Beta-secretase 1 Lowers β-Dystroglycan Protein Levels*
YUAN Fang, WU Zu-Jun, LONG Zhen-Yu, BI Dan-Lei, SHEN Yong
(1)Institute on Aging and Brain Disorders, The First Affiliated Hospital of USTC, School of Life Science, Division of Life Science and Medicine,University of Science and Technology of China, Hefei 230026, China;2)Neurodegenerative Disorder Research Center, CAS Key Laboratory of Brain Function and Disease, University of Science and Technology of China,Hefei 230026, China;3)Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai 200031, China)
Abstract Objective Beta-secretase 1 (BACE1) is the key enzyme for amyloid β (Aβ) production in the Alzheimer’s disease(AD) brain. The dystroglycan (DG) protein anchors astroglial endfeet onto cerebral blood vessels forming glia limitans, a supportive element in the blood-brain barrier. An untargeted proteomics study predicted that BACE1 downregulates DG expression. Here, this study investigated whether BACE1 modulates the protein levels of DG and its hypothetic mechanism. Methods Transient transfection technique was used to express target protein in HEK-293T cells and in primary mouse astrocytes. And the protein levels of targets were analyzed by Western blot. Quantitative polymerase chain reaction and co-immunoprecipitation were used to explore the potential mechanisms of BACE1-dependent regulation of DG. Results This study found that addition of BACE1 resulted in significantly lower levels of β subunit DG (β-DG) protein, both in HEK-293T cells and in primary mouse astrocytes. And in HEK-293T cells, this down-regulation of β-DG protein dependent on the enzyme activity of BACE1. Conclusion BACE1 lowers β-DG protein levels in HEK-293T cells and in mouse astrocytes.
Key words BACE1, β-dystroglycan, astrocyte, Alzheimer’s disease
BACE1 is known for cleaving amyloid precursor protein (APP) to produce amyloid β (Aβ), which is regarded as the key pathological mechanism in Alzheimer’s disease (AD)[1]. Levels of BACE1 protein and enzymatic activity levels are upregulated in the brains of AD patients[2-4]. BACE1 is one of the most promising candidate drug targets for AD.However, BACE1 is a transmembrane aspartyl protease and has a growing list of known substrates and interacting proteins besides APP[5]. Data from a mass spectrometry quantitative proteomics study showed that the level of released dystroglycan (DG)ectodomain was significantly lower in the cerebrospinal fluid of BACE1-/-mice than in control mice[6]. This suggests that the protein levels of DG,the core component of the dystrophin-glycoprotein complex, may be down-regulated by BACE1.
DG is a type I membrane protein encoded by a single gene,DAG1[7]. Mature DG contains two subunits (α-DG and β-DG), which are bondedvianoncovalent interactions[8]. β-DG has a transmembrane domain, which connects the intracellular cytoskeleton to the extracellular matrixviaα-DG. Deficiency of DG is implicated in congenital muscular dystrophy (CMD), a group of lethal muscular degenerative disorders characterized by progressive muscular atrophy[9]. Many CMD patients and CMD mouse models exhibit mental defects and breaches of the glia limitans[10-11].Mutations of DG (p. Cys669Phe) lead to severe muscle-eye-brain malformation[12]. In the brain, DG is mainly expressed in astrocytes and neurons and regulates the glia limitans, neuron migration, and axon guidance of neurons[13-16]. Astrocytic DG serves as a transmembrane receptor, anchoring astrocyte endfeet onto the basal membranes of cerebral vessels[17-18]. Proteolytic degradation of β-DG by matrix metalloproteinase (MMP) leads to compromised blood-brain barrier (BBB) integrity, and leukocyte extravasation, in the brains of an experimental autoimmune encephalomyelitis mouse model[13].
In both AD patients and the Tg2576 AD mouse model, reactive astrocytes exhibit BACE1 expression[19]. However, it is not known whether BACE1 can affect astrocytic DG. In this study, we demonstrated that BACE1 downregulated the level of β-DG protein in HEK-293T cells and in mouse astrocytes. This newly identified link between BACE1 and β-DG may potentially account for the impairment of the glia limitans in AD brain and may consequently exacerbate Aβ pathology and cognitive impairment.
1 Materials and methods
1.1 Expression plasmids
The expression constructs used were BACE1-GFP-FUGW, GFP-FUGW, DG-pcDNA3.1 and empty pcDNA3.1 vector.
