肝樣細(xì)胞的體外誘導(dǎo)及小分子化合物在肝樣細(xì)胞誘導(dǎo)中的應(yīng)用
唐薇 王冬梅
摘要:體外誘導(dǎo)肝樣細(xì)胞(HLC)是獲取大量具有應(yīng)用價(jià)值的肝細(xì)胞的有效途徑之一,這些 HLC可以用于構(gòu)建疾病模型、藥物設(shè)計(jì)和藥物毒理學(xué)評價(jià)等。目前,HLC的體外誘導(dǎo)主要通過引入外源轉(zhuǎn)錄因子、細(xì)胞因子或小分子化合物組合的處理。小分子化合物因其結(jié)構(gòu)多樣性、時(shí)間和劑量的可控性及操作上的便利和安全等優(yōu)勢,讓科學(xué)家們致力于篩選小分子化合物來取代外源轉(zhuǎn)錄因子和細(xì)胞因子,其在再生醫(yī)學(xué)領(lǐng)域的應(yīng)用前景廣闊。本文主要對體外誘導(dǎo)多能干細(xì)胞及其他成體細(xì)胞分化成HLC的研究進(jìn)行概述,并總結(jié)了小分子化合物在體外誘導(dǎo)HLC中的應(yīng)用,以期為HLC的體外誘導(dǎo)研究提供思路和借鑒。
關(guān)鍵詞:肝樣細(xì)胞; 小分子化合物; 再生醫(yī)學(xué)
基金項(xiàng)目:福建省自然科學(xué)基金項(xiàng)目(2020J05038); 福建省教育廳項(xiàng)目(JAT190090)
Advances in in vitro induction of hepatocyte-like cells and the application of small-molecule compounds in inducing hepatocyte-like cells
TANG Wei, WANG Dongmei. (Fujian Key Laboratory of Development and Neurobiology, College of Life Sciences, Fujian Normal University, Fuzhou 350117, China)
Corresponding author:
TANG Wei, 40720007@qq.com (ORCID:0000-0001-8841-4125)
Abstract:
The induction of hepatocyte-like cells (HLCs) in vitro is one of the effective ways to obtain a large number of useful hepatocyte, and these HLCs can be used in disease modeling, drug design, and toxicological evaluation. At present, the induction of HLCs in vitro is mainly achieved by introducing exogenous transcription factors, cytokines or small-molecule compounds. Since small-molecule compounds have the advantages of structural diversity, controllable time and dose, and convenient and safe operation, scientists are devoted to screening out the small-molecule compounds to replace exogenous transcription factors and cytokines, and such compounds have a promising application prospect in the field of regenerative medicine. This article reviews the studies on the in vitro induction of HLCs from pluripotent stem cells and other adult stem cells and summarizes the application of small-molecule compounds in the in vitro induction of HLCs, in order to provide ideas and references for the in vitro induction of HLCs.
Key words:
Hepatocyte-Like Cells; Small Molecules; Regenerative Medicine
Research funding:
