Effects of Shengmai Yin (生脈飲) on pulmonary and cardiac function in coronavirus disease 2019 convalescent patients with cardiopulmonary symptoms: a randomized,double blind,multicenter control trial
AN Xuedong,MAO Lina,XIA Ping,SU Wen,WANG Beibei,KOU Leiya,ZHANG Zequan,QI Meng,HU Song,CHEN Jing,LI Xiujuan,LIU Jinwei,ZHOU Juan,QIAO Jie,LUO Dan,LUO Guangwei,YAN Youqin,YANG Guiping,DONG Dandan,ZHOU Wei,TAO Junxiu,JIN De,TONG Xiaolin,WEI Li
AN Xuedong,JIN De,Institute of Metabolic Diseases,Guang'anmen Hospital,China Academy of Chinese Medical Sciences,Beijing 100053,China
MAO Lina,Department of Traditional Chinese Medicine,Wuhan No.1 Hospital (Wuhan Integrated TCM and Western Medicine Hospital),Wuhan 430022,China
XIA Ping,ZHOU Juan,Department of Orthopedics,Wuhan No.1 Hospital (Wuhan Integrated TCM and Western Medicine Hospital),Wuhan 430022,China
SU Wen,Department of Paediatrics,Wuhan No.1 Hospital (Wuhan Integrated TCM and Western Medicine Hospital),Wuhan 430022,China
WANG Beibei,KOU Leiya,CHEN Jing,QI Meng,LUO Dan,LUO Guangwei,Department of Respiratory and Critical Care Medicine,Wuhan No.1 Hospital (Wuhan Integrated TCM and Western Medicine Hospital),Wuhan 430022,China
HU Song,LIU Jinwei,Department of Pharmacy,Wuhan No.1 Hospital (Wuhan Integrated TCM and Western Medicine Hospital),Wuhan 430022,China
LI Xiujuan,Department of Geriatrics,Wuhan No.1 Hospital (Wuhan Integrated TCM and Western Medicine Hospital),Wuhan 430022,China
ZHANG Zequan,QIAO Jie,College of Traditional Chinese Medicine,Hubei University of Traditional Chinese Medicine,Wuhan 430060,China
YAN Youqin,DONG Dandan,Department of Infectious Diseases,Wuhan No.7 Hospital,Wuhan 430071,China
ZHOU Wei,Department of Gastroenterology,Wuhan No.7 Hospital,Wuhan 430071,China
YANG Guiping,Department of Gastroenterology,Xiaogan Traditional Chinese Medicine Hospital,Xiaogan 432100,China
TAO Junxiu,Department of Hepatology,Hubei Provincial Hospital of Traditional Chinese Medicine,Wuhan 430061,China
TONG Xiaolin,Institute of Metabolic Diseases,Guang’an Men Hospital of China Academy of Chinese Medical Sciences,Beijing 100053,China
WEI Li,Department of Critical Care Medicine,Wuhan No.1 Hospital(Wuhan Integrated TCM and Western Medicine Hospital),Wuhan 430022,China
Abstract OBJECTIVE: To evaluate the efficacy and safety of Shengmai Yin (生脈飲,SMY) on visual analogue scale(VAS) for cardiopulmonary symptoms in coronavirus disease 2019 (COVID-19) convalescent patients.METHODS: In this randomized,double blind and multicenter controlled trial,a total of 200 COVID-19 convalescent patients who with cardiopulmonary symptoms were enrolled from three medical centers in Hubei,China.These patients were randomized divided into trial group and the control group,100 patients in each one,SMY and its placebo were applied to respectively,for two weeks.VAS of clinical symptoms included shortness of breath,hidrosis,chest distress,palpitation,and dry cough was performed at 0,1,2 weeks.Decrease in VAS of 30% or more was defined as effective,and a reduction in VAS of 0 was defined as curative.RESULTS: A total of 192 completed the study.The VAS of TCM symptoms showed there was no difference in baseline between the two groups.The VAS in both groups was down-regulated,and there was no significant difference in VAS and cure rates at the first and second week between the two groups.There was no significant difference in breath,hidrosis,palpitation,and dry cough between the two groups but SMY treatment for two weeks has remarkable therapeutic effects in chest distress than placebo.CONCLUSIONS: SMY could effectively ameliorate the symptoms of chest distress,and improve the quality of life of the COVID-19 convalescent patients.
