auricular neurovascular network has been demonstrated in this study, the quantitative analysis of nerve fibers and blood vessels was not carried out further.
3 Discussion
A new protocol combining the immunofluorescence staining technique with the tissue clearing strategy has been described in this study. Both techniques were highly compatible for demonstrating the neural and vascular labeling in the whole mount auricular skin, and tissue clearing treatment was further enhanced the visualization of the neural and vascular labeling within the auricular skin for insight into the auricular neurovascular network in morphological detail.
Previously, many efforts have been made to reveal the neurovascular network with immunostaining from the whole mount auricular skin under the physiological and pathological conditions[8-9]. In this study, we further compared the quality of auricular neural and vascular labeling in the auricular skin with the conventional approach and the combination of the conventional immunofluorescence staining with tissue clearing. All morphological information that obtained from the montage images,Z-stack images and reconstructed images supports the idea that the tissue clearing treatment is a better choice for visualizing the neural and vascular labeling in the whole mount auricular skin of mice.
The various tissue clearing techniques, including hydrophobic, hydrophilic, and hydrogel-based methods, have become popular in histological study[5-7,13-16]. With tissue clearing treatment, the lipids, pigments, and water were removed from the specimen for minimizing light scattering, and allow the light almost unrestrictedly penetrating intact organs and entire organisms to excite fluorophore[17-19]. Numerous studies have achieved fruitful results by the combination of the tissue clearing techniques with light-sheet microscopy and automated approaches to image analysis of large-sized specimen[17-19]. Relevantly, our present data indicate that tissue clearing is also suitable for analyzing the neural and vascular labeling within the whole-mount auricular skin even with an epifluorescence microscope or a laser scanning confocal microscope in ordinary laboratory. Here, it should be emphasized that the concentration of Triton-X 100 and the incubation time of antibodies are important for the immunofluorescence staining of the whole mount auricular skin.
As the technical limitation, it is to be noted that PGP 9.5-positive labeling and phalloidin-positive labeling could not reflect the full view of auricular neurovascular network. As it was known, the auricular skin is innervated by different kinds of nerve fibers with their own chemical expression, such as neurofilament 200 (NF200), neuron-specifc class III β-tubulin (Tuj1)[3,9], however, the correlation between these nerve fibers with the PGP 9.5-positive nerve fibers remains to be elucidated. For the blood vessels,phalloidin is strongly expressed on the thick vascular wall in the arterioles, comparatively, platelet endothelial cell adhesion molecule and α-smooth muscle actin are also proper candidates for labeling the vascular structures in the auricular skin[9]. Whether these biomarkers could be compatible with tissue clearing reagent, it still remains to be examined in the future study.
4 Conclusion
In summary, this paper described a valuable protocol that permit to image the neurovascular network in the whole mount auricular skin in a threedimensional pattern. We have tried to optimize the immunofluorescence staining, tissue clearing, and confocal scanning techniques to obtain the highresolution image of the nerve fibers and blood vessels from the superficial layer to the deep layer of auricular skin. As an improved protocol, it might be potentially used to investigate the spatial correlation of nerve fibers and blood vessels in other kinds of whole mount tissues and thick tissue sections.

