Investigation of cyclohexane catalytic degradation driven by N atoms from N2 discharges
Yuying LI (李鈺瑩),Jiacheng XU (徐家成),Chunle ZHANG (張春樂),Shuiliang YAO (姚水良),Jing LI (李晶),Zuliang WU (吳祖良),Erhao GAO (高爾豪) and Jiali ZHU (朱佳麗)
Key Laboratory of Advanced Plasma Catalysis Engineering for China Petrochemical Industry,School of Environmental and Safety Engineering,Changzhou University,Changzhou 213164,People’s Republic of China
Abstract The effect of N2 discharge products on cyclohexane degradation over a MnO2/γ-Al2O3 catalyst has been evaluated by feeding N2 discharge products to the catalyst using a specially designed dielectric barrier discharge reactor.At a reaction temperature of 100 °C,the cyclohexane conversion increased from 2.46%(without N2 discharge products)to 26.3%(with N2 discharge products).N- and O-containing by-product (3,4-dehydroproline) was found on the catalyst surface using gas chromatograph-mass spectrometry identification,in which C=N-C and C=NH bonds were also confirmed from x-ray photoelectron spectroscopy analysis results.Operando analysis results using diffuse reflectance infrared Fourier transform spectroscopy revealed that N atoms can react with surface H2O possibly to NH and OH reactive species that have reactivities to promote CO oxidation to CO2.The mechanism of N-atom-driven cyclohexane degradation to CO and CO2 is proposed.
Keywords: N2 discharge,N atom,ion current,MnO2,cyclohexane degradation
1.Introduction
Volatile organic compounds (VOCs) in the atmosphere can cause great concern in air quality and human health [1,2].Non-thermal plasma (NTP) is a powerful technology for VOC degradation of low concentrations [3].Many studies reported that the combination of NTP and catalysis can improve the efficiency of VOC degradation and reduce the formation of by-products [4].Xuet aldeveloped a Camodified Ni/ZSM-5 catalyst for toluene oxidation using a plasma catalytic system[5].Guoet alused a mixed surface/packed-bed discharge plasma reactor of Ag-Ce/γ-Al2O3catalysts for the degradation of a mixture of benzene,toluene,and xylene at room temperature,and found the conversion,by-product emission,and CO2selectivity can be greatly improved by using the catalyst[6].Wuet alreported that the plasma catalysis technology can not only promote toluene conversion but also improve CO2selectivity and reduce by-product nanoparticles even at a temperature up to 250 °C [7].Xiaet alused a dielectric barrier discharge(DBD) reactor equipped with a CeO2catalyst to degrade n-undecane with a high energy efficiency [8].Liet alsuggested that toluene can be decomposed by short-lived and long-lived active substances,where short-lived active substances contribute toluene oxidation to COx.The CoMnOx/TiO2catalyst can effectively decompose O3to reactive oxygen species in and post the plasma spaces,those reactive species enhancing toluene oxidation to COx[9].Many studies have affirmed the role of transition metal oxides in VOC degradation,since oxygen vacancies on transition metal oxides have strong abilities to oxidize VOCs to CO2and H2O[10].MnO2is an efficient catalyst for VOC decomposition,and has been used for the plasma-catalytic or thermo-catalytic degradation of toluene [11],o-xylene [12],and benzene [13,14].
As a typical VOC,cyclohexane mainly comes from the volatile waste gas when it is used as a solvent as well as the discharge during nylon manufacturing [20,21].A recent paper reported that the repeated inhalation of cyclohexane can produce steady hyperactivity and reduce ataxia,sedation,and seizures as the exposure to cyclohexane progressed [22,23].
In this study,the effect of N2discharge products on cyclohexane degradation over the MnO2catalyst is investigated.An operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) is used to monitor the changes in surface functional groups due to the effect of N2discharge products.Cyclohexane degradation by-products on the catalyst surfaces are analyzed using a gas chromatograph and mass spectrometry (GC-MS) and x-ray photoelectron spectroscopy (XPS).The promotion mechanism of N atoms on the degradation of cyclohexane is proposed.
2.Experiments
2.1.Experimental setup
Figure 1(a) is the experimental system used for investigating the effect of N2discharge products on cyclohexane degradation.The system includes a simulated gas generation system,a plasma catalytic reaction system,and a product analysis system.The simulated gas generation system includes N2and O2pure gas cylinders,four mass flow meters(MFC1-4,D07,Sevenstars Beijing,China),a cyclohexane bubbler (100 ml),and a gas mixer.The plasma catalytic reaction system is mainly composed of a DBD reactor (DBD reactor 1),an electric furnace(KSL,Longkou Xianke,China),a pulse power supply (M10K-08,Suzhou Allftek,China),a voltage probe (P6015A,Tektronix,USA),a current probe(CP8030H,Cybertek,China),an ion current probe (CT-1,Tektronix,USA),and an oscilloscope (MDO3024,Tektronix,USA).
