管花鹿藥中2個新的甾體皂苷
張 欣,劉媛媛,李玉澤,張東東,姜 祎,宋小妹,王 薇,鄧 翀
管花鹿藥中2個新的甾體皂苷
張 欣,劉媛媛,李玉澤,張東東,姜 祎,宋小妹,王 薇,鄧 翀*
陜西中醫(yī)藥大學(xué)藥學(xué)院,陜西 咸陽 712046
研究管花鹿藥中的甾體皂苷類成分及其細胞毒活性。采用硅膠柱色譜、葡聚糖凝膠LH-20柱色譜以及半制備高效液相色譜等方法分離,根據(jù)MS、IR、NMR和GC等數(shù)據(jù)鑒定化合物的結(jié)構(gòu),MTT法測定化合物的細胞毒活性。從管花鹿藥乙醇提取物的正丁醇部位分離鑒定了2個甾體皂苷類化合物,分別鑒定為(23,24)-螺甾-5,25(27)-二烯-lβ,3β,23,24-四醇1--α--鼠李糖基-(1→2)-β--呋糖苷(1)和(23,24)-螺甾-5,25(27)-二烯- lβ,3β,21,23,24-五醇1--α--鼠李糖基-(1→2)-β--呋糖苷(2),化合物1和2對2種腫瘤細胞的細胞毒活性的半數(shù)抑制濃度(median inhibition concentration,IC50)值均大于100 μmol/L。化合物1和2為新化合物,分別命名為管花鹿藥皂苷K(1)和管花鹿藥皂苷L(2);兩者均未表現(xiàn)出細胞毒活性。
管花鹿藥;甾體皂苷;細胞毒性;管花鹿藥皂苷K;管花鹿藥皂苷L
管花鹿藥(Baker) LaFrankie為天門冬科(Asparagaceae)舞鶴草屬Web.植物管花鹿藥的根及根莖[1],其為甘、苦,性溫,又稱偏頭七、竹葉菜等,主要分布于陜西、湖北和云南等地[2-4]。文獻報道其主要化學(xué)成分為甾體皂苷類、黃酮類和有機酸等,具有抗腫瘤和抗氧化等藥理作用[5-7]。其中甾體皂苷類成分是管花鹿藥抗腫瘤的主要活性成分,受到國內(nèi)外學(xué)者的重點關(guān)注[4-8]。目前關(guān)于管花鹿藥的化學(xué)成分研究并不系統(tǒng),為進一步明確其化學(xué)成分,本研究基于前期的研究基礎(chǔ)[8-10],繼續(xù)對管花鹿藥醇提取物進行系統(tǒng)研究,從中共鑒定了2個新化合物分別為管花鹿藥皂苷K(1)和管花鹿藥皂苷L(2),細胞毒活性測試結(jié)果顯示2個化合物均未表現(xiàn)出抑制作用。
1 儀器與材料
Bruker-AVANCE400型核磁共振儀(布魯克公司);HH-2恒溫水浴鍋(青島聚創(chuàng)環(huán)保集團有限公司);Sephadex LH-20(GE公司);薄層色譜硅膠GF254、柱色譜用硅膠(青島海洋化工廠);色譜乙腈(阿德馬斯試劑公司);甲醇、二氯甲烷、正丁醇、石油醚等均為分析純(天力化學(xué)試劑有限公司),水為娃哈哈純凈水;人肝癌HepG2細胞和人結(jié)直腸癌SW620細胞購于中國科學(xué)院上海細胞庫。
