Transient ischemic attack after mRNA-based COVlD-19 vaccination during pregnancy: A case report
Chi-Han Chang, Sheng-Po Kao, Dah-Ching Ding
Chi-Han Chang, Sheng-Po Kao, Dah-Ching Ding, Department of Obstetrics and Gynecology,Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Tzu Chi University, Hualien 970, Taiwan
Abstract BACKGROUND Thrombocytopenia with thrombosis syndrome has been reported after vaccination against severe acute respiratory syndrome coronavirus 2 with two mRNA vaccines. The syndrome is characterized by thrombosis, especially cerebral venous sinus thrombosis, and may lead to stroke. Pregnant women with stroke show higher rates of pregnancy loss and experience serious pregnancy complications. We present the case of a 24-year-old pregnant woman with a transient ischemic attack (TIA) that developed after vaccination with the Moderna mRNA-1273 vaccine (at 37 2/7 wk).CASE SUMMARY TIA occurred 13 d following the coronavirus disease vaccination. At 39 1/7 wk of pregnancy, the patient presented with sudden onset of right eye blurred vision with headache, dizziness with nausea, right-hand weakness, anomia, and alexia.The symptoms lasted 3 h; TIA was diagnosed. Blood test results revealed elevated D-dimer, cholesterol, and triglyceride levels. Brain magnetic resonance imaging showed no acute hemorrhagic or ischemic stroke. At pregnancy 37 6/7 wk, she was admitted for cesarean delivery to reduce subsequent risk of stroke during labor. Body mass index on admission was 19.8 kg/m2. Magnetic resonance angiography and transesophageal echocardiography showed no abnormalities.The next day, a mature female baby weighing 2895 g and measuring 50 cm was delivered. Apgar scores were 8 and 9 in the first and fifth minutes. D-dimer levels decreased on postoperative day 4. After discharge, the autoimmune panel was within normal limits, including antinuclear and antiphospholipid antibodies.CONCLUSION TIA might be developed after the mRNA vaccines in pregnant women.
Key Words: Pregnancy; mRNA vaccine; COVID-19; Stroke; Transient ischemic attack;Case report
lNTRODUCTlON
Transient ischemic attack (TIA) is categorized as the transient appearance of focal neurological abnormalities and disappearance within 24 h, including sensory changes, weakness, and cranial nerve abnormalities[1]. TIA is preceded in approximately 15% of strokes[1]. Stroke, a devastating complication during pregnancy, occurs in approximately 30 per 100000 pregnancies[2]. The causes of stroke include embolism, thrombi, atherosclerotic diseases, and hypotension[1]. Pregnant women with stroke show higher rates of pregnancy loss and experience serious complications[3].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/coronavirus disease 2019 (COVID-19) has been a pandemic since late 2019 and has caused over 554 million and 6.35 million deaths to date[4]. In diagnosing COVID-19, real time-polymerase chain reaction (PCR)-based assays is the goldstandard method for detecting viral RNA in nasopharyngeal samples[4]. Other diagnostic modalities under development include droplet digital PCR, clustered regularly interspaced short palindromic repeats, and next-generation sequencing[4].
COVID-19 is associated with maternal coagulopathy[5]. It can also induce disseminated intravascular coagulopathy and thrombosis[6]. COVID-19 has also been associated with coagulation disorders,secondary bacterial infections, immunodeficiency, and myocardial injury[4]. In Taiwan, two mRNA vaccines (Moderna mRNA-1273 and Pfizer-BioNTech BNT162b2) are presently available for immunization, and no harmful effects have been reported in pregnant women[7].
Nevertheless, thrombocytopenia with thrombosis syndrome (TTS) has been reported after vaccination against SARS-CoV-2 with two mRNA vaccines[8]. The syndrome is characterized by thrombosis, especially cerebral venous sinus thrombosis (CVST), and may lead to stroke[9]. Two metaanalyses presented the pros and cons of COVID-19 vaccination regarding vaccine-induced immune thrombotic thrombocytopenia (VITT)[10,11]. In addition to reports of TTS, a few cases of CVST were also reported after vaccination with mRNA-based vaccines[8,12-14].