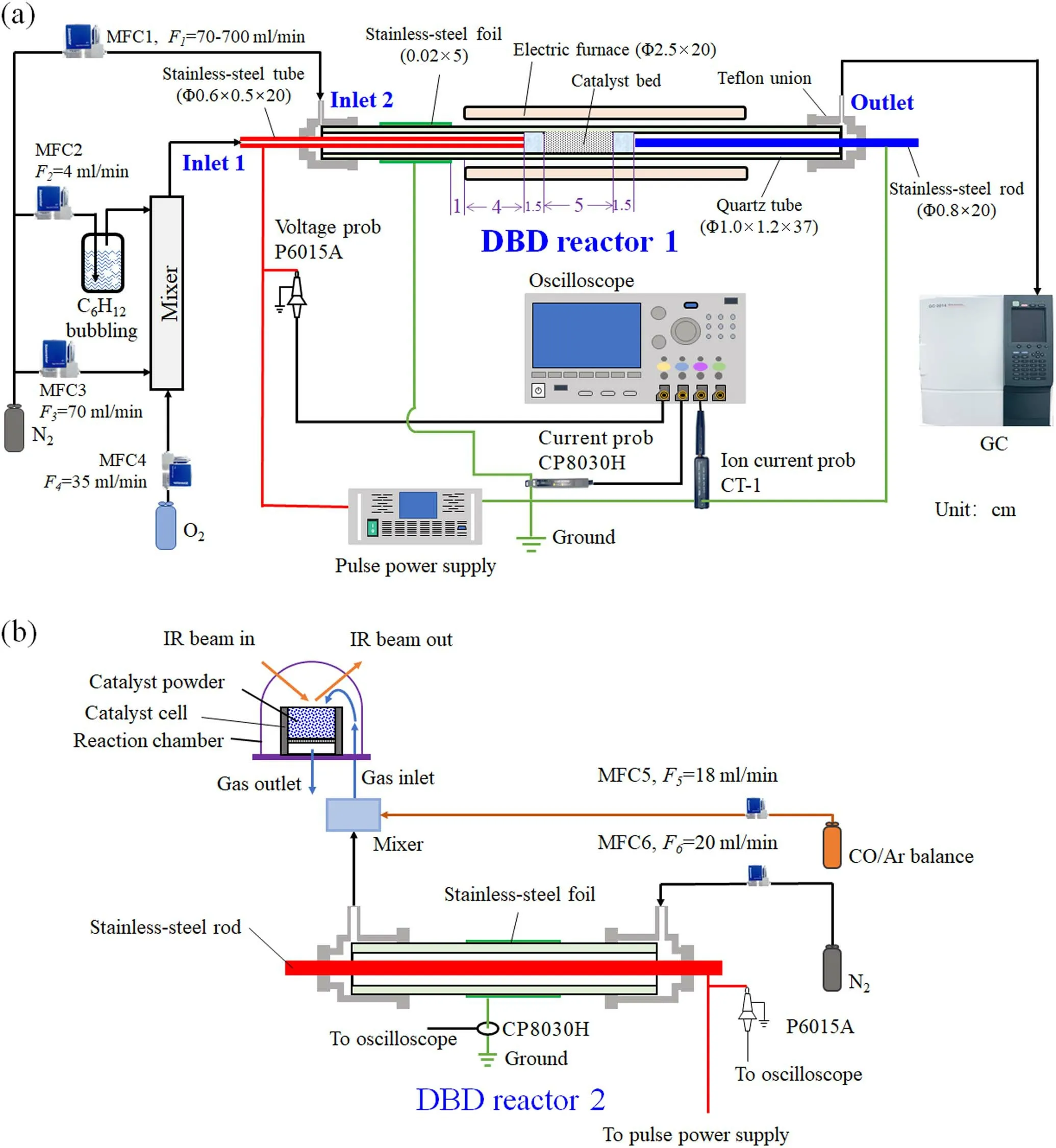
Figure 1.(a) Schematic diagram of the experimental setup for cyclohexane gradation and (b) the operando DRIFTS system.
A stainless-steel foil (as the ground electrode) and stainless-steel tube (as the high voltage electrode) were installed in the DBD reactor 1.A catalyst bed was filled with catalyst balls downstream the stainless-steel tube.A stainlesssteel rod was set after the catalyst bed.When voltage pulses are applied to the stainless-steel foil and tube,pulsed corona discharges occur in the space between the stainless-steel foil and tube.The waveforms of the discharge voltage and current were measured using the voltage prob (P6015A) and current probe(CP8030H).N2gas with a flow rate ofF1was supplied to the inlet 2 and passed through the discharge space,then mixed with the gas mixture of O2with a flow rateF4,N2with a flow rateF3,and cyclohexane (bubbling using N2with a flow rate ofF2).The mixed gas mixture flowed through the catalyst bed and out from the outlet.A part of the DBD reactor 1 was placed in a tubular electric furnace to keep the reaction temperature.When N2passed through the discharge space,N2is ionized to some charged species(such as)and decomposed to N atoms.Charged species and N atoms flowed downstream to the catalyst bed resulting in N-atom driven reactions and to the stainless-steel rod resulting in ion currents,which were measured using the ion current probe(CT-1).
The gas products in the gas mixture from the outlet of the DBD reactor 1 were online analyzed using a gas chromatograph (GC-2014,Shimadzu,Japan),where CO,CO2,and hydrocarbons with carbon number less than 5 were separated using a Porapak-N column and detected with a flame ion detector(FID).A methanizer was used between the column and FID to convert CO and CO2to CH4.Cyclohexane and hydrocarbon products with a carbon number higher than 5 were analyzed using a SE-30 capillary column and detected with another FID.The intermediates on the catalyst surface were washed off using ethanol and the wash liquids were analyzed using a GC-MS (GC-7890A,MS-5975C,Agilent,USA).
2.2.Operando DRIFTS system
Figure 1(b) shows a DRITTS (Nicolet IS50,Thermo Scientific,USA) and a reaction chamber (HVC-DRP-5,Harrick,USA) to monitor the intensity changes of surface functional groups during cyclohexane degradation.Catalyst powder (about 30 mg,100 mesh) from grinding 6wt.%MnO2/γ-Al2O3catalyst balls was loaded in a catalyst cell,where the reaction temperature of the catalyst cell was controlled using an electric heater.The catalyst powder was heated in Ar (20 ml min-1) at 300 °C for 1 h.After cooling the temperature of the catalyst powder to 25 °C,the background spectrum was collected in the Ar atmosphere.Then,a gas mixture containing CO (Ar balanced,20 ml min-1)and N2(with or without discharges)was introduced into the reaction chamber.DRIFTS spectra in the range of 800-4000 cm-1were then collected at 100 °C.