Keywords: COVID-19;convalescence;visual analog scale;cardiopulmonary symptoms;Shengmai Yin
1.INTRODUCTION
Coronavirus disease 2019 (COVID-19) has become a global pandemic since December 2019.1With the accumulation of treatment experience,some treatment options have been recognized,for example,nucleoside analogs,chloroquine,and protease inhibitors.2However,there is still an incomplete understanding of COVID-19,in particular its sequelae and long-term outcomes.
The National Health Commission of the People’s Republic of China has released multiple versions of a treatment guideline for COVID-19.Traditional Chinese medicine (TCM),as a typical complementary and alternative medicine,has played an essential role in clinical treatment.The "Wuhan Kangyi Formula",a universal TCM prescription based on the theory of "colddampness pestilence",has played an important role in the treatment of COVID-19.3By October 16,2020,a total of 80 766 patients were discharged from hospital in China.4There is already evidence that the use of TCM has significantly reduced the mortality rate of COVID-19.5Because of the physical,cognitive,and psychosocial damage caused by COVID-19,rehabilitation should be noticed.6The TCM Shengmai Yin (生脈飲,SMY) has the beneficial effect of gas fixation and pulse strengthening.Network analysis found that SMY could alleviate the mechanism of arrhythmia,with more than 50% of the active components acting on adrenergic receptors and more than 40% acting on calcium ion channels.7The addition of SMY can rapidly improve the pulmonary function of patients with chronic obstructive pulmoriary disease (COPD) combined with pulmonary interstitial fibrosis and inhibit the progression of fibrosis by reducing the state of inflammation in the host.8Hence,we conducted a randomized,double blind,multicenter control trial,to evaluate the efficacy and safety of SMY on cardiopulmonary function of COVID-19 convalescent patients.
2.METHODS
2.1.Study design and Ethics
This was a randomized,double blind,multicenter control trial which was performed in discharged COVID-19 patients with cardiopulmonary dysfunction in three medical centers (Wuhan No.1 Hospital,Wuhan No.7 Hospital,Xiaogan Hospital of Traditional Chinese Medicine) from April 27,2020,to May 14,2020.The registration number on the CHICTR is ChiCTR-2000032205.Due to the temporary lack of relevant research and data,a total of 200 patients were assigned to the two groups in a 1:1 ratio in this trial and gave informed consent,and the trial was approved by the Ethics Committee of Wuhan No.1 Hospital (2020-9).
2.2.Visual analogue scale (VAS)
Clinical symptoms included shortness of breath,hidrosis,chest distress,palpitation,and dry cough were evaluated by VAS.The results of VAS show from 0 to 10,depending on the severity of the patient's symptoms.
2.3.Inclusion criteria
All COVID-19 convalescent patients were diagnosed and typed according to the seventh edition of the Diagnosis and Treatment Guideline for COVID-19.9The criteria for COVID-19 convalescent were the absence of fever for at least 72 h,substantial improvement in both lungs in chest computed tomography (CT),clinical remission of respiratory symptoms,and two throat-swab samples negative for SARS-CoV-2 RNA obtained at least 24 h apart.The target population was COVID-19 convalescent patients with cardiopulmonary symptoms:(a) with 2 to 3 symptoms,or at least one symptom with a VAS score greater than 4;(b) discharged from hospital for 2 to 4 weeks;(c) aged between 18 and 70;(d) signed the informed consent.
2.4.Exclusion criteria
Exclusion criteria were as follows: (a) patients who had difficulty taking oral drugs due to underlying diseases;(b)accompanied by severe underlying diseases affecting survival,including uncontrolled heart,lung,kidney,digestive,hematologic,neuropsychiatric,immunological,metabolic diseases,malignant tumors,severe malnutrition;(c) allergic constitution,allergic to the drugs involved in the treatment plan;(d) Pregnant or lactating women;(e) unable to cooperate with others in mental state,suffering from mental diseases,lacking self-knowledge and unable to express clearly;(f)participating in other clinical trials.
2.5.Washout criteria
(a) The right to withdraw from the study for any reason at any time during the study and to ensure that it did not affect future treatment.(b) In addition,treatment was terminated in the event of any expected adverse event.(c)Noncompliance with treatment.(d) Pregnancy during the study.(e) Adverse events that are not tolerable (both the investigator and the subject can decide whether to terminate).(f) The presence of a disease or factor not related to treatment.(g) Poor compliance.