Natural Science Foundation of Fujian Province (2020J05038); Fujian Education Department (JAT190090)
肝臟疾病一直威脅著全人類的健康,終末期肝病更是有著較高的致死率。肝移植被認(rèn)為是治療肝臟代謝類疾病、終末期肝病和急性肝病最為有效的方法[1-3]。然而可用于移植手術(shù)的肝臟供體器官非常緊缺,一般肝移植較為常見的方法是進(jìn)行肝細(xì)胞移植[4],即向患者體內(nèi)移植正常的肝細(xì)胞進(jìn)行治療,這樣可以有效擴(kuò)大供體肝臟的使用效率,并具有操作簡便、創(chuàng)傷小的優(yōu)點(diǎn)[5]。
目前將肝細(xì)胞移植技術(shù)廣泛運(yùn)用于臨床治療仍然面臨著許多問題,如缺少高質(zhì)量的供體肝臟,缺少臨床級別的凍存和復(fù)蘇肝細(xì)胞的方法,以及合適的免疫抑制試劑等。如果在肝細(xì)胞來源方面有進(jìn)一步突破,細(xì)胞移植將會(huì)更有效地應(yīng)用于臨床,為患者提供更好的治療,提高生存機(jī)會(huì)。另一方面,肝細(xì)胞是藥物研發(fā)的重要工具,可以用于評價(jià)藥物毒性、穩(wěn)定性等。如果能在體外大量獲得肝樣細(xì)胞(hepatocyte-like cells,HLC)將非常有利于肝臟疾病的治療和新藥研發(fā)[6-7]。近年來,國內(nèi)外科研工作者一直致力于獲得不依賴于供體肝臟的功能型HLC。隨著干細(xì)胞技術(shù)、細(xì)胞轉(zhuǎn)分化技術(shù)的大力發(fā)展,誘導(dǎo)其他細(xì)胞分化為HLC的研究工作已成為再生醫(yī)學(xué)的研究熱點(diǎn)。眾多研究[1,8-9]表明胚胎干細(xì)胞、誘導(dǎo)多能性干細(xì)胞以及其他終末分化的細(xì)胞可通過導(dǎo)入外源基因、小分子化合物處理、細(xì)胞共培養(yǎng)或添加特定細(xì)胞因子、3D培養(yǎng)等方式來實(shí)現(xiàn)向肝細(xì)胞的誘導(dǎo)分化。小分子化合物因其作用靶點(diǎn)相對清晰、操作簡便等獨(dú)特優(yōu)勢,讓科研工作者們更致力于探索用小分子化合物的方法來不斷優(yōu)化細(xì)胞肝向分化的誘導(dǎo)過程。
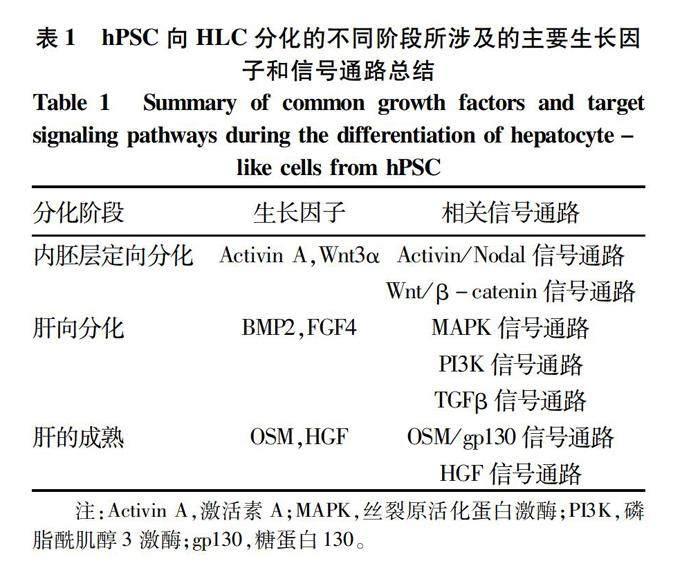
1 HLC的體外誘導(dǎo)
1.1 多能干細(xì)胞來源的HLC 人多能干細(xì)胞(human pluripotent stem cells,hPSC)包含了人胚胎干細(xì)胞(human embryonic stem cells,hESC)和人誘導(dǎo)多能性干細(xì)胞(human-induced pluripotent stem cells,hiPSC)。hPSC體外誘導(dǎo)分化成肝細(xì)胞常見的方法有:細(xì)胞因子與胞外基質(zhì)共同誘導(dǎo)、化合物誘導(dǎo)、遺傳修飾以及與分化的成體細(xì)胞共培養(yǎng)等[5]。目前科學(xué)家在誘導(dǎo)hPSC體外分化成肝細(xì)胞時(shí)采用的主要策略是模擬人體胚胎發(fā)育過程中肝臟的形成[1]。主要經(jīng)歷了3個(gè)階段的分化:(1)激活A(yù)ctivin/Nodal通路,促使胚胎干細(xì)胞向內(nèi)胚層分化;(2)生長因子骨形態(tài)發(fā)生蛋白(bone morphogenetic protein,BMP)和成纖維細(xì)胞生長因子(fibroblast growth factor,F(xiàn)GF)刺激內(nèi)胚層向胎肝分化發(fā)育;(3)添加肝細(xì)胞生長因子(HGF)和抑瘤素 M(oncostatin M,OSM)促進(jìn)肝細(xì)胞的成熟。具體的誘導(dǎo)肝向分化所涉及的細(xì)胞因子與相關(guān)信號通路如表1所示。目前已有研究通過優(yōu)化細(xì)胞培養(yǎng)基[10]、細(xì)胞共培養(yǎng)[11-12]、過表達(dá)相關(guān)轉(zhuǎn)錄因子[13]等方式來提高誘導(dǎo)得到的HLC的成熟性。已有研究[14-15]表明肝細(xì)胞的極性對于膽管和膜轉(zhuǎn)運(yùn)體的形成至關(guān)重要。Dao Thi等[15]將hESC誘導(dǎo)分化成柱狀極化的HLC,這些極化的HLC可以用于藥代動(dòng)力學(xué)、藥物之間相互作用的研究。