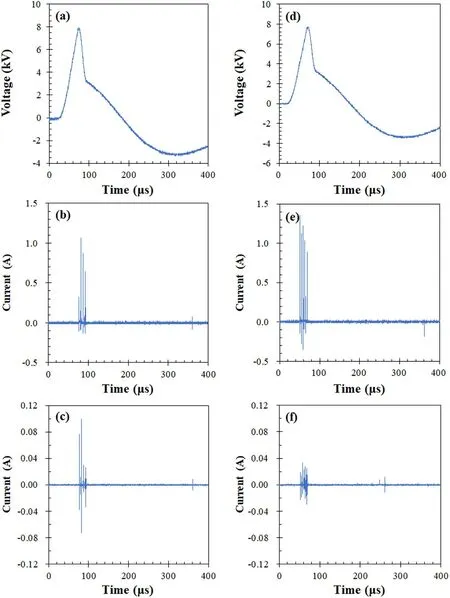
Figure 2.Typical waveforms of voltage (a),current(b),and ion current(c)using the DBD reactor without a catalyst; typical waveforms of voltage (d),current (e),and ion current (f) using the DBD with 6wt.% MnO2/Al2O3 catalyst.Experimental condition: F1 = 70 ml min-1,F2 = 4 ml min-1, F3 = 70 ml min-1, F4 = 35 ml min-1,temperature 25 °C.
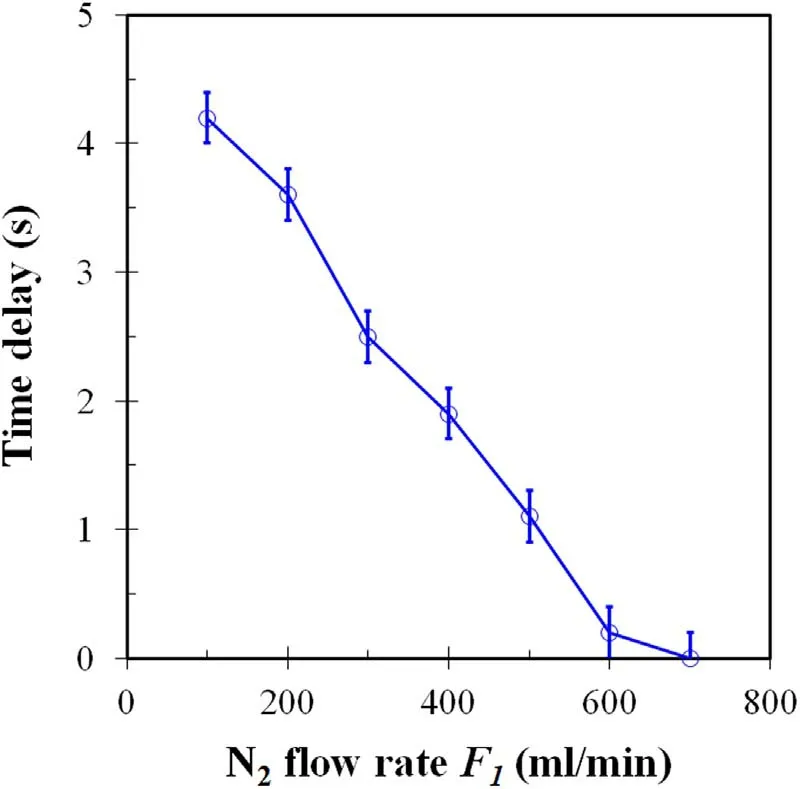
Figure 3. Time delay while ion current can be found after voltage pulses for N2 discharges were applied as a function of N2 flow rate(F1).Experimental condition: F1 = 100-700 ml min-1,F2 = F3 = F4 = 0 ml min-1,temperature 25 °C.
2.3.Calculations
Cyclohexane conversion is calculated using equation (1).
where,C0andCare the saturated cyclohexane concentration in the gas mixture from the outlet of the DBD reactor 1 without N2discharges and the cyclohexane concentration in the gas mixture from the outlet of the DBD reactor 1 with N2discharges,respectively.
2.4.Preparation and characterization of MnO2/γ-Al2O3 catalysts
MnO2/γ-Al2O3catalysts of different loadings were prepared using an equal volume impregnation method[12].Mn(NO3)2solution(AR,50wt.%in H2O,Shanghai Jiuzhou,China)was used as the precursor of MnO2.γ-Al2O3balls with a diameter of 1.5-2.0 mm (purity ≥99.7%,Shanghai Jiuzhou,China)were used as a carrier.The γ-Al2O3balls were pretreated with an ethanol liquid overnight,washed with deionized water several times,dried at 100 °C for 6 h,and then the γ-Al2O3balls were calcined at 500 °C in air for 3 h.An appropriate amount of Mn(NO3)2solution and deionized water were added to 5 g γ-Al2O3balls,placed in the dark overnight,dried at 100°C for 6 h,and calcined at 500°C for 3 h.The loadings of MnO2were 2wt.%,4wt.%,6wt.%,and 8wt.%,respectively,by changing the amount of Mn(NO3)2solution.The 6wt.% MnO2/γ-Al2O3catalyst was characterized using an x-ray diffractometer (XRD,Rigaku Ultima IV,Rigaku,Japan) and XPS (Thermo Scientific K-Alpha,Thermo Fisher Scientific,USA).The x-ray source with an energy of 1000-1500 eV was used to measure the photoelectron energy distribution,and semi-quantitatively analyze the content of Mn,O,and N elements on the catalyst surface and their corresponding valence states.