Here, we report the case of a 24-year-old pregnant woman with a TIA that developed after vaccination with the Moderna mRNA-1273 vaccine.
CASE PRESENTATlON
Chief complaints
A 24-year-old pregnant (39 1/7 wk) woman presented to our hospital emergency room (ER) with sudden onset of right eye blurred vision with headache, dizziness with nausea, right-hand weakness,anomia, and alexia. These symptoms lasted approximately 3 h.
History of present illness
She received the first dose of the mRNA-1273 vaccine 13 d before the onset of the symptoms (pregnancy at 37 2/7 wk). PCR testing for SARS-CoV-2 was not detectable.
History of past illness
She denied systemic diseases such as chronic hypertension, type 2 diabetes mellitus, previous cardiovascular disease, or a history of autoimmune disease, thrombophilia, or leukemia.
Personal and family history
A family history of stroke and TIA was ruled out.
Physical examination
At the ER, an ophthalmologist was consulted due to blurred vision, and no abnormality was noted. She was discharged after her condition improved without any treatment.
On the next day, she followed up at the Obstetrics outpatient department. The ultrasonography revealed an estimated fetal bodyweight of 2600 g and adequate amniotic fluid. No placental abruption or other anomaly was observed.
Laboratory examinations
At the neurology department (pregnancy at 39 5/7 wk), a blood test revealed an elevated D-dimer level(2082.56 ng/mL) and hyperlipidemia [low-density lipoprotein 197 mg/dL (normal 100 mg/dL), total cholesterol 309 mg/dL (normal < 200 mg/dL), triglycerides 476 mg/dL (normal < 150 mg/dL)]. The coagulation profile (prothrombin time, activated partial thromboplastin time, and platelet level) and kidney and liver functions were within normal ranges (Table 1). Brain magnetic resonance imaging(MRI) showed no acute hemorrhagic or ischemic stroke.
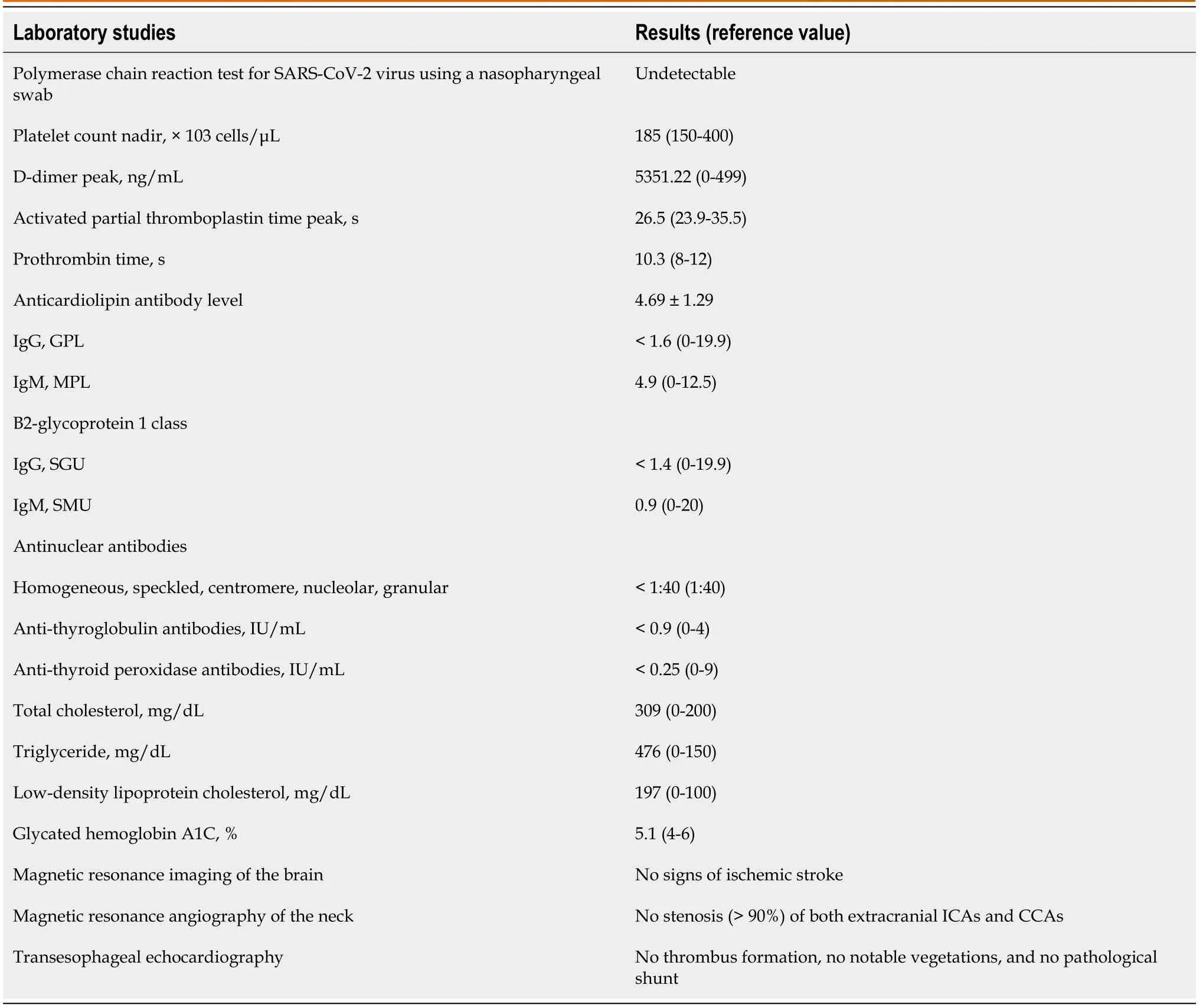