管花鹿藥于2017年8月購于陜西省眉縣,經(jīng)陜西中醫(yī)藥大學(xué)藥學(xué)院王薇教授鑒定為天門冬科舞鶴草屬植物管花鹿藥(Baker) LaFrankie的根及根莖,藥材標本(SH-201708)保存于陜西中醫(yī)藥大學(xué)藥學(xué)院中藥標本館。
2 方法
2.1 提取與分離
干燥的管花鹿藥根及根莖6.6 kg,粉碎后以80%乙醇回流提取3次,各2、2和1 h。減壓回收乙醇,干燥至浸膏,分散于水中,分別用石油醚和水飽和正丁醇萃取3次,得到石油醚部位9.0 g和正丁醇部位130.2 g。取正丁醇部位130 g,經(jīng)硅膠柱色譜,以二氯甲烷-甲醇-水(100∶0∶0~60∶40∶10)梯度洗脫,合并相同流分,分別得到6個組分Fr. 1~6。Fr. 4(19.1 g)經(jīng)硅膠柱色譜分離,二氯甲烷-甲醇-水(100∶10∶0~70∶30∶5)梯度洗脫,得到6個組分Fr. 4-1~4-6。Fr. 4-2(1.4 g)經(jīng)葡聚糖凝膠色譜,二氯甲烷-甲醇(1∶1)洗脫,合并相同流分后再經(jīng)高效液相色譜(HPLC),乙腈-水(65∶35)洗脫得到化合物1(8.2 mg,R=41 min)和2(13.1 mg,R=47 min)。
2.2 酸水解實驗
化合物1和2各5 mg,采用課題組前期水解和衍生化方法得到糖的衍生物[14-15],經(jīng)Agilent 7890B氣相色譜儀分析,色譜柱:HP-5 ms,60 m×0.25 mm×0.25 μm;程序升溫:起始溫度140 ℃,5 ℃/min升溫至240 ℃,1 ℃/min升溫至260 ℃,保溫10 min,2 ℃/min升溫至280 ℃,保溫5 min;進樣溫度240 ℃;載氣:氮氣。測得各類單糖的保留時間分別為-呋糖:35.39 min,-呋糖:35.63 min,-鼠李糖:36.08 min,-鼠李糖:36.20 min。
3 結(jié)構(gòu)鑒定
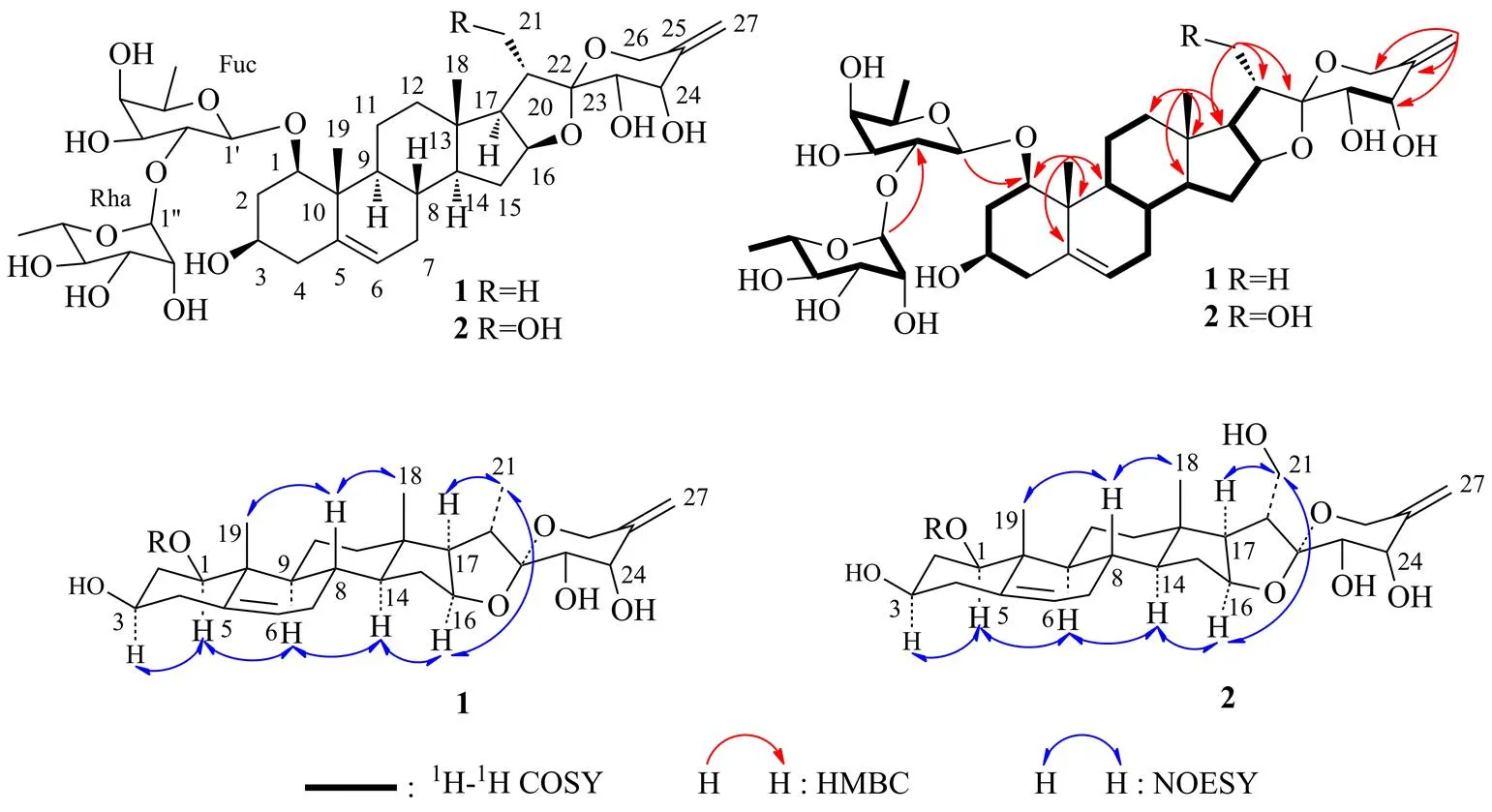
圖1 化合物1和2的結(jié)構(gòu)及主要的1H-1H COSY、HMBC和NOESY相關(guān)信號
表1 化合物1和2的1H (400 MHz, pyridine-d5)和13C-NMR數(shù)據(jù) (100 MHz, pyridine-d5)
Table 1 1H (400 MHz, pyridine-d5) and 13C-NMR (100 MHz, pyridine-d5) data of compounds 1 and 2