3.Results and discussion
3.1.Evidence of ion current and ion adsorption on catalyst
Figures 2(a)-(c) show typical waveforms of voltage,current,and ion current using the DBD reactor 1 without a catalyst in the catalyst bed.The voltage pulse has a peak value,rise time,and half width of 8.0 kV,40 μs,and 35 μs,respectively.The discharge current increased with the pulse voltage and peaked at 1.1 A.The ion current received using the stainless-steel rod rises to a peak value of 0.10 A with increasing pulse voltage.When the DBD reactor 1 was filled with 6wt.%MnO2/γ-Al2O3catalyst balls in the catalyst bed,the discharge current is slightly increased to 1.4 A (figure 2(e)),while the ion current is greatly decreased to 0.37 A(figure 2(f)).The remarkable decrease in ion current is obviously due to the presence of the catalyst balls in the catalyst bed as the catalyst has an ability to adsorb ions [3].When the voltage pulses were applied to the DBD reactor 1,N2molecules are ionized,resulting in the formation of ions(such as),those flowed downstream to the space around stainless steel rod,and get electrons from the stainless-steel rod,resulting the current related with ions.The ion current waveform was monitored using the ion current probe.Figure 3 shows the time delay while the ion current could be found after voltage pulses for N2discharges were applied.When the gas flow rate was 100 ml min-1,the time delay was 4.2 s,which is equal to that of the gas residence time when the gas passed through the space between discharge space and stainless-steel rod.The findings of ion currents and time delay strongly implied that ions are produced due to the N2discharges.
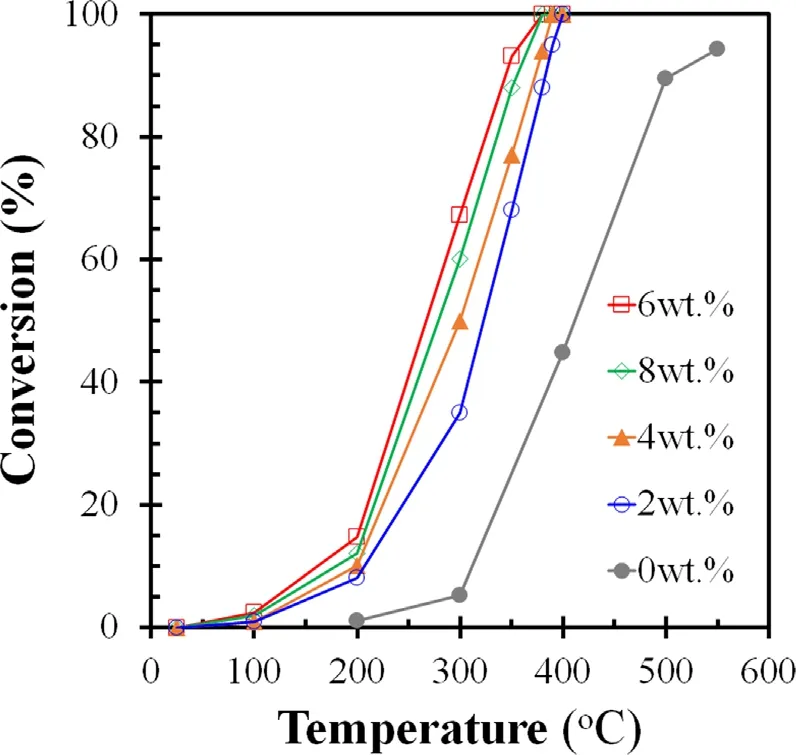
Figure 4.Effect of MnO2 loadings on γ-Al2O3 on cyclohexane conversion at various reaction temperatures.Experimental conditions: F1 = 70 ml min-1, F2 = 4 ml min-1, F3 = 70 ml min-1,F4 = 35 ml min-1,and no N2 discharges.
3.2.Effect of N-atom driven cyclohexane degradation
The loading of MnO2on Al2O3can promote cyclohexane conversion,and 6wt.% MnO2/Al2O3has the best cyclohexane conversion in comparison with other catalysts (figure 4).The effect of N2discharge products on cyclohexane conversion on 6wt.% MnO2/Al2O3catalyst was then evaluated.Figure 5(a) indicates cyclohexane conversion on the Al2O3catalyst at various discharge voltages as a function of temperature.Cyclohexane conversion increased with increasing temperature.When temperature was fixed,cyclohexane conversion increased when increasing discharge voltage.Cyclohexane conversion at a peak voltage higher than 0 kV is higher than that at 0 kV.This fact implied that discharge produced active species promoted cyclohexane degradation.The maximum promotion effect on cyclohexane conversion is 10.7% at 200 °C,and the promotion effect generally decreased with increasing temperature.When 6wt.%MnO2/Al2O3catalyst was used,cyclohexane conversion at 100 °C increased from 2.46% at 0 kV to 26.3% at 12 kV.When the reaction temperature increased to 200 °C,cyclohexane conversion increased from 14.7%at 0 kV to 32.2%at 12 kV.The cyclohexane conversion at 200 °C is higher than the sum of that (14.7%) by thermal catalysis (figure 4) and that (10.7%) on Al2O3at 12 kV (figure 5(a)),indicating that there is a synergistic effect between N2discharge products and the MnO2catalyst.
Figure 6 shows GC analysis results of the gas mixture from the outlet of the DBD reactor 1.Only CO,CO2,and C2H4were found as the products of cyclohexane degradation.CO2is the main product as it accounted for 75% of the total peak area,higher than that of CO(accounted 24%)and C2H4(accounted 1.6%).