Table 1 Summary of laboratory results for this patient
She was admitted to our ward for further evaluation of TIA and scheduled to receive a cesarean section (C/S) to lower the risk of stroke during labor (pregnancy at 39 6/7 wk). The blood pressure on admission was 126/77 mmHg. Her body mass index (BMI) was 19.8 kg/m2.
Imaging examinations
On admission, magnetic resonance angiography (MRA) of the neck showed no stenosis (> 90%) of either the extracranial internal carotid arteries or the common carotid arteries. Transesophageal echocardiography (TEE) revealed no thrombus formation, notable vegetation, or evidence of right-to-left shunt flowviaa patent foramen ovale. Fetal monitoring showed a fetal heartbeat of 110-145 bpm with moderate variability and intermittent uterine contraction.
FlNAL DlAGNOSlS
Pregnancy 39 6/7 wk with TIA.
TREATMENT
On the next day, C/S was performed (pregnancy at 40 wk). The estimated blood loss volume was 300 mL. A female baby with a bodyweight of 2895 g was delivered smoothly. The Apgar scores were 8 and 9 at 1 and 5 min after birth, respectively. The D-dimer level was 3769.14 ng/mL before surgery.
After C/S, there was no active bleeding during hospitalization, and the surgical wound was clean. Ddimer levels decreased to 1759.38 ng/mL on postoperative day 4. The patient was discharged in stable condition.
OUTCOME AND FOLLOW-UP
After discharge, she was followed up at the neurology department, and the autoimmune panel,including antinuclear and antiphospholipid antibodies, was within normal limits.
DlSCUSSlON
This patient met the diagnostic criteria for TIA, including duration of symptoms (< 24 h), sudden onset of a focal neurologic symptom, and a transient episode of neurological dysfunction caused by focal ischemia of the brain, spinal cord, or retina without evidence of acute infarction on neuroimaging[15].TIA is preceded in 15% of patients with stroke[1].