科研人員在將hESC誘導(dǎo)分化成肝細(xì)胞的研究上已經(jīng)取得了很大的進(jìn)展,構(gòu)建了多種分化誘導(dǎo)體系[4]。但hESC的使用依然面臨著倫理道德及個(gè)體間免疫排斥等問題,在一定程度上也限制了其臨床的應(yīng)用。而hiPSC來源的肝細(xì)胞可以有效避免此問題。可以先用患者自身的體細(xì)胞體外誘導(dǎo)成 hiPSC,再進(jìn)一步將 hiPSC定向誘導(dǎo)分化為人肝樣細(xì)胞(hHLC),這類細(xì)胞被稱為hiPSC-HLC[9,16]。從患者自身取材誘導(dǎo)得到的細(xì)胞可以有效緩解免疫排斥的情況,且不存在倫理道德爭議。hiPSC-HLC還可用于構(gòu)建肝類器官,這種3D的肝類器官可以用于模擬肝臟的發(fā)育過程,探究肝臟疾病發(fā)病機(jī)制、藥物療效、細(xì)胞間以及細(xì)胞與基質(zhì)間的相互作用[17-19]。
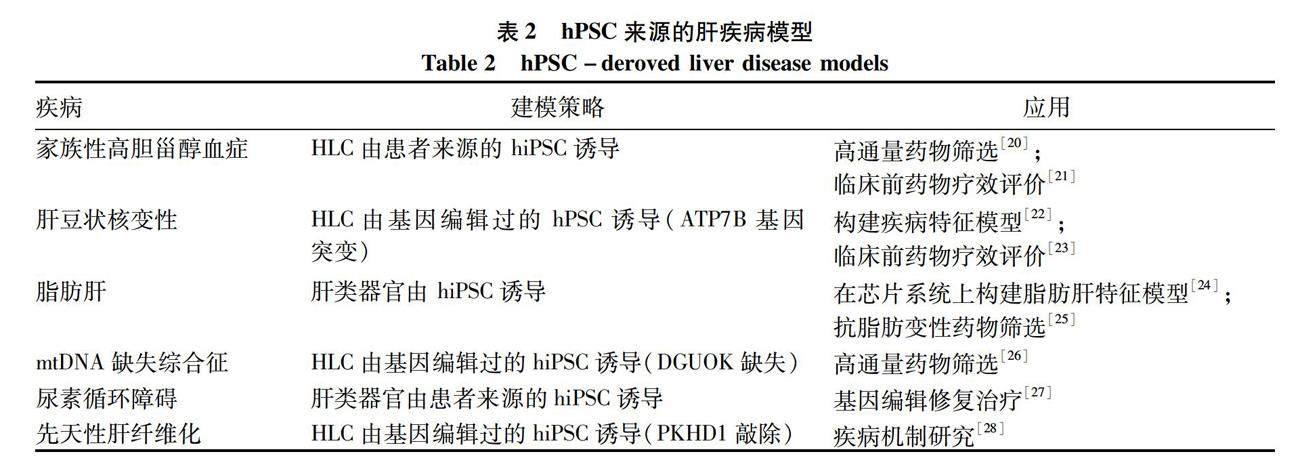
hPSC來源的HLC已成功開發(fā)為多種肝相關(guān)疾病的細(xì)胞模型和類器官模型,在一定程度上解決了病理樣品有限的問題,可將其用于探究肝疾病發(fā)病機(jī)制,并設(shè)計(jì)針對性的治療方案。表2總結(jié)了一些 hPSC來源的肝疾病模型及其應(yīng)用。hPSC來源的HLC在高通量藥物篩選、藥物毒性分析、藥代動(dòng)力學(xué)和個(gè)體化差異作用評價(jià)等領(lǐng)域都發(fā)揮了重要價(jià)值[20-28]。
1.2 其他成體細(xì)胞來源的HLC 細(xì)胞轉(zhuǎn)分化技術(shù)也稱為譜系重編程,實(shí)質(zhì)是將分化成熟的體細(xì)胞直接轉(zhuǎn)變成其他譜系的功能細(xì)胞或者祖細(xì)胞,該過程無需經(jīng)歷干細(xì)胞的多能性狀態(tài)。轉(zhuǎn)分化技術(shù)操作簡便,較好地避免了多能干細(xì)胞技術(shù)在細(xì)胞療法中潛在的致瘤性,能夠?yàn)檎T導(dǎo)培養(yǎng)功能型肝細(xì)胞、解決肝病患者需求提供新思路。
2011年中國科學(xué)家惠利健研究團(tuán)隊(duì)[29]在Nature報(bào)道了采用3種轉(zhuǎn)錄因子 Foxa3、Hnf1α、Gata4 可以實(shí)現(xiàn)將小鼠皮膚細(xì)胞轉(zhuǎn)分化成HLC。這些HLC具有典型的上皮樣細(xì)胞形態(tài),表達(dá)肝細(xì)胞特異性基因并具有類似肝細(xì)胞的功能,且在移植到Fah-/-小鼠體內(nèi)后能夠發(fā)揮一定的治療作用。有研究[30]表明,F(xiàn)oxa蛋白家族作為“先鋒因子”,可招募Hnf4α共同結(jié)合到染色質(zhì)上來激活肝特異性基因的表達(dá)。而Foxa3不同于Foxa1和Foxa2的是,其能遷移到目標(biāo)基因的轉(zhuǎn)錄起始區(qū)與RNA聚合酶Ⅱ結(jié)合,共同調(diào)控基因的表達(dá)。胡以平課題組[31]報(bào)道了利用另外兩種轉(zhuǎn)錄因子Hnf1β和Foxa3可將小鼠的胚胎成纖維細(xì)胞轉(zhuǎn)分化為肝臟祖細(xì)胞,而肝臟祖細(xì)胞可以進(jìn)一步分化為肝細(xì)胞和膽管細(xì)胞。隨著小鼠體系的轉(zhuǎn)分化誘導(dǎo)技術(shù)的成熟,科學(xué)家們也開始實(shí)現(xiàn)將人的成纖維細(xì)胞轉(zhuǎn)分化成hHLC。惠利健課題組[32]通過對人成纖維細(xì)胞過表達(dá)Foxa3、Hnf1α和Hnf4α而成功將其轉(zhuǎn)分化為hHLC。Inada等[33]采用外源轉(zhuǎn)錄因子Foxa3、Hnf1α和Hnf6將人臍靜脈和外周血源性內(nèi)皮細(xì)胞轉(zhuǎn)分化成肝前體細(xì)胞,通過體外3D培養(yǎng)來實(shí)現(xiàn)肝前體細(xì)胞向肝細(xì)胞和膽管細(xì)胞的分化。這些研究成果表明多種成體細(xì)胞是可以通過導(dǎo)入外源轉(zhuǎn)錄因子來實(shí)現(xiàn)向肝細(xì)胞的轉(zhuǎn)分化,但是外源轉(zhuǎn)錄因子的導(dǎo)入可能會(huì)引起細(xì)胞基因組插入后突變,而病毒的使用可能具有一定的致瘤性。目前有許多研究者致力于采用化合物誘導(dǎo)的方法來取代外源轉(zhuǎn)錄因子,讓細(xì)胞肝向轉(zhuǎn)分化的過程更加簡便、可控,且具有醫(yī)學(xué)應(yīng)用價(jià)值。
2 小分子化合物在體外誘導(dǎo)HLC中的應(yīng)用
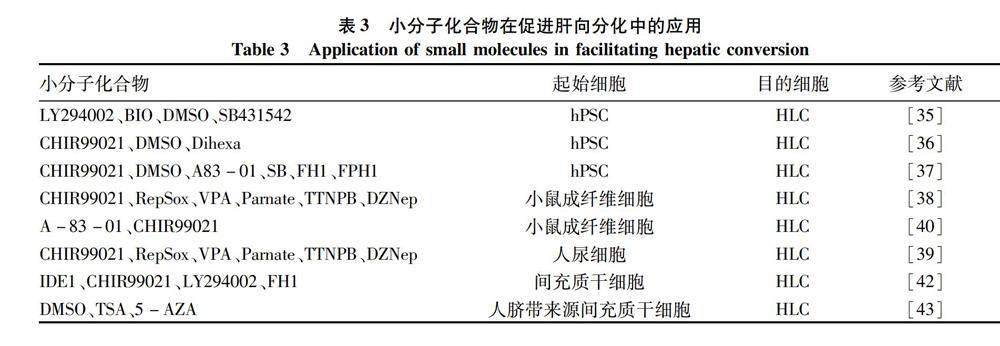