1.2 Cell culture and treatment
A HEK-293T cell line was grown in plastic plates (Costor #3506/3512) with Dulbecco’s modified Eagle medium (HyClone #SH30243.FS) containing 10% fetal bovine serum (FBS) (Biological Industries#04-001-1ACS), 1% penicillin and streptomycin(HyClone #SV30010) and maintained in a cell incubator (37°C, 5% CO2). DG and BACE1 plasmids were transfected into cells using lipofectamine 2000 reagent (Thermo Fisher Scientific #11668019)according to manufacturer instructions. Either BACE1 inhibitor IV (Merck #565788) or proteasome inhibitor epoxomicin (EPOXO)(Merck #324800) was added and protected from light 6 h after transfection and incubated overnight; an equal volume of dimethyl sulphoxide (DMSO) was used as vehicle control.
1.3 Generation of 293T-BACE1-/- cells
We used the following spacers for the human BACE1 (gene ID: 23621) knockout:sgBACE1-1,GGATCCGGAGCCCGCTACAT;sgBACE1-2, CGGGCTCTTCGTCGGTCTCC;sgBACE1-3, TACTACGTGGAGATGACCGT (Sangon Biotech Co. Ltd.,Shanghai). The DNA oligomers targeting BACE1 spacers were cloned into a modified form of pX458(Addgene #48138) or pX459 (Addgene #62988) using double BbsI enzymatic cleavage sites, generating pX458-sgBACE1-(2, 1) and pX459-sgBACE1-(3, 2)constructs. HEK-293T cells were co-transfected with pX458-sgBACE1-(2, 1) and pX459-sgBACE1-(3, 2)using Lipofectamine 3000 (Thermo Fisher Scientific#L3000001). 24 h after transfection, puromycin(1 mg/L, Solarbio #P8230) was added to the cell medium. 48 h after transfection, single cells were isolated according to GFP signalviacell sorting (BD,Facsaria) and expanded in a 96-well plate (Costor#3596). DNA and proteins were extracted from these cells to identify a homozygous BACE1 knockout in the HEK-293T cell line.
1.4 Astrocyte isolation and culture
Primary astrocytes were isolated from neonatal C57BL/6J mice pups at postnatal day 1 to day 3,following a published protocol[20]. The experiments involving animals were performed in compliance with the requirements of the Institutional Animal Care and Use Committee of the University of Science and Technology of China. Briefly, 4 mouse brains were extracted and scissors and forceps were used to remove the meninges. The cortical hemispheres were isolated, minced using a blade, and digested in 0.125% trypsin (GIBCO #25200056) at 37°C for 10 min. A pipet was used to dissociate tissue in the suspension. Cells were strained through a 40-μm cell filter (Biosharp #70120205) and centrifuged (800×gfor 3 min), before being seeded in a T75 flask (Costor#3814) containing growth medium DMEM/F12(HyClone #SH30023.01B) supplemented with 10%FBS, 0.4% penicillin and streptomycin and maintained under incubation at 37°C with 5% CO2.When 90% confluency was reached (normally 5-6 d after the first split), the flasks were shaken at 200 r/min for 6 h to remove microglia and oligodendrocyte precursor cells. Cells were detached from the flask using 0.25% trypsin, incubated at 37°C for 2 min, centrifuged, re-suspended in growth medium, counted, and divided equally into two T75 flasks. Passage 2 can yield 1.5×106-2×106cells. For BACE1 plasmid transfection, we seeded cells in a 12-well plate after passage 2 at a density of 30 000 cells per well and transfected these cells with 4 μg BACE1 plasmid or 4 μg empty vector per wall as described above.
1.5 Western blot
Cells in the culture plate were collected in radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with protease inhibitors (Thermo#A32961) to extract total protein. After ultrasonic cell breaking and centrifuging, the supernatant was preserved and the total protein concentration was quantified using BCA kits (Biosharp #BL521A).Protein samples were adjusted to equal concentrations by diluting in RIPA lysis buffer and adding and equal volume of 2×loading buffer. Protein samples were subjected to 10%-15% sodium dodecyl sulfatepolyacrylamide gels and separated by electrophoresis and then transferred onto polyvinylidene fluoride membranes (Immobilon-P #IPVH00010). The blots were then blocked in 5% milk (BD #232100) for 1 h at room temperature prior to incubation with the target antibodies (mouse anti β-DG, Santa Cruz Biotechnology #sc-165998, 1∶1 000; rabbit anti BACE1, Cell Signaling Technology #5606S, 1∶1 000;mouse anti GAPDH, Sigma #G8795, 1∶1 000;mouse anti α-tubulin, Proteintech #66031-1,1∶100 000) at 4°C overnight, followed by incubation with peroxidase-conjugated secondary antibodies(Jackson ImmunoResearch #211-032-171; CST#58802), 1∶5 000, at room temperature for 1.5 h.The blots were developed using the Supersignal West Femto Substrate (Thermo Fisher Scientific #A38556)and recorded using a Chemiluminescence imaging system (Clinx). The grayscale value of target protein in Western blots were quantified using ImageJ software and normalized to that of loading control(GAPDH or TUBULIN); values were expressed as fold above the mean of control cells.