2.6.Randomization and blinding
Enrolled patients were numbered and randomly divided into the trial group and the control group in a 1:1 ratio,100 cases in each group.The research drugs are distributed according to the drug Numbers received by the central random system.The drug number remained the same throughout the trial.The SMY and placebo were distributed to each medical center by the staff who were not involved in this clinical trial.Research drugs were distributed according to the drug number obtained by the central random system.The drug number remained unchanged throughout the trial.
2.7.Treatment
Totally 100 patients in trial group received SMY (Tong Ren Tang Technologies Co.,Ltd.,National medicine approval number Z11020363) 10 mL each time,three times a day for two weeks,while the 100 patients in the control group received placebo with the same plan.The administration,distribution,taking and recovery of drugs were recorded by special personnel.Other medications should be avoided if not required for treatment.
2.8.Outcomes
The standard of cure was VAS score of 0 for a given symptom after treatment.If the VAS of a symptom decreases by 30% or more after treatment,the intervention was considered effective.The cure rate was the percentage of the number of patients with a VAS score of 0 for a particular symptom after treatment and the total number of patients with this symptom.The effective rate was the percentage of the number of patients with a 30% decrease in the VAS score for a given symptom and the total number of patients with this symptom.
2.9.Statistical analysis
SPSS (vers 25.0,IBM Corp.,Armonk,NY,USA) was used to analyze the data of this study.The missing data were carried forward for the last time to fill.The description of the quantitative data would calculate the mean,standard deviation,median,upper quartile and lower quartile.The quantitative data accorded with normal distribution were tested by two independent samplest-test,and the quantitative data that did not accord with normal distribution were tested by two independent sample rank sum test Mann-WhitneyUtest.Qualitative data describe the number and percentage of various types.χ2test was used for other qualitative variables,and fisher exact probability method was used when χ2test conditions were not satisfied.The twosided test was used in all the statistical tests,andP< 0.05 was regarded as statistically significant.
3.RESULTS
3.1.Baseline data
A total of 200 patients were enrolled in this trial,and the baseline characteristics of two groups were summarized in Table 1.There were no significant differences in age,sex,body mass index,smoking history,drinking history,vital signs include body temperature,blood pressure,respiratory rate,heart rate,COVID-19 medical history includes severity grading,imaging characteristics,blood routine examination.In the trial group,98 of the 100 participants (98%) fulfilled the protocol.In comparison,94 of the 100 participants (94%) fulfilled the protocol in the control group (Figure 1,Table 2).The result of t test showed that there were no statistically significant differences in shortness of breath,hyper perspiration,chest tightness,palpitation or dry cough between the two groups before treatment (P> 0.05) (Table 3).
3.2.VAS
VAS of clinical symptoms included shortness of breath,hidrosis,chest distress,palpitation,dry cough at baseline showed patients’ main symptom was shortness of breath and distress.During the treatment for two weeks,VAS of all symptoms was decreased in both groups,but there was no significant difference in VAS of each symptom between the trial group and the control group.(Figure 2)
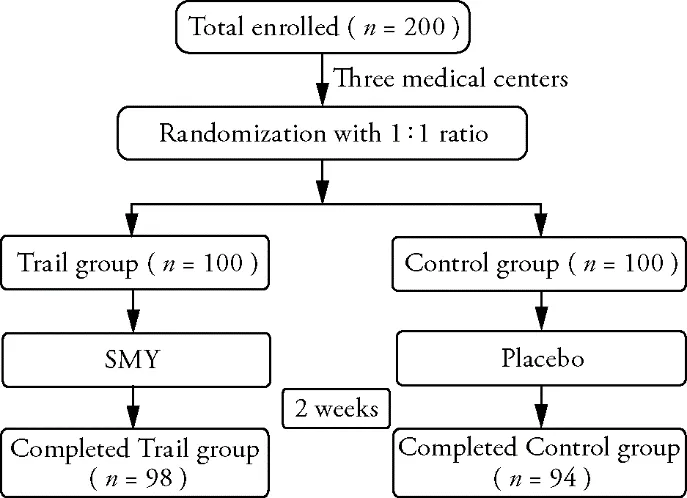
Figure 1 Flow Diagram
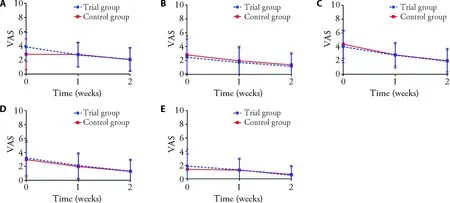
Figure 2 VAS of symptoms
3.3.Treatment efficacy
After 2 weeks of treatment,the effective and cure rate in patients was seen to rise slightly with time.SMY treatment for two weeks had remarkable therapeutic effects in chest distress than placebo (75.00%vs55.56%,P=0.008) (Table 4).