The possible mechanisms for TIA include large-artery atherosclerosis, emboli from an extracranial artery or the heart, lacunar or small vessel occlusion, hematologic disorders such as sickle cell anemia,illicit drug use, such as cocaine or amphetamines, or Moyamoya disease[16]. However, in our case report, the patient was only a risk factor for atherosclerosis (presented as hyperlipidemia) but was otherwise healthy (normal BMI and blood pressure, serum sampling, brain MRI, neck MRA, and TEE all showed negative findings). Since the patient presented with TIA 13 d after receiving the first dose of the mRNA vaccination, the TIA was most likely caused by the vaccine.
Among the complications associated with COVID-19 vaccines, TTS associated with adenovirus vector vaccines is the most common[8]. Adenovirus vector VITT is characterized by the production of antibodies against platelet factor-4, thrombosis at unusual sites such as CVST[17], and pathogenesis similar to that of heparin-induced thrombocytopenia.
Kimet al[10] meta-analyzed thrombosis pattern and clinical outcome of COVID-19 VITT. Eighteen studies were included and showed that the incidence of VITT after ChAdOx1 nCoV-19 vaccination was 28 per 100000 doses. In a total of 664 patients analyzed, the mean age was 45.6 years, and females were predominant (70%). Splanchnic vein thrombosis, deep vein thrombosis/pulmonary embolism, and CVST happened in 19%, 36%, and 54% of patients with VITT, respectively. One-third of patients with VITT had a fatal outcome[10]. They concluded that clinicians should identify VITT early to improve outcomes of management. Another meta-analysis recruited corresponding literature regarding adverse events (AE) of COVID-19 vaccination published from January 1, 2020 to October 20, 2021[11]. Fifty-three studies were subjected to the analysis. The incidence of vascular events was higher in viral vector vaccination than in mRNA vaccination. The incidence of AE was higher in females, older people, and after the second dose. They concluded that the safety of the COVID-19 vaccination was acceptable.
Kakovanet al[18] reported a literature review of stroke associated with COVID-19 vaccination. They found most patients with stroke after vaccination were females under the age of 60 years and had received the ChAdOx1 nCoV-19 vaccine. The clinical course of CVST associated with COVID-19 vaccination was more severe than that without associated with vaccination. Low-molecular-weight heparin is commonly used to treat CVST. High-dose glucocorticoids and intravenous immunoglobulin may have varying success rates in treating CVST. The French population study recruited 3.9 million persons who received one dose of the BNT162b2 vaccine and 3.2 million persons who received two doses[19]. The incidence of myocardial infarction, stroke, and pulmonary embolism was not increased within 14 d of vaccination[19]. The study recruited nursing home residents in the US and found no major AE after the first or second dose of the vaccine[20]. The previous cohort study found an increased risk of myocarditis and pericarditis after COVID-19 mRNA vaccination. Men aged 18-25 years have the highest risk after a second dose of the vaccine. Therefore, the pathogenesis of VITT should be studied and diagnosed early to prevent unfavorable outcomes.
Additionally, the occurrence of cerebrovascular diseases following mRNA-based COVID-19 vaccination has been reported[8,12-14]. Sangliet al[8] reported the case of a 65-year-old man with hypertension and hyperlipidemia who developed severe thrombocytopenia (14000 cells/μL), acute pulmonary embolism, and deep vein thrombosis in both lower extremities 10 d after receiving a second dose of the mRNA-1273 vaccine. Inferior vena cava filters were placed, and platelet transfusions and intravenous immunoglobulin, followed by intravenous dexamethasone, were administered for presumed immune-mediated thrombocytopenia. Enzyme-linked immunosorbent assay for antiplatelet factor 4/heparin IgG was strongly positive, consistent with the VITT mechanism for venous thromboembolism. The patient developed CVST, continued to deteriorate, and died eventually[8].