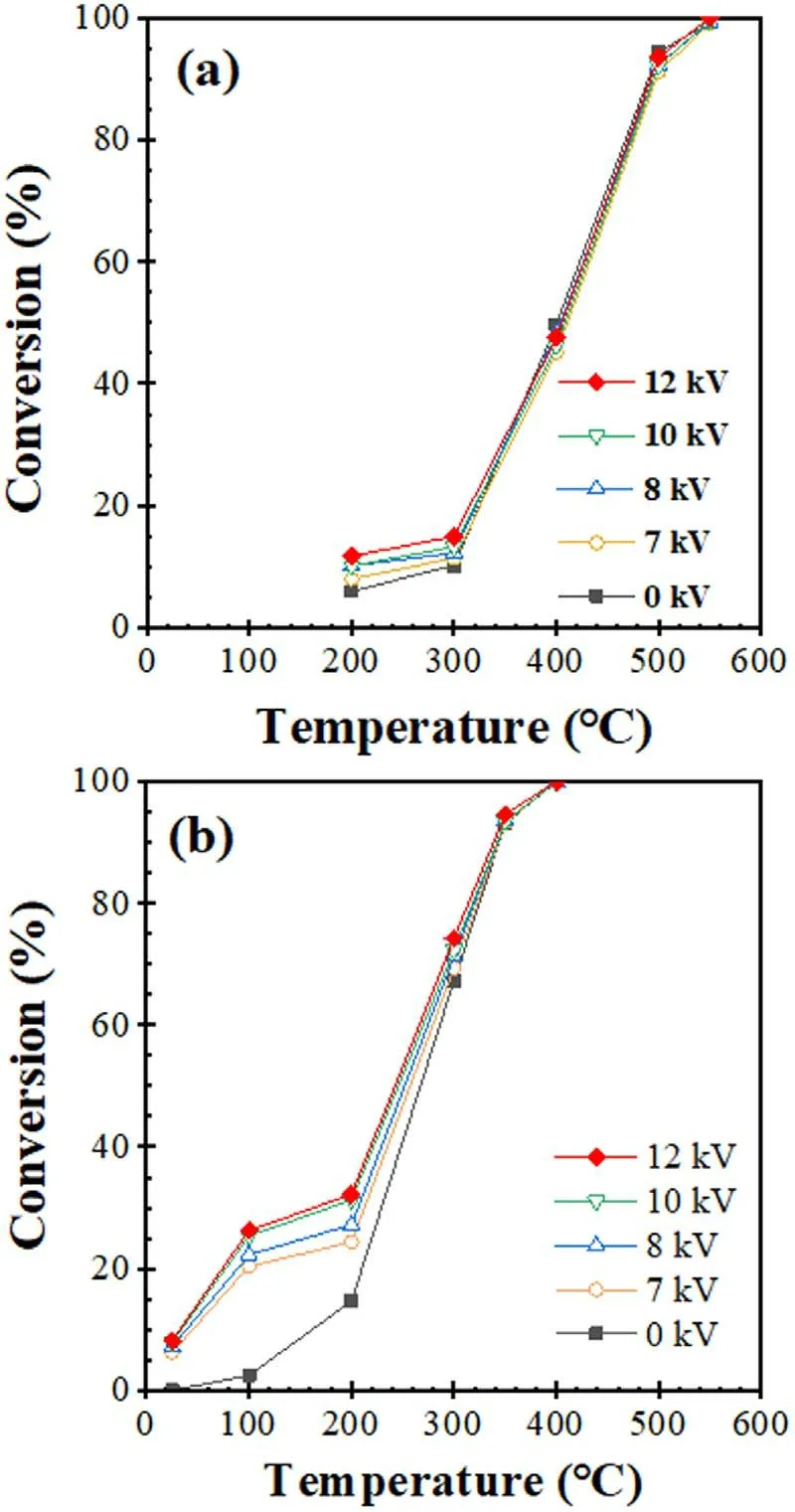
Figure 5. Cyclohexane conversion on (a) γ-Al2O3 and (b) 6wt.%MnO2/γ-Al2O3 at various peak voltages as a function of reaction temperature.Experimental conditions: F1 = 70 ml min-1,F2 = 4 ml min-1, F3 = 70 ml min-1, F4 = 35 ml min-1.
3.3.Catalyst characterization
The 6wt.% MnO2/γ-Al2O3catalyst used for cyclohexane degradation at 300 °C,12 kV,and 200 Hz for 150 min was washed with ethanol liquid and the washed ethanol liquid was analyzed using the GC-MS.A by-product (3,4-dehydroproline,C5H7NO2) was clearly found (figure 7).The by-product obviously contained N and O atoms in its molecule.This by-product is possibly generated via the ring open reaction by the O atom and cyclization reaction by NH and oxidation to carboxylic acid with the O atom.
Figure 8 shows the XRD patterns of the fresh and used 6wt.% MnO2/γ-Al2O3catalyst.The peaks at 29°,43°,57°,59°,and 72°can be assigned to the β-MnO2crystals(JCPDS No.24-0735).The peaks at 37°,46°,and 67°can be indexed to both γ-Al2O3(JCPDF NO.29-0063)and β-MnO2crystals.The XRD patterns did not change after 150 min reaction at 300 °C,12 kV,and 200 Hz.