3.4.Safety evaluation
Safety outcomes were evaluated including heart rate,systolic blood pressure,and diastolic blood pressure abnormalities.There was no statistically significant difference between the two groups (P> 0.05) (Table 5).
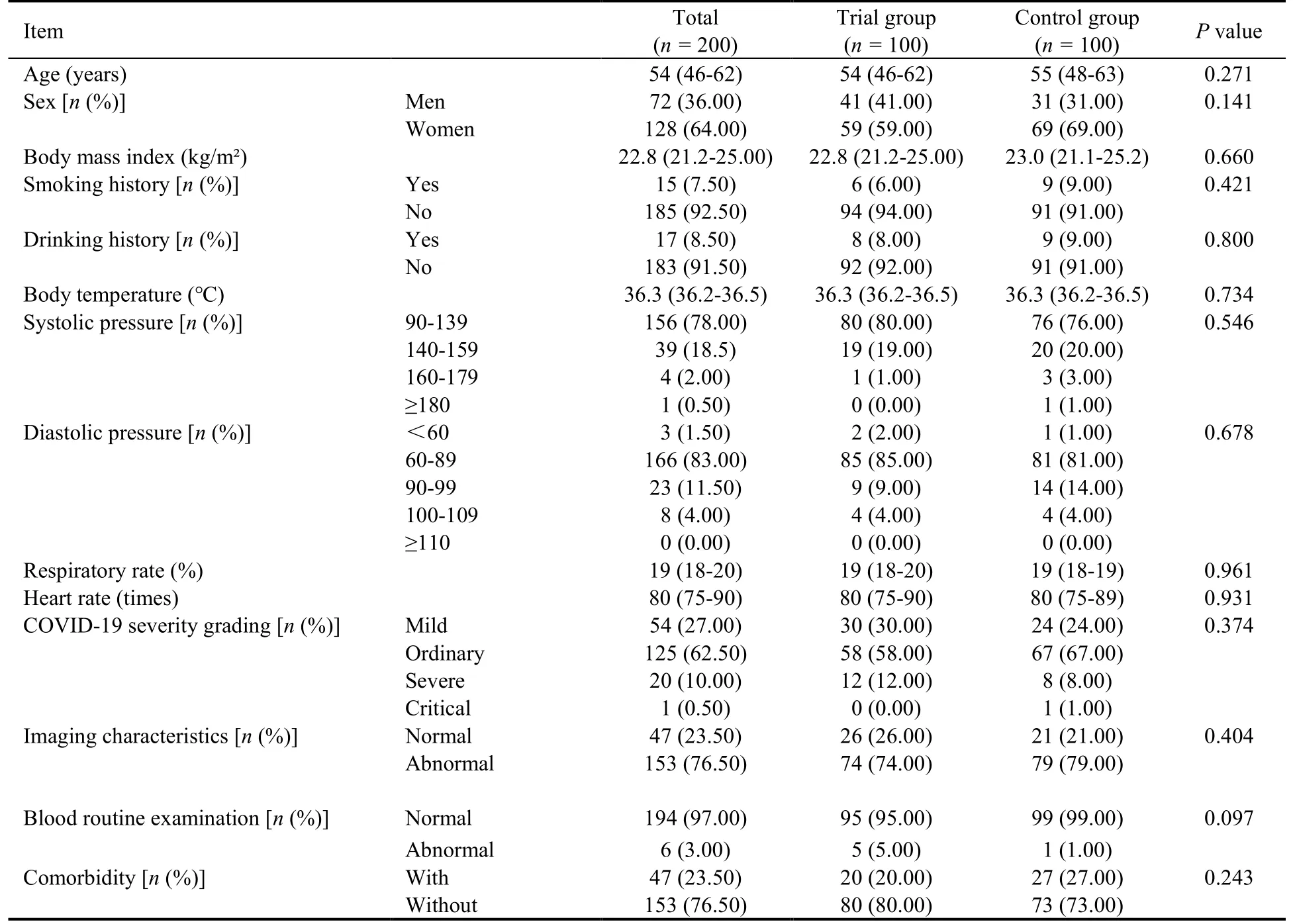
Table 1 Baseline characteristics of study patients
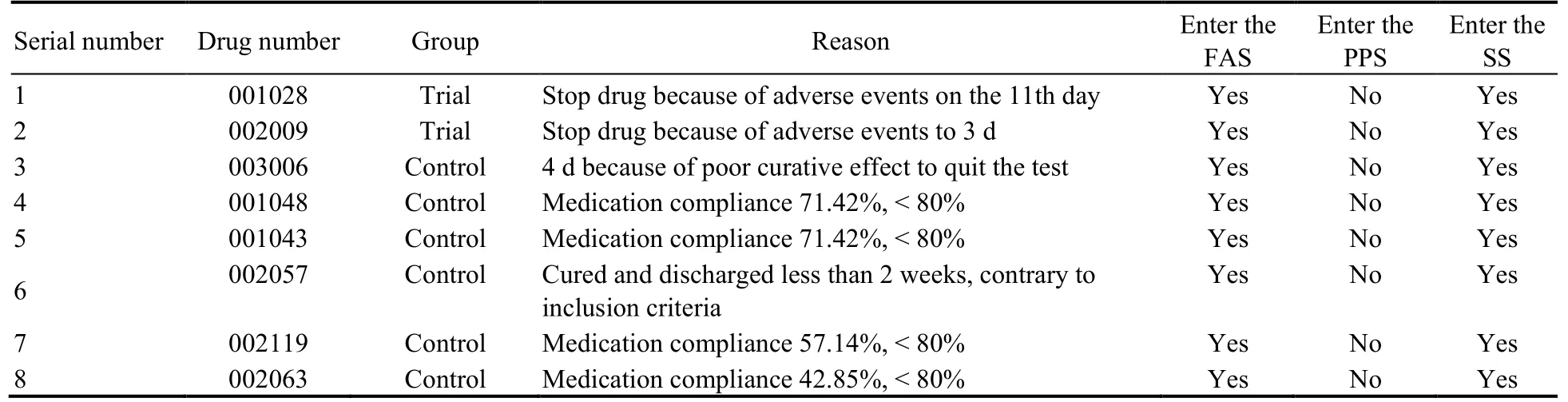
Table 2 Not enter the list of FAS,PP,SS
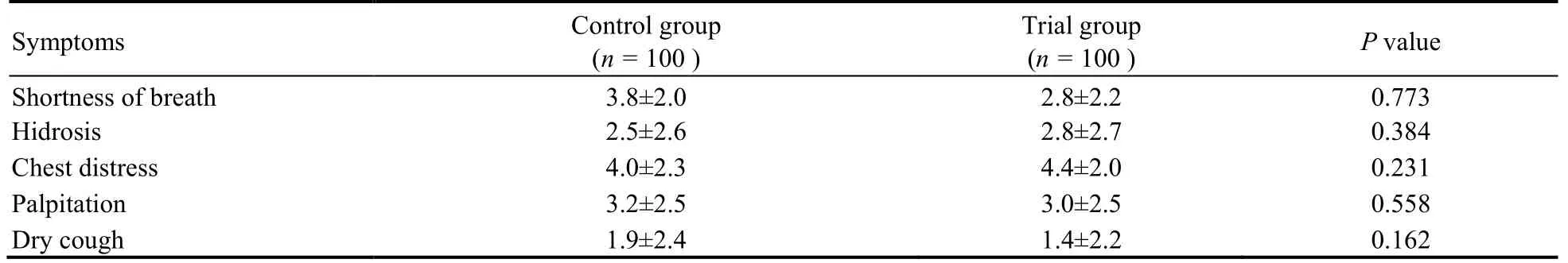
Table 3 VAS before treatment
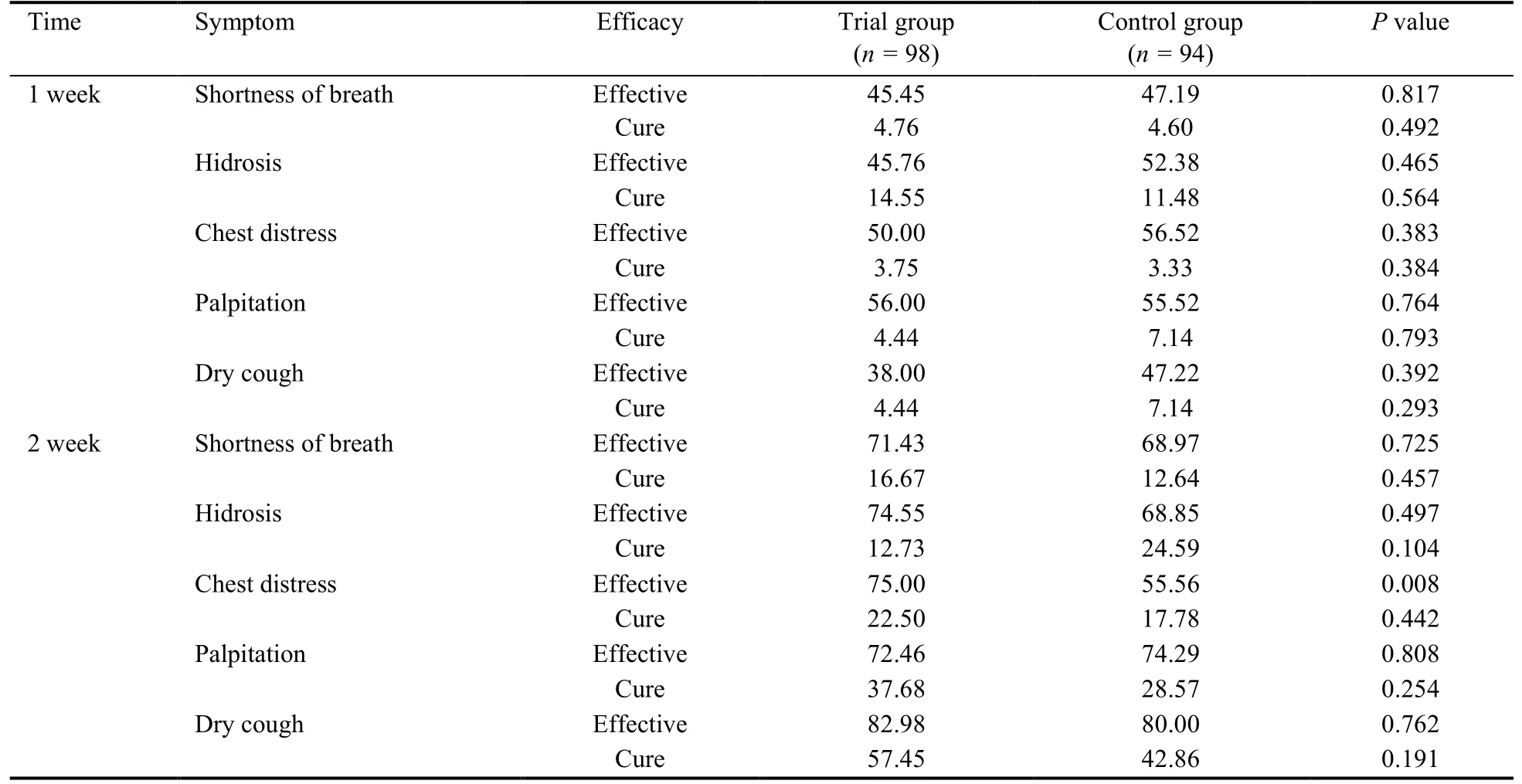
Table 4 Treatment efficacy (%)
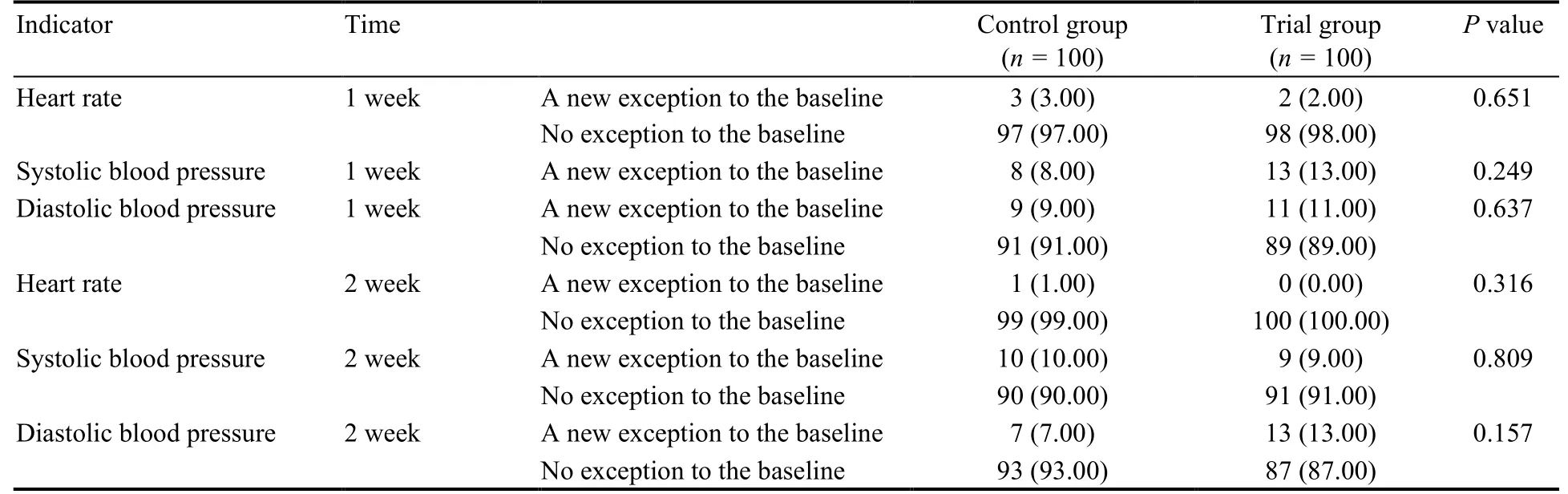
Table 5 Comparison of new heart rate and blood pressure abnormalities during the test [n (%)]
4.DISCUSSION
Our study showed that after 2 weeks of treatment,the total effective rate of improvement of chest distress in the trial group was statistically different from that in the control group,with SMY significantly improve VAS scores for chest distress in COVID-19 convalescent patients.Since the treatment lasted only 2 weeks,the clinical symptoms of many