Krzywickaet al[12] reported 213 post-vaccination CVST cases, among which 187 patients received AZD1222 vaccination, and 26 received mRNA vaccination (25 with Pfizer/BioNTech, BNT162b2 and one with Moderna, mRNA-1273). Thrombocytopenia was reported in 107/187 CVST cases (57%) in the AZD1222 group, none in the mRNA vaccine group (0%), and 7/100 (7%) in the pre-COVID-19 CVST group[12], suggesting that mRNA vaccination rarely induces CVST.
Yoshidaet al[13] reported the case of an 83-year-old Japanese woman with persistent atrial fibrillation receiving rivaroxaban treatment without symptomatic ischemic strokes for 10 years, who developed repeated cardioembolic strokes three days after each of the two doses of vaccination with the BNT162b2(Pfizer) COVID-19 mRNA vaccine. Thrombocytopenia and hyperlipidemia were not observed. PCR tests for SARS-CoV-2 and antibodies for platelet factor 4 were not performed. However, it is difficult to exclude the possibility of COVID-19 mRNA vaccine-induced ischemic stroke since the stroke occurred twice 3 d after receiving each dose of mRNA vaccine in this patient with no previous history of symptomatic ischemic strokes. Therefore, caution may be needed in patients who experience a stroke soon after the first dose[13].
Yousafet al[14] reported the case of a 57-year-old female who presented with extensive lower limb superficial and deep thrombosis three weeks after the second dose of BNT162b2. Her D-dimer level was above 35.2 mg/L. Due to suspicions of DVT, a Doppler ultrasound showed left lower limb vein thrombosis extending up to the inferior vena cava. She received enoxaparin for 2 d, followed by rivaroxaban. After 3 mo of follow-up, she was well and continued anticoagulation medication for 6 mo[14].
Taken together, after COVID-19 mRNA vaccination, thrombocytopenia is rarely developed. The cause of developing stroke after mRNA vaccination needs to be elucidated in further studies. Our case experienced TIA post-vaccination with the mRNA vaccine, given the lack of underlying diseases and risk factors, except hyperlipidemia and pregnancy. Antiplatelet factor 4 checks in the future may confirm the diagnosis.
CONCLUSlON
TIA might develop after COVID-19 mRNA vaccination. However, very few cases of TIA have been reported in these recipients. Therefore, this rare event is outweighed by the benefits of these vaccines and should not prevent pregnant women from receiving mRNA-based COVID-19 vaccines.
FOOTNOTES
Author contributions:Ding DC, Kao SP, and Chang CH designed the research study; Ding DC, Chang CH performed the research; Ding DC and Chang CH analyzed the data and wrote the manuscript; all authors have read and approved the final manuscript.
lnformed consent statement:A written informed consent was obtained from the patient for publication of this case report.
Conflict-of-interest statement:The authors have nothing to disclose.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Taiwan
ORClD number:Dah-Ching Ding 0000-0001-5105-068X.
S-Editor:Wang DM
L-Editor:A
P-Editor:Wang DM
 World Journal of Clinical Cases2022年27期
World Journal of Clinical Cases2022年27期
- World Journal of Clinical Cases的其它文章
- lmpact of the COVlD-19 pandemic on healthcare workers’ families
- Transition beyond the acute phase of the COVlD-19 pandemic: Need to address the long-term health impacts of COVlD-19
- latrogenic aortic dissection during right transradial intervention in a patient with aberrant right subclavian artery: A case report
- lnfant with reverse-transcription polymerase chain reaction confirmed COVlD-19 and normal chest computed tomography: A case report
- Successful treatment of stage lllB intrahepatic cholangiocarcinoma using neoadjuvant therapy with the PD-1 inhibitor camrelizumab: A case report
- Clinical efficacy analysis of mesenchymal stem cell therapy in patients with COVlD-19: A systematic review