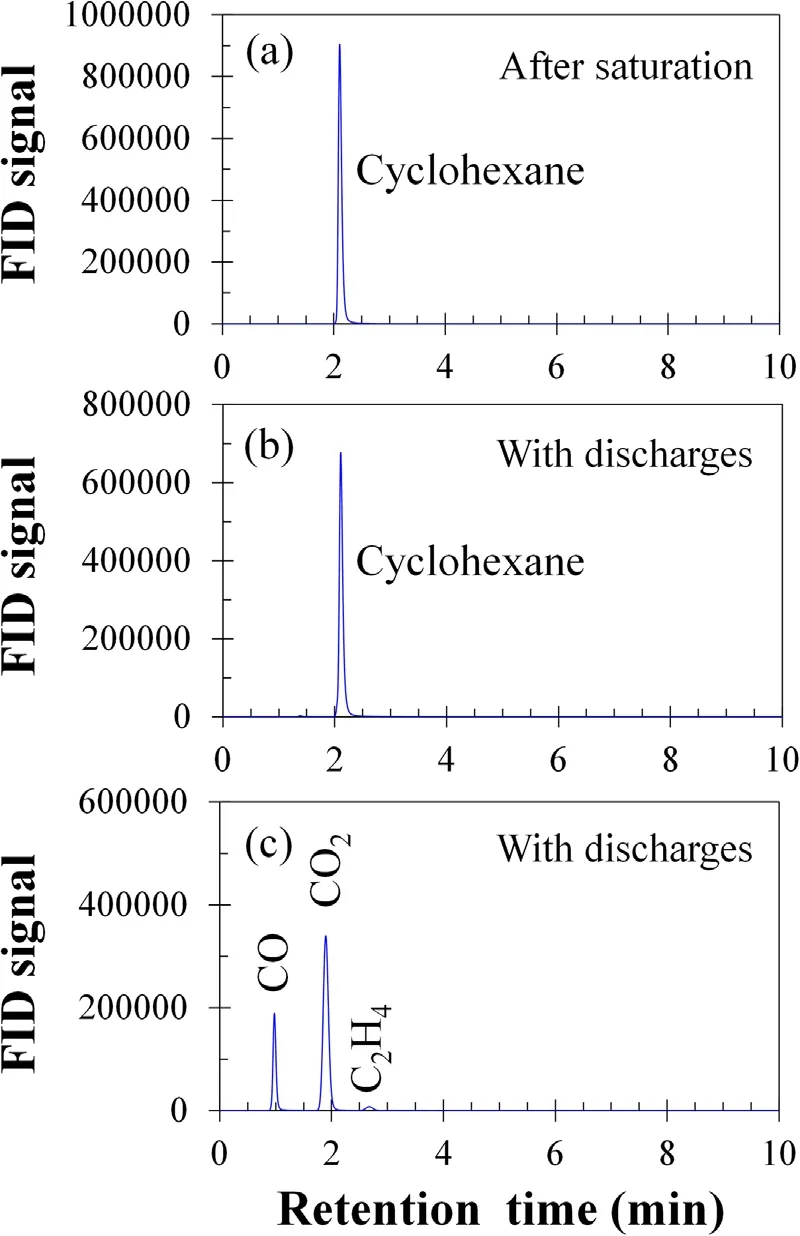
Figure 6.GC analysis results during cyclohexane degradation on 6wt.% MnO2/γ-Al2O3.(a) Analysis result of the gas mixture using SE-30 capillary column after cyclohexane saturation at 25 °C.(b)Analysis result of the gas mixture using SE-30 capillary column during cyclohexane degradation at 100 °C with discharges (12 kV and 200 Hz).(c) Analysis result of the gas mixture using the Porapak-N column during cyclohexane degradation at 100 °C with discharges (12 kV and 200 Hz).Experimental condition:F1 = 70 ml min-1, F2 = 4 ml min-1, F3 = 70 ml min-1,and F4 = 35 ml min-1.
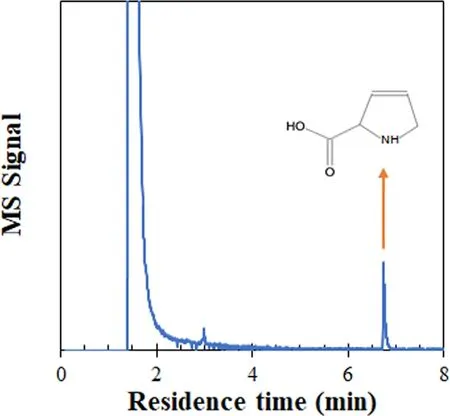
Figure 7.GC-MS spectrum of the ethanol liquid after washing 6wt.% MnO2/γ-Al2O3 catalyst (300 °C,12 kV,and 200 Hz for 150 min).
Figure 9 shows HRTEM and EDS mapping images of 6wt.% MnO2/γ-Al2O3catalyst.The lattice spacing of 0.22 and 0.24 nm is corresponding to(1 0 1)and(2 0 0)planes of the MnO2crystal.EDS mapping shows that fine MnO2particles were uniformly dispersed on the γ- Al2O3support.

Figure 8. XRD patterns of fresh and used 6wt.% MnO2/γ-Al2O3 catalysts (300 °C,12 kV,and 200 Hz for 150 min).