2.1 小分子化合物在促進(jìn)多能干細(xì)胞肝向分化中的應(yīng)用 小分子化合物在細(xì)胞重編程過程中取代轉(zhuǎn)錄因子的作用要追溯到iPSC的體外誘導(dǎo)。2013年鄧宏魁課題組[34]采用7個(gè)小分子化合物VPA(丙戊酸)、CHIR99021、Repsox、Parnate、Forskolin、DZNep和TTNPB成功將小鼠體細(xì)胞誘導(dǎo)為iPSC,首次實(shí)現(xiàn)了全化合物組合的方法誘導(dǎo)體細(xì)胞重編程,實(shí)現(xiàn)了里程碑式的突破。運(yùn)用全化合物誘導(dǎo)得到的iPSC被命名為化合物誘導(dǎo)多能性干細(xì)胞(chemical induced pluripotent stem cells,CiPSC)。CiPSC的出現(xiàn)也讓科學(xué)家們更多的嘗試將小分子化合物用于誘導(dǎo)多能干細(xì)胞的定向分化。Tasnim等[35]采用小分子化合物三步誘導(dǎo)法促使hPSC分化為HLC:LY294002和BIO(6-溴-靛玉紅-3′-肟)誘導(dǎo)定形內(nèi)胚層的形成;丁酸鈉(sodium butyrate,SB)和DMSO誘導(dǎo)內(nèi)胚層分化為肝前體細(xì)胞;最后聯(lián)合SB431542 將肝前體細(xì)胞誘導(dǎo)分化為HLC。Asumda等[36]發(fā)現(xiàn)小分子化合物組合(CHIR99021、DMSO和Dihexa)可以誘導(dǎo)hPSC分化為HLC。Du等[37]利用CHIR99021和DMSO促進(jìn) hPSC向定形內(nèi)胚層分化,然后采用A83-01、SB和 DMSO誘導(dǎo)內(nèi)胚層肝向分化,最后加入FH1和 FPH1促進(jìn)HLC的成熟。
2.2 小分子化合物在促進(jìn)其他細(xì)胞肝向分化中的應(yīng)用 相較于細(xì)胞因子較為昂貴的價(jià)格和外源轉(zhuǎn)錄因子導(dǎo)入較為繁瑣的操作,小分子化合物因其結(jié)構(gòu)多樣性、操作便利可控及價(jià)格上的優(yōu)勢,使其成為體外誘導(dǎo)其他細(xì)胞重編程為 HLC的優(yōu)先選擇。目前已有不少研究結(jié)果表明小分子化合物可以促進(jìn)其他成體細(xì)胞體外轉(zhuǎn)分化為HLC。Guo等[38]采用小分子化合物組合CRVPTD(C,CHIR99021;R,RepSox;V,VPA;P,Parnate;T,TTNPB;D,DZNep)結(jié)合外源轉(zhuǎn)錄因子Foxa3可以誘導(dǎo)小鼠成纖維細(xì)胞向肝細(xì)胞轉(zhuǎn)分化,并在肝損傷小鼠(Fah-/-小鼠)中驗(yàn)證了這些HLC的功能;Tang等[39]將小分子化合物組合CRVPTD結(jié)合單個(gè)轉(zhuǎn)錄因子(Foxa3/Hnf1α/Hnf4α)誘導(dǎo)人尿細(xì)胞轉(zhuǎn)分化為 HLC;Lim等[40]發(fā)現(xiàn)單個(gè)轉(zhuǎn)錄因子(Hnf1α 或Hnf4α)結(jié)合BMP4、小分子化合物A-83-01和CHIR99021可以將小鼠成纖維細(xì)胞轉(zhuǎn)分化為HLC。然而,將小分子化合物A-83-01與化合物組合CRVPTD結(jié)合卻不能實(shí)現(xiàn)全化合物誘導(dǎo)成纖維細(xì)胞向肝細(xì)胞的轉(zhuǎn)分化[39]。也有研究將小分子化合物應(yīng)用于間充質(zhì)干細(xì)胞的體外肝向分化誘導(dǎo)。Panta等[41]發(fā)現(xiàn)丁酸鈉可以誘導(dǎo)間充質(zhì)干細(xì)胞分化為肝前體細(xì)胞,并促進(jìn)其體外成熟;Luo等[42]采用小分子化合物組合(IDE1、CHIR99021、LY294002、FH1)誘導(dǎo)人間充質(zhì)干細(xì)胞體外分化為HLC;Cipriano等[43]采用表觀遺傳調(diào)控分子曲古菌素A(TSA)、5-氮雜胞苷(5-AZA)聯(lián)合DMSO誘導(dǎo)人臍帶來源間充質(zhì)干細(xì)胞分化為HLC。表3將不同小分子化合物在體外誘導(dǎo)其他細(xì)胞分化為HLC中的應(yīng)用進(jìn)行了總結(jié)和歸納。
小分子化合物也可用于誘導(dǎo)原代肝細(xì)胞轉(zhuǎn)化為具有增殖能力的肝前體細(xì)胞。Katsuda等[44]發(fā)現(xiàn)小分子化合物Y-27632、A-83-0和CHIR99021可使小鼠肝細(xì)胞轉(zhuǎn)化為可增殖的、并具有雙向分化潛能的前體細(xì)胞,以實(shí)現(xiàn)小鼠肝細(xì)胞的體外增殖,并將該技術(shù)用于治療肝疾病小鼠。惠利健課題組[45]則發(fā)現(xiàn)在條件培養(yǎng)基中加入Y-27632和A-83-01可促使人原代肝細(xì)胞進(jìn)入一種可增殖的中間過渡態(tài)(具有一定分化潛能),實(shí)現(xiàn)人肝細(xì)胞的體外增殖并誘導(dǎo)其定向分化。這些研究成果為體外大量獲得功能性肝細(xì)胞提供了非常好的策略,但其原代肝細(xì)胞的獲得仍需依賴于供體肝臟。如何實(shí)現(xiàn)全化合物誘導(dǎo)其他細(xì)胞分化成具有成熟肝細(xì)胞功能的HLC,而不再依賴于供體肝臟是科學(xué)界未來想要解決的問題。