碳位12δC (type)δHδC (type)δH 184.3 (CH) 3.77 (m)84.9 (CH)3.75 (m) 2a38.0 (CH2)2.43 (m)38.5 (CH2)2.42 (m) 2b2.69 (m)2.68 (m) 368.3 (CH) 3.86 (m)68.8, (CH) 3.85 (m) 4a43.8 (CH2)2.58 (m)44.3, (CH2)2.58 (m) 4b2.69 (overlapped)2.67 (overlapped) 5139.6 (C) 140.2 (C) 6124.7 (CH)5.56 (d, J = 5.5 Hz)125.3 (CH)5.56 (d, J = 5.3 Hz) 7a32.0 (CH2)1.33 (m)32.6 (CH2)1.35 (m) 7b1.86 (m) 1.81 (m) 833.0 (CH)1.52 (overlapped)33.6 (CH)1.54 (overlapped) 950.6 (CH)1.57 (m)51.1 (CH)1.56 (m) 1042.8 (C) 43.4 (C) 11a24.0 (CH2)1.60 (m)24.5 (CH2)1.59 (m) 11b2.98 (overlapped)2.94 (overlapped) 12a40.7 (CH2)1.46 (m)41.0 (CH2)1.42 (m) 12b1.82 (m)1.78 (m) 1340.7 (C) 41.0 (C) 1457.3 (CH)1.22 (overlapped)57.9 (CH)1.23 (overlapped) 15a32.3 (CH2)1.41 (m)33.1 (CH2)1.40 (m) 15b1.97 (m)2.01 (m) 1683.3 (CH)4.68 (m)84.2 (CH)4.67 (m) 1761.5 (CH)1.88 (m)62.7 (CH)1.89 (m) 1817.0 (CH3)1.03 (s)17.6 (CH3)1.10 (s) 1915.0 (CH3)1.40 (s)15.5 (CH3)1.37 (s) 2037.0 (CH)3.00 (d, J = 6.9 Hz)46.4 (CH)2.94 (m) 2114.6 (CH3)1.09 (d, J = 7.0 Hz)62.8 (CH2)4.20 (m), 3.91 (m) 22112.7 (C) 112.9 (C)
續(xù)表1
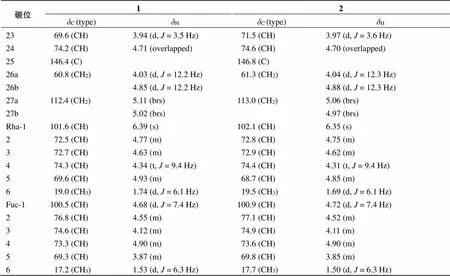
碳位12 δC (type)δHδC (type)δH 2369.6 (CH)3.94 (d, J = 3.5 Hz)71.5 (CH)3.97 (d, J = 3.6 Hz) 2474.2 (CH)4.71 (overlapped)74.6 (CH)4.70 (overlapped) 25146.4 (C) 146.8 (C) 26a60.8 (CH2)4.03 (d, J = 12.2 Hz)61.3 (CH2)4.04 (d, J = 12.3 Hz) 26b4.85 (d, J = 12.2 Hz)4.88 (d, J = 12.3 Hz) 27a112.4 (CH2) 5.11 (brs)113.0 (CH2)5.06 (brs) 27b5.02 (brs)4.97 (brs) Rha-1101.6 (CH)6.39 (s)102.1 (CH)6.35 (s) 272.5 (CH)4.77 (m)72.8 (CH)4.75 (m) 372.7 (CH)4.63 (m)72.9 (CH)4.62 (m) 474.3 (CH)4.34 (t, J = 9.4 Hz)74.4 (CH)4.31 (t, J = 9.4 Hz) 569.6 (CH)4.93 (m)68.7 (CH)4.85 (m) 619.0 (CH3)1.74 (d, J = 6.1 Hz)19.5 (CH3)1.69 (d, J = 6.1 Hz) Fuc-1100.5 (CH)4.68 (d, J = 7.4 Hz)100.9 (CH)4.72 (d, J = 7.4 Hz) 276.8 (CH)4.55 (m)77.1 (CH)4.52 (m) 374.6 (CH)4.12 (m)74.9 (CH)4.11 (m) 473.3 (CH)4.90 (m)73.6 (CH)4.90 (m) 569.3 (CH)3.87 (m)69.8 (CH)3.85 (m) 617.2 (CH3)1.53 (d, J = 6.3 Hz)17.7 (CH3)1.50 (d, J = 6.3 Hz)
4 細胞毒實驗
根據(jù)課題組前期研究[8-10],采用MTT法分別測定化合物1和2對人肝癌HepG2細胞和人結(jié)直腸癌細胞SW620的細胞毒活性。結(jié)果化合物1和2對兩種腫瘤細胞的半數(shù)抑制濃度(median inhibition concentration,IC50)值均大于100 μmol/L。
5 討論
本實驗從管花鹿藥中共分離鑒定了2個新的甾體皂苷類化合物,分別命名為管花鹿藥皂苷K和管花鹿藥皂苷L,進一步豐富了管花鹿藥的化學(xué)成分。但體外細胞毒活性研究表明二者均未顯示出對HepG2和SW620細胞的抑制活性,關(guān)于2個新化合物的其他活性還有待于進一步研究。
利益沖突 所有作者均聲明不存在利益沖突
[1] 中國科學(xué)院中國植物志編輯委員會. 中國植物志 [M]. 北京: 科學(xué)出版社, 1978: 35.
[2] 高天慧, 樊浩, 梁卓菲, 等. 鹿藥屬植物化學(xué)成分及藥理作用研究進展 [J]. 中國野生植物資源, 2021, 40(12): 45-54.
[3] 林潔, 王國全, 白璐, 等. 鄂西鹿藥甾體化學(xué)成分研究 [J]. 中草藥, 2018, 49(17): 3987-3991.
[4] Zhang X, Su Y F, Chen L,. Steroidal saponins from the rhizomes of[J]., 2013, 96(3): 478-487.