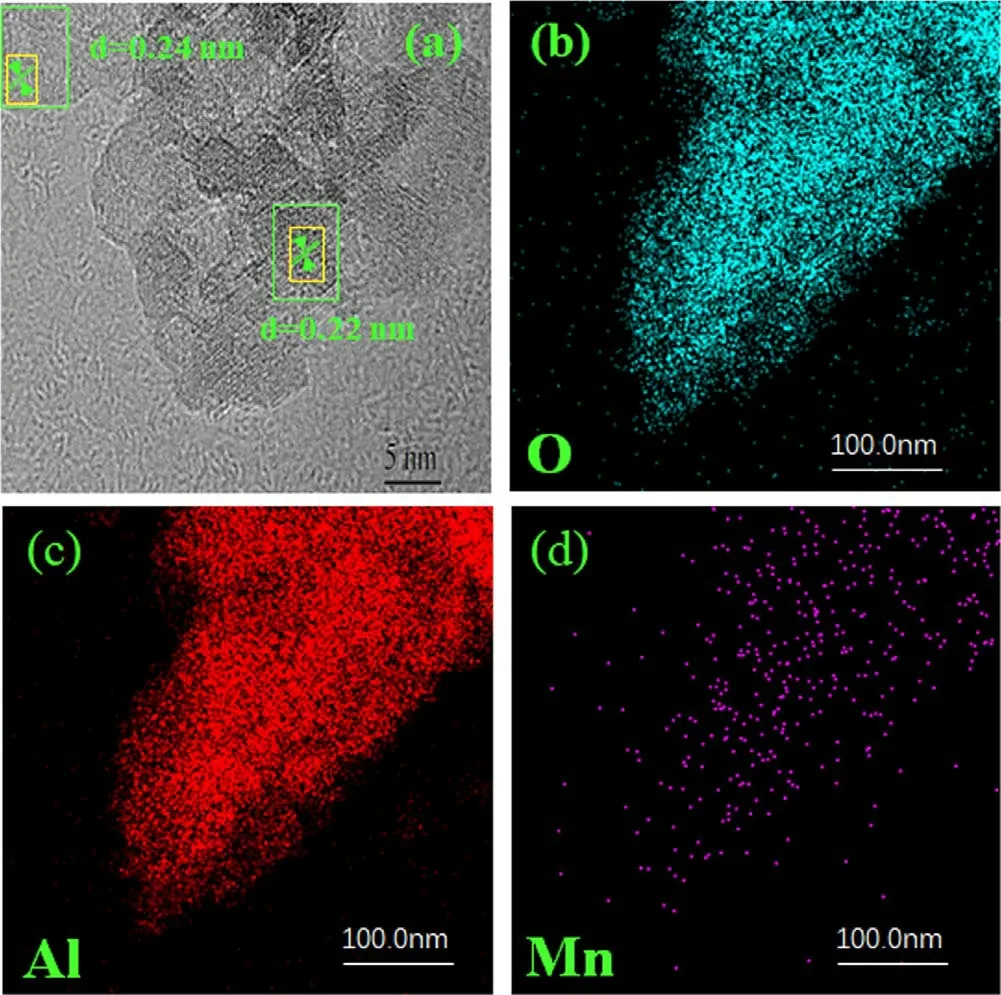
Figure 9.HRTEM images(a)and EDS mapping of O(b),Al(c),and Mn (d) elements on the 6wt.% MnO2/γ-Al2O3 catalyst.
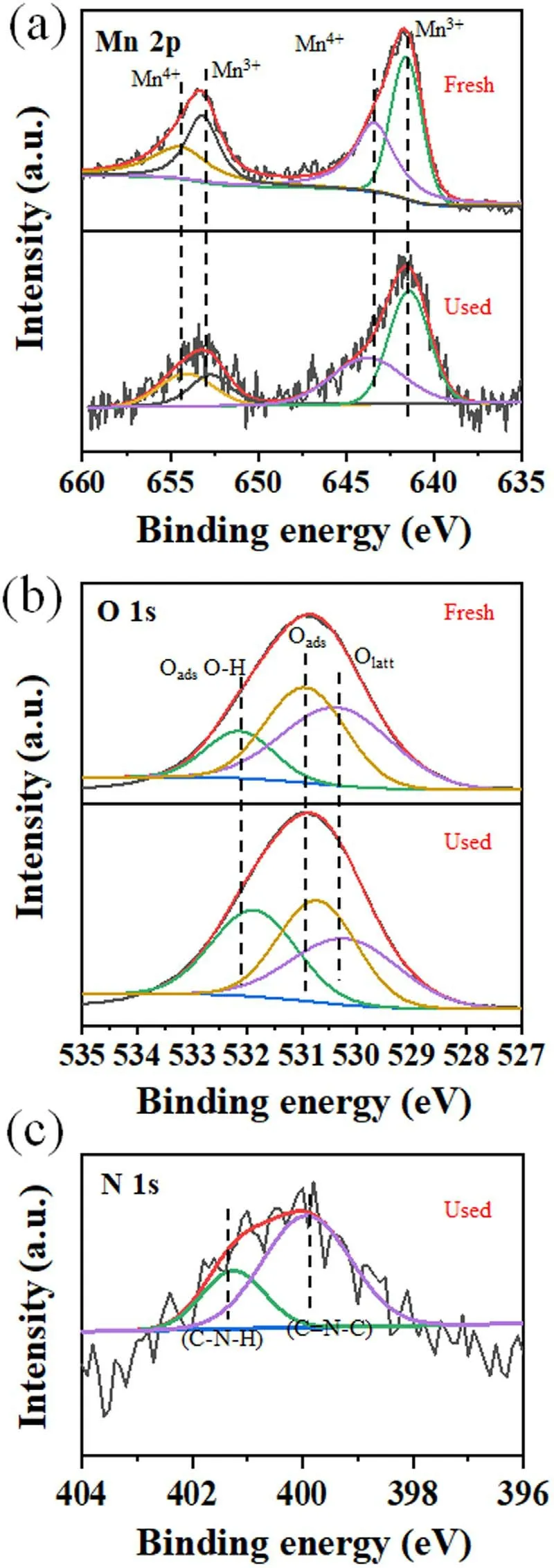
Figure 10.XPS spectra of Mn(a)and O(b)on the fresh catalyst,and N(c)on the used catalyst(300°C,12 kV,and 200 Hz for 150 min).
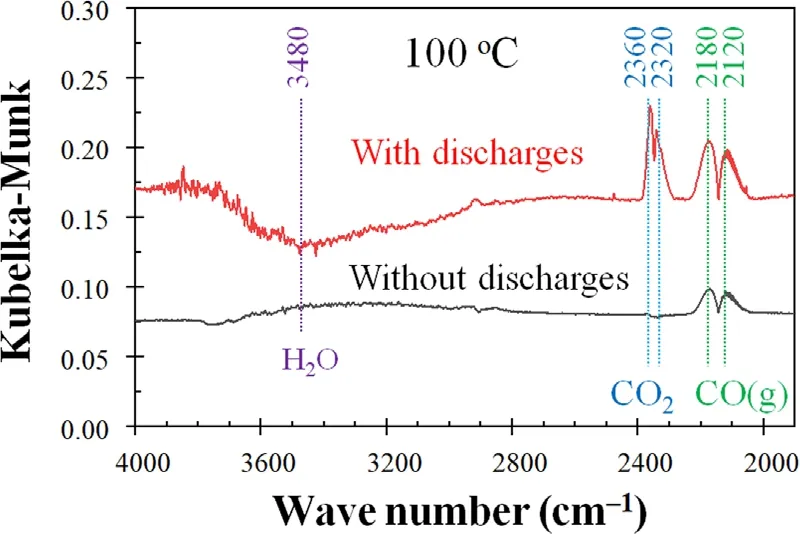
Figure 11.DRIFTS spectra when the 6wt.%MnO2/γ-Al2O3 catalyst fed with the gas mixture with or without N2 discharges.Experimental condition: cell temperature 100 °C,discharges at 12 kV and 200 Hz.