3 小分子化合物在HLC誘導(dǎo)中的作用機(jī)制
小分子化合物在HLC誘導(dǎo)過程中的作用機(jī)制可以大致分為兩個(gè)方面(表4)。在誘導(dǎo)多能干細(xì)胞肝向分化的第一階段——定形內(nèi)胚層的形成,常用到的小分子化合物有CHIR99021、BIO、IDE1和LY294002。作為糖原合成激酶3(GSK-3)抑制劑的CHIR99021和BIO,主要是通過激活Wnt/β-catenin通路來上調(diào)內(nèi)胚層標(biāo)志性基因的表達(dá),促進(jìn)定形內(nèi)胚層的形成[35-37]。IDE是一種定形內(nèi)胚層誘導(dǎo)物,作用與Activin A相似,通過介導(dǎo)Smad2磷酸化,激活A(yù)ctivin/Nodal通路來促進(jìn)內(nèi)胚層的形成[42,46]。第二階段是細(xì)胞肝向分化和HLC的成熟。許多研究[35-37]表明適當(dāng)濃度的DMSO能夠驅(qū)動(dòng)定形內(nèi)胚層的肝向分化,促進(jìn)HLC的成熟。但也有研究者對此持不同意見。Wang等[51]認(rèn)為細(xì)胞的肝向分化效率并不受DMSO影響。DMSO作為含硫有機(jī)化合物,可以與蛋白質(zhì)的疏水基團(tuán)相互作用,引起蛋白質(zhì)變性,進(jìn)而影響細(xì)胞的代謝活動(dòng),這對其在細(xì)胞肝向分化中的作用帶來了一定的爭議性,具體作用機(jī)制有待闡明。
有些小分子化合物能推進(jìn)體細(xì)胞的重編程,加速細(xì)胞從間質(zhì)向上皮的轉(zhuǎn)化(MET,調(diào)節(jié)細(xì)胞命運(yùn)轉(zhuǎn)換的關(guān)鍵過程),如 A83-01和RepSox作為活化素受體樣激酶抑制劑(抑制TGFβ)能夠促進(jìn)細(xì)胞MET的過程[8,37,48],從而有利于肝前體細(xì)胞的生成和肝細(xì)胞的成熟。組蛋白去乙酰化抑制劑(如SB、VPA和TSA)也常用于誘導(dǎo)細(xì)胞肝向分化,其可以促進(jìn)肝細(xì)胞代表性基因的表達(dá),有利于內(nèi)胚層向肝細(xì)胞分化[41,43,47]。還有一些表觀遺傳調(diào)控分子,如Parnate(賴氨酸特異性脫甲基酶抑制劑)、DZNep(組蛋白甲基化抑制劑)和 5-AZA(DNA甲基化酶抑制劑),能夠創(chuàng)造一種開放的狀態(tài)來調(diào)節(jié)染色質(zhì)結(jié)構(gòu),實(shí)現(xiàn)在基因轉(zhuǎn)錄水平上促進(jìn)細(xì)胞向肝臟譜系的轉(zhuǎn)化[8,43,49]。TTNPB 可通過激活視黃酸受體參與調(diào)控肝核受體介導(dǎo)的信號通路,從而推動(dòng)細(xì)胞的肝向轉(zhuǎn)分化[50]。小分子化合物FH1和FPH1可以分別取代細(xì)胞因子HGF和OSM來促進(jìn)肝細(xì)胞的成熟[37]。目前,小分子化合物在促進(jìn)其他細(xì)胞向肝細(xì)胞的轉(zhuǎn)化過程中所發(fā)揮的精確作用和機(jī)制尚不完全清楚,未來需要篩選更多的小分子化合物并確定最優(yōu)的組合和適用濃度,以實(shí)現(xiàn)對細(xì)胞肝向分化誘導(dǎo)的精準(zhǔn)調(diào)控。
4 總結(jié)與展望
肝移植是治療終末期肝病唯一有效的方法,但因?yàn)楣w肝臟的短缺以及需要忍受長期的免疫抑制治療而無法被廣泛應(yīng)用。肝細(xì)胞體外誘導(dǎo)技術(shù)的發(fā)展是解決供體肝臟不足的有效方法,尤其是hPSC來源的HLC在產(chǎn)量上能夠?qū)崿F(xiàn)無限供給,可以廣泛用于肝疾病藥物開發(fā)、肝疾病模型建立、個(gè)性化藥物設(shè)計(jì)、藥物毒理學(xué)評價(jià)等領(lǐng)域。且hiPSC-HLC還可取材于患者自身細(xì)胞,用于體外功能性肝臟器官的誘導(dǎo),具有重大的臨床意義。體外誘導(dǎo)HLC主要采用的是外源性細(xì)胞因子誘導(dǎo)、外源基因?qū)胍约靶》肿踊衔锏亩ㄏ蛘T導(dǎo)。小分子化合物的誘導(dǎo)方式相比轉(zhuǎn)基因操作要更加安全、可控且易于操作。目前小分子化合物還不能完全取代外源轉(zhuǎn)錄因子和細(xì)胞因子在誘導(dǎo)其他細(xì)胞肝向分化中的作用,需要尋找更多有效、安全的小分子化合物來實(shí)現(xiàn)全化合物誘導(dǎo)以及提高誘導(dǎo)得到的HLC的成熟性。未來,如何更高效、安全地獲得HLC以及科學(xué)、系統(tǒng)地評價(jià)獲得的HLC的功能用途是體外誘導(dǎo)細(xì)胞肝向分化研究的努力方向。