1.6 Co-immunoprecipitation
HEK-293T cells were co-transfected with 4 μg of DG and 2 μg of BACE1 per well in a 6-well plate as described above. The transfected cells were cultured for 24 h to reach full confluency. Cells were harvested using 500 μl RIPA lysis buffer per well and sonicated for 10 s. Cell lysate from 2 wells was prepared as one single sample and 100 μl of lysate was spared and mixed with 2×loading buffer as an input control; 2 tubes were prepared with 60 μl of Protein G PLUSAgarose (Santa Cruz Biotechnology #sc-2002), and centrifuged at 5 000×gfor 15 s at 4°C. The supernatant was aspirated and the agarose pellet was re-suspended with 500 μl PBS buffer containing 0.12% BSA (Sangon Biotech #A600332-0100). In one tube, 4 μl of β-DG antibody was added and in the other tube, 2 μl of normal mouse IgG (Santa Cruz Biotechnology #sc-2025) was added. The tubes were incubated for 4 h at 4°C in an orbital shaker.Following centrifugation at 5 000×gfor 1 min, the supernatant was aspirated, and the agarose pellet was washed 3 times with PBS buffer. Cell lysate (450 μl)was added to each tube and incubated with the agarose pellet overnight at 4°C in an orbital shaker for immunoprecipitation. After centrifugation at 5 000×gfor 1 min, the supernatant was aspirated, and the agarose pellet was washed 5 times with the RIPA lysis buffer. Loading buffer (100 μl) was added in the last wash. Samples were then boiled for 10 min and evaluated using Western blot.
1.7 Quantitative polymerase chain reaction
The total RNA of HEK-293T cells were extracted using RNAex Pro Reagent (AG #21101)according to manufacturer instructions. Reverse transcription (RT)-quantitative polymerase chain reaction (qPCR) was performed using a Prime Script?RT reagent Kit (Takara, # RR047Q) on a Light Cycler 96 real-time PCR system (Roche, Swit). Primer sequences were:
Humanactb:5” -CATGTACGTTGCTATCCAGGC-3” (forward), 5” -CTCCTTAATGTCACGCACGAT-3” (reverse);
Humandag1: 5” -TCGAGTGACCATTCCAACAGA-3” (forward), 5” -CACACCCTTATCAGTGTCAA-3” (reverse);
Humanbace1: 5” -TGATCATTGTGCGGGTGGAGA-3” (forward), 5” -TGATGCGGAAGGACTGGTTGGTAA-3” (reverse).
Primers were used at a concentration of 10 μmol/L.cDNA, primers and TB Green were mixed according to manufacturer instructions.Actbwas used as the reference gene. Changes were normalized to the control values mean.
1.8 Statistical analyses
Each experimental assay was performed at least 3 times and all values were combined for analysis.Data were analyzed with GraphPad Prism v8.4 software. Statistical analyses between two groups were performed with unpairedttest andPvalues<0.05 were considered statistically significant. All data are shown as means ± s.e.m.
2 Results
2.1 BACE1 downregulates β-DG protein level in HEK-293T cells
The DG ectodomain is significantly lower in the cerebrospinal fluid of BACE1-/-mice than in control mice, which suggests that DG is regulated by BACE1[6]. To explore the relationship between BACE1 and DG, we designed a co-transfection experiment in HEK-293T cells to achieve simultaneous expression of DG and BACE1. From the Western blot of whole-cell lysate, we found that the band intensity of β-DG (43 ku) was significant lower by 30% in DG+BACE1 cellsvs. DG+vehicle cells, as detected using β-DG C-terminal antibody (Figure 1a,b). As BACE1 transfection level increased, the β-DG protein level decreased in a dose dependent manner and at each incremental BACE1 level (0.5, 1, 2 μg)β-DG was significantly lower than none-BACE1 group (Figure 1c, d). On the other hand, when the endogenous BACE1 in HEK-293T cells were knocked out using the CRSPR-Cas9 gene editing technique, the β-DG protein level was 31% higher in 293T-BACE1-/-cells than wild type (WT) 293T cells(Figure 1e, f). Taken together, these results show that BACE1 downregulates β-DG protein level in transfected HEK-293T cells.