patients did not completely disappear,so there was no statistical difference in the symptom disappearance rate between the two groups.If the treatment and follow-up time can be prolonged on this basis,the positive results of symptom disappearance rate may be obtained hopefully.
SMY is a famous TCM formula,is mainly consisted of Hongshen (RadixGinseng),Maidong (Radix Ophiopogonis Japonici),and Wuweizi (Fructus Schisandrae Chinensis).Pharmacological studies revealed that SMY could attenuate contractile dysfunction and structural damage,10against inflammatory reaction to protect heart function.11,12There is evidence that promotes SMY may be beneficial for heart failure.13A retrospective study of 2501 patients with type 2 diabetes in 9 centers found that the patients who used SMY showed higher frequency of symptoms such as fatigue,short breath and idle speech.14In a systematic review and Meta-analysis of 23 Randomized controlled trials,SMY could increase lung function,modulate inflammation and improve the quality of life of chronic obstructive pulmonary disease patients.15
However,there are still some limitations in these results.First,most of the patients with COVID-19 have underlying diseases.However,because the SMY used inthis study is a sugary preparation,diabetes patients were not included in this study.Therefore,it is questionable whether the study population could represent all COVID-19 patients in rehabilitation period.Second,both groups of patients had a certain proportion of underlying diseases.Whether the intervention of these basic diseases,especially heart-related drugs,would affect the outcome of this study needs further study.Third,although the VAS score application quantified clinical symptoms,more laboratory and imaging tests such as cardiopulmonary function,lung CT,and immune function may provide a more comprehensive understanding.In addition,two weeks of treatment was performed in this study,but a long follow-up was still valuable.
In conclusion,SMY could effectively ameliorate the symptoms of chest distress,improving the quality of life of COVID-19 convalescent patients without significant adverse reactions.Because there are some limitations,in order to improve the reliability of the research results,a larger sample cohort study or a randomized controlled trial is necessary in the future.
 Journal of Traditional Chinese Medicine2023年1期
Journal of Traditional Chinese Medicine2023年1期
- Journal of Traditional Chinese Medicine的其它文章
- Potential of natural medicines for treatment of osteoporosis: a narrative review
- Outlook on cultural inheritance and development
- Upholding fundamental principles and breaking new ground: ensuring positive efficacy and reaching new consensus
- General principle of high-quality academic development of traditional chinese medicine: “carrying on the essence,while pursuing innovations”
- Effects of moderate-intensity aerobic exercise combined with acupuncture on attention function of mentally-retarded adolescents: a randomised controlled trial
- Reveal the mechanisms of prescriptions for liver cancer' treatment based on two illustrious senior TCM physicians