利益沖突聲明:所有作者均聲明不存在利益沖突。
作者貢獻(xiàn)聲明:唐薇參與文獻(xiàn)調(diào)研和文章撰寫;王冬梅參與文章的文獻(xiàn)查閱與內(nèi)容修改。
參考文獻(xiàn):
[1]LI Y, YANG X, PLUMMER R, et al. Human pluripotent stem cell-derived hepatocyte-like cells and organoids for liver disease and therapy[J]. Int J Mol Sci, 2021, 22(19): 10471. DOI: 10.3390/ijms221910471.
[2]FAN Q, LI Z. Liver transplantation for acute-on-chronic liver failure[J]. Ogran Transplant, 2022, 13(3): 333-337. DOI: 10.3969/j.issn.1674-7445.2022.03.008.
范祺, 李照. 慢加急性肝衰竭的肝移植治療[J]. 器官移植, 2022, 13(3): 333-337. DOI: 10.3969/j.issn.1674-7445.2022.03.008.
[3]XIA Q, SHA M. Progress and prospect of living donor liver transplantation[J]. Chin J Dig Surg, 2022, 21(1): 39-42. DOI: 10.3760/cma.j.cn115610-20211205-00622.
夏強(qiáng), 沙朦. 活體肝移植的進(jìn)展與展望[J]. 中華消化外科雜志, 2022, 21(1): 39-42. DOI: 10.3760/cma.j.cn115610-20211205-00622.
[4]
LUCE E, MESSINA A, DUCLOS-VALLE JC, et al. Advanced techniques and awaited clinical applications for human pluripotent stem cell differentiation into hepatocytes[J]. Hepatology, 2021, 74(2): 1101-1116. DOI: 10.1002/hep.31705.
[5]MESSINA A, LUCE E, HUSSEIN M, et al. Pluripotent-stem-cell-derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration[J]. Cells, 2020, 9(2): 420. DOI: 10.3390/cells9020420.
[6]LIU JT, LAMPRECHT MP, DUNCAN SA. Using human induced pluripotent stem cell-derived hepatocyte-like cells for drug discovery[J]. J Vis Exp, 2018, 135: 57194. DOI: 10.3791/57194.
[7]DEGUCHI S, TAKAYAMA K, MIZUGUCHI H. Generation of human induced pluripotent stem cell-derived hepatocyte-like cells for cellular medicine[J]. Biol Pharm Bull, 2020, 43(4): 608-615. DOI: 10.1248/bpb.b19-00740.
[8]ROMBAUT M, BOECKMANS J, RODRIGUES RM, et al. Direct reprogramming of somatic cells into induced hepatocytes: Cracking the Enigma code[J]. J Hepatol, 2021, 75(3): 690-705. DOI: 10.1016/j.jhep.2021.04.048.
[9]XIE Y, YAO J, JIN W, et al. Induction and maturation of hepatocyte-like cells in vitro: focus on technological advances and challenges[J]. Front Cell Dev Biol, 2021, 9: 765980. DOI: 10.3389/fcell.2021.765980.