[5] 常曉薇. 鄂西鹿藥化學(xué)成分研究 [J]. 中南藥學(xué), 2017, 15(6): 762-764.
[6] 張嬌, 張歡, 楊帆, 等. 偏頭七的研究進展 [J]. 陜西中醫(yī)藥大學(xué)學(xué)報, 2017, 40(5): 120-122.
[7] 張肖. 鄂西鹿藥根莖甾體皂苷類成分研究 [D]. 天津: 天津大學(xué), 2013.
[8] Chen Z L, Xue X J, Zhang S,. Steroidal components from the roots and rhizomes ofand their cytotoxic activities [J]., 2020, 14(3): 225-230.
[9] Zhang X, Sun J, Zhang S,. Two new cholestanol glycosides from the roots and rhizomes of[J]., 2019, 13(6): 499-505.
[10] Zhang X, Sun J, Zhang X F,. Cytotoxic steroidal saponins from the roots and rhizomes of[J]., 2021, 35(11): 1808-1815.
[11] Lin J, Wang G Q, Bai L,. Two new steroidal saponins fromand their cytotoxic activity against human HepG2 tumor cells [J]., 2019, 33(24): 3551-3558.
[12] Mimaki Y, Inoue T, Kuroda M,. Steroidal saponins from[J]., 1996, 43(6): 1325-1331.
[13] Luo Y, Shen H Y, Zuo W J,. A new steroidal saponin from dragon's blood of[J]., 2015, 17(4): 409-414.
[14] Song X M, Zhang D D, He H,. Steroidal glycosides from reineckia carnea [J]., 2015, 105: 240-245.
[15] Zhang D D, Wang W, Li Y Z,. Two new pregnane glycosides from[J].. 2016, 15: 142-146.
Two new steroidal saponins from the roots and rhizomes of
ZHANG Xin, LIU Yuan-yuan, LI Yu-ze, ZHANG Dong-dong, JIANG Yi, SONG Xiao-mei, WANG Wei, DENG Chong
School of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang 712046, China
To study the steroidal saponins from the roots and rhizomes ofand their cytotoxicity.The chemical constituents were studied by chromatography on silica gel, Sephadex LH-20 and semi-preparative HPLC, and the structures were elucidated on the basis of spectroscopic methods, including MS, IR, NMR and GC data analysis. The cytotoxicity was studied by MTT method.Two new steroidal saponins were obtained from-butanol fraction of ethanol extractofand namely (23,24)-spirost-5,25(27)-diene-lβ,3β,23,24-tetraol 1--α--rhamnopyranosyl-(1→2)-β-- furopyranoside (1) and (23,24)-spirost-5,25(27)-diene-lβ,3β,21,23,24-pentaol-1--α--rhamnopyranosyl-(1→2)-β--furopyranoside (2), and compounds 1 and 2 showed cytotoxicity against two cancer cell lines with IC50values greater than 100 μmol/L.Compounds 1 and 2 were new compounds named henryioside K and henryioside L which possessed no cytotoxicity.
(Baker) LaFrankie; steroidal saponins; cytotoxicity; henryioside K; henryioside L
R284.1
A
0253 - 2670(2022)20 - 6375 - 05
10.7501/j.issn.0253-2670.2022.20.009
2022-06-23
陜西省科技廳自然科學(xué)研究基礎(chǔ)計劃項目(2021JQ-742);陜西中醫(yī)藥大學(xué)研究生創(chuàng)新項目(2021CX19);陜西中醫(yī)藥大學(xué)學(xué)科創(chuàng)新團隊項目(2019-YL12)
張 欣(1985—),男,在讀博士,講師,研究方向為中藥藥效物質(zhì)及制劑研究。Tel: (029)38185165 E-mail: 277102745@qq.com
鄧 翀(1978—),男,博士,教授,研究方向為中草藥藥效物質(zhì)基礎(chǔ)及炮制機制。Tel: (029)38185165 E-mail: 393494971@qq.com
[責(zé)任編輯 王文倩]