XPS characterization results on the states of the surface elements Mn 2p,O 1 s,and N 1 s of the 6wt.%MnO2/γ-Al2O3catalyst are shown in figure 10.The peaks with binding energies around 641.5-641.9 eV and 653.3-653.7 eV are attributed from Mn4+and Mn3+(figure 10(a)),proving the existence of Mn3+and Mn4+at the catalyst surfaces.The XPS spectrum of O 1 s is shown in figure 10(b).The peak with binding energy near 530.5 eV is the characteristic peak of surface adsorbed oxygen (Oads),the peak with binding energy near 531.5 eV is the characteristic peak of lattice oxygen (Olatt),and the peak near 531.5 eV is the surface characteristic peak of hydroxyl oxygen (OH)ads.
The element ratios of Mn3+/Mntotalat the fresh and used 6wt.% MnO2/γ-Al2O3catalyst surfaces are 51.90% and 53.08%,and those of Oads/Ototalare 59.11% and 61.27%,respectively.No obvious changes in element ratios of Mn3+/Mntotaland Oads/Ototalwere found between the fresh and used 6wt.%MnO2/γ-Al2O3catalysts,this finding means the cyclohexane degradation process did not change the catalyst.
There is no N element on the fresh catalyst,while the N element was found on the used catalyst after cyclohexane degradation fed with N2discharge products (figure 10(c)).This finding proves that after plasma treatment,N2is decomposed to N,which reacts with cyclohexane or its oxidation intermediates to form N-containing species(3,4-dehydroproline,C5H7NO2as per figure 7) and remains on the catalyst surface,inferring that it has a bonding of C-N-H and C=N-C within the 3,4-dehydroproline molecule.
3.4.Surface reaction observation using operando DRIFTS
The promotion mechanism of N atoms on 6wt.%MnO2/γ-Al2O3catalyst was observed using the operando DRIFTS (figure 1(b)) with or without N2discharges.As shown in figure 11,CO peaks at 2180 and 2120 cm-1[24]surface CO2peaks 2360 and 2320 cm-1[25,26],and the water-related peaks at 3480 cm-1[27]could be observed.When there were no N2discharges,only CO peaks could be found.When there were N2discharges,the surface CO2peaks became positive,and water peaks negative.This finding implied that CO2was generated and surface water took part in the CO reaction.
3.5.Mechanism of N2 discharge products on cyclohexane degradation
The by-product 3,4-dehydroproline from cyclohexane degradation is the result of C-C bond cleavage in the cyclohexane molecule and the incorporation of N and O atoms into the cyclohexane molecule.The N atoms are obviously from the N2discharges.Together with the fact that ion currents were found in the gas steam downstream the N2discharge space,it is true that N2discharges produced charged species and N atoms,those charged species and N atoms flowed downstream to the catalyst bed,resulting in cyclohexane degradation driven by ions and N atoms.
The N atoms can be used to generate O atoms from the reaction of O2with N atoms by the Zeldovich mechanism(equation (2)) [28].The reaction of N atoms and H2O has been suggested by Umemotoet al[29]and Homayoonet al[30],where NH and OH are the main products(equation(3));where N(2D)atoms exist in the N2discharge spaces[15].Our previous study found that during N2-O2pulsed discharge at atmospheric pressure and room temperature,emission spectrum lines of N2(C3Πu →B3Πg)have been clearly observed[31],N(2D) may be generated from N2(C3Πu) [32].The surface OH can react with CO to yield H and CO2(equation(4))[33,34].The H atoms may react with O2to OH(equations (5) and (6)) [33].
O and OH are reactive,which can initiate the dehydrogenation of cyclohexane (C6H12) to produce cyclohexane free radicals(·C6H11)(equation(7)).·C6H11can react with O,OH,and O2completely to H2O and CO2(equation (8)).
4.Conclusions
The effect of N2discharge products on cyclohexane degradation has been investigated using a specially designed DBD reactor.N2discharges generate charged species and N atoms,and those can move to the catalyst bed and deposit on the catalyst surfaces.N atoms can promote the formation of O atoms by Zeldovich mechanism and the formation of surface OH via N + H2O = NH + OH reaction.N,O and OH contribute cyclohexane degradation to CO and CO2.
Acknowledgments
This research was supported by National Natural Science Foundation of China (No.12075037),the Postgraduate Research&Practice Innovation Program of Jiangsu Province(No.KYCX21_2873) and Research and Application Service Platform Project of API Manufacturing Environmental Protection and Safety Technology in China(No.2020-0107-3-1).
 Plasma Science and Technology2023年2期
Plasma Science and Technology2023年2期
- Plasma Science and Technology的其它文章
- Square grid pattern with direction-selective surface discharges in dielectric barrier discharge
- Magneto-hydrodynamic simulation study of direct current multi-contact circuit breaker for equalizing breaking arc
- Oxidation of ciprofloxacin by the synergistic effect of DBD plasma and persulfate:reactive species and influencing factors analysis
- Numerical simulation and performance analysis of the radiofrequency inductive cathode
- Experimental and numerical investigation of a self-supplementing dual-cavity plasma synthetic jet actuator
- Self-absorption effects of laser-induced breakdown spectroscopy under different gases and gas pressures