[10]TOBA Y, DEGUCHI S, MIMURA N, et al. Comparison of commercially available media for hepatic differentiation and hepatocyte maintenance[J]. PLoS One, 2020, 15(2): e0229654. DOI: 10.1371/journal.pone.0229654.
[11]TAKAGI C, YAGI H, HIEDA M, et al. Mesenchymal stem cells contribute to hepatic maturation of human induced pluripotent stem cells[J]. Eur Surg Res, 2017, 58(1-2): 27-39. DOI: 10.1159/000448516.
[12]SGODDA M, DAI Z, ZWEIGERDT R, et al. A scalable approach for the generation of human pluripotent stem cell-derived hepatic organoids with sensitive hepatotoxicity features[J]. Stem Cells Dev, 2017, 26(20): 1490-1504. DOI: 10.1089/scd.2017.0023.
[13]BOON R, KUMAR M, TRICOT T, et al. Amino acid levels determine metabolism and CYP450 function of hepatocytes and hepatoma cell lines[J]. Nat Commun, 2020, 11(1): 1393. DOI: 10.1038/s41467-020-15058-6.
[14]BUSHWELLER L, ZHAO Y, ZHANG F, et al. Generation of human pluripotent stem cell-derived polarized hepatocytes[J]. Curr Protoc, 2022, 2(1): e345. DOI: 10.1002/cpz1.345.
[15]DAO THI VL, WU X, BELOTE RL, et al. Stem cell-derived polarized hepatocytes[J]. Nat Commun, 2020, 11(1): 1677. DOI: 10.1038/s41467-020-15337-2.
[16]CHEN YF, TSENG CY, WANG HW, et al. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol[J]. Hepatology, 2012, 55(4): 1193-1203. DOI: 10.1002/hep.24790.
[17]RAMLI M, LIM YS, KOE CT, et al. Human pluripotent stem cell-derived organoids as models of liver disease[J]. Gastroenterology, 2020, 159(4): 1471-1486. e12. DOI: 10.1053/j.gastro.2020.06.010.
[18]WANG S, WANG X, TAN Z, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury[J]. Cell Res, 2019, 29(12): 1009-1026. DOI: 10.1038/s41422-019-0242-8.
[19]MARSEE A, ROOS F, VERSTEGEN M, et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids[J]. Cell Stem Cell, 2021, 28(5): 816-832. DOI: 10.1016/j.stem.2021.04.005.
[20]CAYO MA, MALLANNA SK, DI FURIO F, et al. A drug screen using human iPSC-derived hepatocyte-like cells reveals cardiac glycosides as a potential treatment for hypercholesterolemia[J]. Cell Stem Cell, 2017, 20(4): 478-489.e5. DOI: 10.1016/j.stem.2017.01.011.
[21]YANG J, WANG Y, ZHOU T, et al. Generation of human liver chimeric mice with hepatocytes from familial hypercholesterolemia induced pluripotent stem cells[J]. Stem Cell Reports, 2017, 8(3): 605-618. DOI: 10.1016/j.stemcr.2017.01.027.
[22]OVEREEM AW, KLAPPE K, PARISI S, et al. Pluripotent stem cell-derived bile canaliculi-forming hepatocytes to study genetic liver diseases involving hepatocyte polarity[J]. J Hepatol, 2019, 71(2): 344-356. DOI: 10.1016/j.jhep.2019.03.031.
[23]KIM D, KIM SB, RYU JL, et al. Human embryonic stem cell-derived Wilsons disease model for screening drug efficacy[J]. Cells, 2020, 9(4): 872. DOI: 10.3390/cells9040872.
[24]WANG Y, WANG H, DENG P, et al. Modeling human nonalcoholic fatty liver disease (NAFLD) with an organoids-on-a-chip system[J]. ACS Biomater Sci Eng, 2020, 6(10): 5734-5743. DOI: 10.1021/acsbiomaterials.0c00682.
[25]MUN SJ, RYU JS, LEE MO, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids[J]. J Hepatol, 2019, 71(5): 970-985. DOI: 10.1016/j.jhep.2019.06.030.
[26]JING R, CORBETT JL, CAI J, et al. A screen using iPSC-derived hepatocytes reveals NAD+ as a potential treatment for mtDNA depletion syndrome[J]. Cell Rep, 2018, 25(6): 1469-1484. e5. DOI: 10.1016/j.celrep.2018.10.036.
[27]ZABULICA M, JAKOBSSON T, RAVAIOLI F, et al. Gene editing correction of a urea cycle defect in organoid stem cell derived hepatocyte-like cells[J]. Int J Mol Sci, 2021, 22(3): 1217. DOI: 10.3390/ijms22031217.
[28]TSUNODA T, KAKINUMA S, MIYOSHI M, et al. Loss of fibrocystin promotes interleukin-8-dependent proliferation and CTGF production of biliary epithelium[J]. J Hepatol, 2019, 71(1): 143-152. DOI: 10.1016/j.jhep.2019.02.024.
[29]HUANG P, HE Z, JI S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors[J]. Nature, 2011, 475(7356): 386-389. DOI: 10.1038/nature10116.
[30]HORISAWA K, UDONO M, UENO K, et al. The dynamics of transcriptional activation by hepatic reprogramming factors[J]. Mol Cell, 2020, 79(4): 660-676. e8. DOI: 10.1016/j.molcel.2020.07.012.