2.2 BACE1 lowers endogenous β-DG protein levels in mouse astrocytes
The first set of analyses examined the impact of BACE1 on β-DG. Since β-DG plays an important role in the architecture of astrocytic endfeet on brain vessel walls[17], and BACE1 is found upregulated in reactive astrocytes in proximity to Aβ plaques in the brains of AD patients and an AD mouse model(Tg2576)[21], we next investigated whether BACE1 could lower endogenous β-DG in astrocytes. Because normal cultured astrocytes express very low levels of BACE1[22], we transfected primary astrocytes with BACE1 or vehicle plasmids. Using Western blot analysis, we found that the β-DG protein level was 45% lower in BACE1 overexpressing astrocytes relative to the control group (Figure 2a, b). It has been suggested that β-DG is a substrate of MMP. MMP cleaves β-DG and produces a 30 ku C-terminal fragment (CTF)[23-24]. Our results do not appear consistent with this, since we did not detect any β-DG CTF in astrocyte whole-cell lysate. Thus, it is unlikely that MMP contributes to this downregulation of β-DG in astrocytes. These data indicate that BACE1 can lower astrocytic β-DG protein levels.
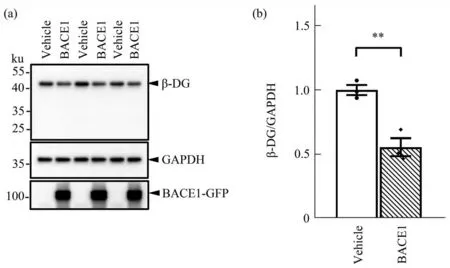
Fig. 2 BACE1 lowers the protein level of endogenous β-DG in mouse astrocytes
2.3 BACE1 regulates β-DG in an enzymaticactivity-dependent manner via protein-protein interaction
To further investigate the mechanism of BACE1-dependent regulation of β-DG, we performed a RTqPCR analysis in HEK-293T co-transfected cells.There was no significant difference in mRNA levels of β-DG between the DG+BACE1 and DG+vehicle groups (Figure 3a). This indicates that BACE1 does not lead to lower β-DGviatranscriptional inhibition.On the other hand, a co-immunoprecipitation (co-IP)assay of transfected HEK-293T cells showed that β-DG antibody IP can pull down BACE1 band(Figure 3b), which suggests a protein-protein interaction between BACE1 and β-DG. Treatment with 5 μmol/L and 25 μmol/L of the BACE1 inhibitor(BI) significantly increased the band intensity of β-DG by 87% and 120%, respectively, compared with the treatment with DMSO as vehicle control in HEK293T cells transfected with β-DG and BACE1(Figure 3c, d). Thus, the downregulation of β-DG depends on BACE1 enzymatic activity. If BACE1 downregulates β-DG by cleavage, a β-DG CTF would be expected in the Western blot results. However, we did not detect any BACE1-related β-DG CTF when BACE1 was overexpressed in astrocytes or HEK-293T cells. Thus, we assume that the putative β-DG fragment was degraded by proteasomes. We treated HEK-293T cells with proteasome inhibitor EPOXO to inhibit small fragment degradation and separated the total protein of the cell lysate using polyacrylamide gels. However, there was still no BACE1 specific β-DG CTF detected in the resulting immunoblots(Figure 3e). Collectively, BACE1 downregulates β-DG neither by directly cleavage nor by transcriptional pathway. A possible explanation for this regulation of β-DG by BACE1 might be translational or post-translational modification.Further investigation is needed to determine which mechanism underlies our findings.
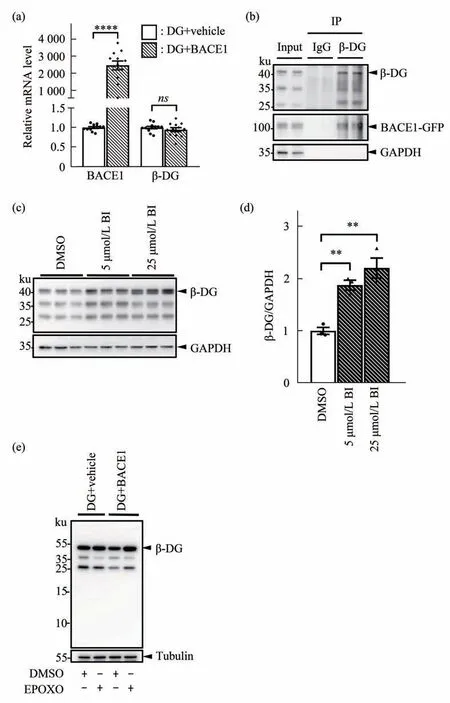
Fig. 3 BACE1 regulates β-DG in an enzymatic-activity-dependent manner via protein-protein interaction
3 Discussion
3.1 Potential mechanisms of BACE1-dependent downregulation of β-DG
We demonstrated that BACE1 lowered β-DG protein levels without alteringDGmRNA levels. The downregulation of β-DG was inhibited by inhibition of BACE1, suggesting that this regulation depends on enzymatic activity. Furthermore, we have also identified an interaction between BACE1 and β-DG using a co-IP assay. Recent studies have demonstrated that BACE1 regulates the gating of Na+and K+channels in a non-proteolytic manner[25]. Therefore,we propose that BACE1 downregulates β-DG at a post-translational levelviaa direct protein-protein interaction.