[31]YU B, HE ZY, YOU P, et al. Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors[J]. Cell Stem Cell, 2013, 13(3): 328-340. DOI: 10.1016/j.stem.2013.06.017.
[32]HUANG P, ZHANG L, GAO Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes[J]. Cell Stem Cell, 2014, 14(3): 370-384. DOI: 10.1016/j.stem.2014.01.003.
[33]INADA H, UDONO M, MATSUDA-ITO K, et al. Direct reprogramming of human umbilical vein- and peripheral blood-derived endothelial cells into hepatic progenitor cells[J]. Nat Commun, 2020, 11(1): 5292. DOI: 10.1038/s41467-020-19041-z.
[34]HOU P, LI Y, ZHANG X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds[J]. Science, 2013, 341(6146): 651-654. DOI: 10.1126/science.1239278.
[35]TASNIM F, PHAN D, TOH YC, et al. Cost-effective differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules[J]. Biomaterials, 2015, 70: 115-125. DOI: 10.1016/j.biomaterials.2015.08.002.
[36]ASUMDA FZ, HATZISTERGOS KE, DYKXHOORN DM, et al. Differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules[J]. Differentiation, 2018, 101: 16-24. DOI: 10.1016/j.diff.2018.03.002.
[37]DU C, FENG Y, QIU D, et al. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails[J]. Stem Cell Res Ther, 2018, 9(1): 58. DOI: 10.1186/s13287-018-0794-4.
[38]GUO R, TANG W, YUAN Q, et al. Chemical cocktails enable hepatic reprogramming of mouse fibroblasts with a single transcription factor[J]. Stem Cell Reports, 2017, 9(2): 499-512. DOI: 10.1016/j.stemcr.2017.06.013.
[39]TANG W, GUO R, SHEN SJ, et al. Chemical cocktails enable hepatic reprogramming of human urine-derived cells with a single transcription factor[J]. Acta Pharmacol Sin, 2019, 40(5): 620-629. DOI: 10.1038/s41401-018-0170-z.
[40]LIM KT, LEE SC, GAO Y, et al. Small molecules facilitate single factor-mediated hepatic reprogramming[J]. Cell Rep, 2016, 15(4): 814-829. DOI: 10.1016/j.celrep.2016.03.071.
[41]PANTA W, IMSOONTHORNRUKSA S, YOISUNGNERN T, et al. Enhanced hepatogenic differentiation of human Wharton's Jelly-derived mesenchymal stem cells by using three-step protocol[J]. Int J Mol Sci, 2019, 20(12): 3016. DOI: 10.3390/ijms20123016.
[42]LUO S, AI Y, XIAO S, et al. Functional hit 1 (FH1)-based rapid and efficient generation of functional hepatocytes from human mesenchymal stem cells: a novel strategy for hepatic differentiation[J]. Ann Transl Med, 2021, 9(13): 1087. DOI: 10.21037/atm-21-2829.
[43]CIPRIANO M, CORREIA JC, CAMOES SP, et al. The role of epigenetic modifiers in extended cultures of functional hepatocyte-like cells derived from human neonatal mesenchymal stem cells[J]. Arch Toxicol, 2017, 91(6): 2469-2489. DOI: 10.1007/s00204-016-1901-x.
[44]KATSUDA T, KAWAMATA M, HAGIWARA K, et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity[J]. Cell Stem Cell, 2017, 20(1): 41-55. DOI: 10.1016/j.stem.2016.10.007.
[45]ZHANG K, ZHANG L, LIU W, et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity[J]. Cell Stem Cell, 2018, 23(6): 806-819. e4. DOI: 10.1016/j.stem.2018.10.018.
[46]XU F, LIU J, DENG J, et al. Rapid and high-efficiency generation of mature functional hepatocyte-like cells from adipose-derived stem cells by a three-step protocol[J]. Stem Cell Res Ther, 2015, 6: 193. DOI: 10.1186/s13287-015-0181-3.
[47]KONDO Y, IWAO T, YOSHIHASHI S, et al. Histone deacetylase inhibitor valproic acid promotes the differentiation of human induced pluripotent stem cells into hepatocyte-like cells[J]. PLoS One, 2014, 9(8): e104010. DOI: 10.1371/journal.pone.0104010.
[48]SGODDA M, MOBUS S, HOEPFNER J, et al. Improved hepatic differentiation strategies for human induced pluripotent stem cells[J]. Curr Mol Med, 2013, 13(5): 842-855. DOI: 10.2174/1566524011313050015.
[49]LI W, DING S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming[J]. Trends Pharmacol Sci, 2010, 31(1): 36-45. DOI: 10.1016/j.tips.2009.10.002.
[50]ANG LT, TAN A, AUTIO MI, et al. A roadmap for human liver differentiation from pluripotent stem cells[J]. Cell Rep, 2018, 22(8): 2190-2205. DOI: 10.1016/j.celrep.2018.01.087.
[51]WANG ZY, LI WJ, LI QG, et al. A DMSO-free hepatocyte maturation medium accelerates hepatic differentiation of HepaRG cells in vitro[J]. Biomed Pharmacother, 2019, 116: 109010. DOI: 10.1016/j.biopha.2019.109010.
收稿日期:
2022-09-05;錄用日期:2022-10-12
本文編輯:王瑩