DG links extracellular matrix and intracellular actin cytoskeleton to transduce signals. β-DG, the transmembrane DG subunit, represents one full-length protein and two fragments with the molecular mass of 43, 35 and 26 ku, respectively. The 35 ku and 26 ku fragments are generated following cleavage of 43 ku full-length β-DG by γ-secretase and MMP2/9[26-27].BACE1 overexpression in HEK-293T cells led to all the 3 β-DG bands in the Western blot results being lower than control cells. Maintenance of glia limitans by β-DG does not require the last 15 amino acids at the cytosolic tail, as a mouse model with this deletion does not show disruption of glia limitans[28]. In this study, the 43 ku full-length β-DG was lower when BACE1 was expressed in astrocytes, suggesting a disruption of glia limitans. The γ-secretase-dependent 26 ku β-DG fragment enters the nucleus and mediates transcription of some androgen-regulated genes,which may be related to the progression of prostate cancer[29-30]. Therefore, BACE1 may be involved in the development of cancer by mediating levels of the 26 ku β-DG fragment.
3.2 Downregulation of β-DG by BACE1 in astrocytes may underlie blood-brain barrier leakage
In this study, we have demonstrated that BACE1 downregulated β-DG protein levels in HEK-293T cells and in primary astrocytes. Astrocytic DG stabilizes the endfeet architecture on blood vessel maintaining the integrity of glia limitans[31-32].Aberrant degradation of DG leads to the leakage of BBB[13]. Therefore, it seems highly probable that this newly identified BACE1-dependent reduction of DG in astrocytes leads to compromised BBB integrity in AD.
In addition to maintaining the BBB, DG may also mediate Aβ clearance in the AD brain. Aquaporin-4(AQP4) is the predominant water channel protein in brain and facilitates Aβ clearance along the paravascular pathway and perivascular glymphatic pathway[33-35]. AQP4 promotes Aβ clearance in a manner dependent upon the polarized localization at astrocyte endfeet[36-37]. Association between the DG complex and laminin is important for endfeet localization of AQP4[18,38]. Therefore, decreased astrocytic DG likely leads to impaired Aβ clearance.These mechanisms may explain why loss of DG contributes to BBB leakage and cognitive impairment.
3.3 BACE1 may downregulate β-DG in neurons
BACE1 is predominantly expressed in neurons and is significant upregulated in the AD brain[39-41].BACE1 enzymatic activity is upregulated in the cerebrospinal fluid and in the brains of mild cognitive impairment patients[42-43]. Therefore, neuronal DG is likely decreased due to aberrant upregulation of BACE1. In neurons, DG is essential for axon guidance, neuron migration and maintenance of functional GABAergic terminals[9,15-16]. Deletion of DG in neurons also significantly impairs long-term potentiation, which reflects synaptic plasticity and is important for learning and memory[28]. Therefore,upregulation of BACE1 may contribute to neuronal dysfunction and cognitive impairmentviaβ-DG downregulation.
4 Conclusion
This study found that the addition of BACE1 can modulate β-DG protein level. When BACE1 and β-DG were co-expressed in HEK-293T cells, the protein level of β-DG was down-regulated. When BACE1 was knock out in HEK-293T cells, the protein level of β-DG was up-regulated. This regulation depends on BACE1 enzyme activityviaproteinprotein interaction. The down-regulation of β-DG by BACE1 was recurred in mouse astrocytes. This down regulation of astrocytic β-DG by BACE1 may lead to glia limitans impairment in the AD brain.AcknowledgmentsWe thank Prof. FAN Li-Bin,from the College of Life Sciences in the Anhui Medical University, for his generous sharing of the DG-pcDNA3.1 plasmid.

