Crystallization thermodynamics of 2,4(5)-dinitroimidazole in eleven pure solvents
Pengbao Lian,Lizhen Chen,Daozhen Huang,Jianxin Xu,Zishuai Xu,Cai Cao,Jiaxiang Zhao,Jianlong Wang,*
1 School of Chemical Engineering and Technology,North University of China,Taiyuan 030051,China
2 Shandong Teneng Environmental Technology Co.,Ltd.,Dezhou 253000,China
3 Gansu Yinguang Chemical Industry Group Co.,Ltd.,Baiyin 730900,China
Keywords:2,4(5)-Dinitroimidazole Solid-liquid equilibrium Solubility model Three thermodynamic parameters Cooling crystallization
ABSTRACT 2,4(5)-Dinitroimidazole (2,4(5)-DNI) is an important energetic material,and it is also an important precursor for the preparation of drugs and energetic materials.In this study,the solubility of 2,4(5)-DNI in eleven pure solvents (chlorobenzene,benzene,1,2-dichloroethane,toluene,water,isopropyl alcohol,ethyl acetate,acetonitrile,methanol,1,4-dioxane and acetone) were measured by using a dynamic test method from 278.15 K to 323.15 K under 101.1 kPa.Four solubility models were used to fit the experimental data,which were ideal model,modified Apelblat equation,polynomial empirical equation,and λh equation.Meanwhile,the relative average deviation and root-mean-square deviation between the experimental data and the fitted data were also calculated.Furthermore,the three thermodynamic parameters,i.e.,dissolution enthalpy,dissolution entropy and Gibbs energy were obtained based on solubility data.Finally,the crude product of 2,4(5)-DNI was crystallized with acetone as solvent,and the purity of the crystalline product was greater than 99.5%.
1.Introduction
2,4(5)-Dinitroimidazole (2,4(5)-DNI,CAS No: 5213-49-0,see Fig.1) is an important organic intermediate,which can be used to manufacture medicine and energetic materials [1-18].Table 1 listed the physical properties of 2,4(5)-DNI,which can be used as an energetic material[4-11].At present,the main method for the synthesis of 2,4(5)-DNI is: nitration of imidazole to obtain 4(5)-nitroimidazole,and further nitration of 4(5)-nitroimidazole yields 1,4-dinitroimidazole,then thermal rearrangement 1,4-dinitroimidazole to obtain 2,4(5)-DNI in chlorobenzene [7-11].However,in the process of preparing 2,4(5)-DNI from 1,4-dinitroimidazole thermal rearrangement,a small amount of 4(5)-nitroimidazole may be generated due to the failure of thermal rearrangement.Therefore,the impurities of 2,4(5)-DNI may be a small number of 4(5)-nitroimidazole and incompletely reacted 1,4-dinitroimidazole.When 2,4(5)-DNI is used as a precursor for preparing energetic materials and drugs,especially when itself is used as an energetic material,which needs to have a higher purity(>99.5%).However,the purity of 2,4(5)-DNI is difficult to meet the requirements of energetic materials as used in weapons,which is prepared using current methods [7-11].Therefore,the crude product of 2,4(5)-DNI needs to be purified.

Table 1 The physical properties of 2,4(5)-DNI.
Solution crystallization is an important means to separate and purify for solid compounds,which is based on the solubility of the target compound in the solvent.Meanwhile,solution crystallization is also used for the purification of energetic materials,such as 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexazaisowurtzitane (CL-20) [19,20],2,20,4,40,6,60-hexanitrostilbene (HNS) [21,22],3,4-bi s(3-nitrofurazan-4-yl)furoxan (DNTF) [23-25],1,1-diamino-2,2-dinitroethylene (FOX-7) [26,27],5,5′-Bistetrazole-1,1′-diolate(TKX-50)[28],2,4,6-trinitroresorcinol hydrate[29,30],and the like.However,only the solubility of 2,4(5)-DNI in ethanol was reported in the literature[31],and these data cannot meet the requirements for purification of 2,4(5)-DNI.Thence,in this work,the solubility of 2,4(5)-DNI have been measured in chlorobenzene,benzene,1,2-dichloroethane,toluene,water,isopropyl alcohol,ethyl acetate,acetonitrile,methanol,1,4-dioxane and acetone with dynamic test method at temperature from 278.15 K to 323.15 K.These solvents are commonly used reagents in experiments,and the test temperature range is determined by the lowest boiling point of the solvent.The solubility data of 2,4(5)-DNI were fitted by using the ideal model [32],modified Apelblat equation [33],polynomial empirical equation[23]and λhequation[34].These four solubility models were often used to fit the solubility data of solid compounds in pure solvents[22,23,30,31].Furthermore,three thermodynamic parameters were calculated based on the solubility data of 2,4(5)-DNI in eleven pure solvents.Finally,solution crystallization was used to purify the crude product of 2,4(5)-DNI and obtained satisfactory purity,which was prepared in our laboratory.
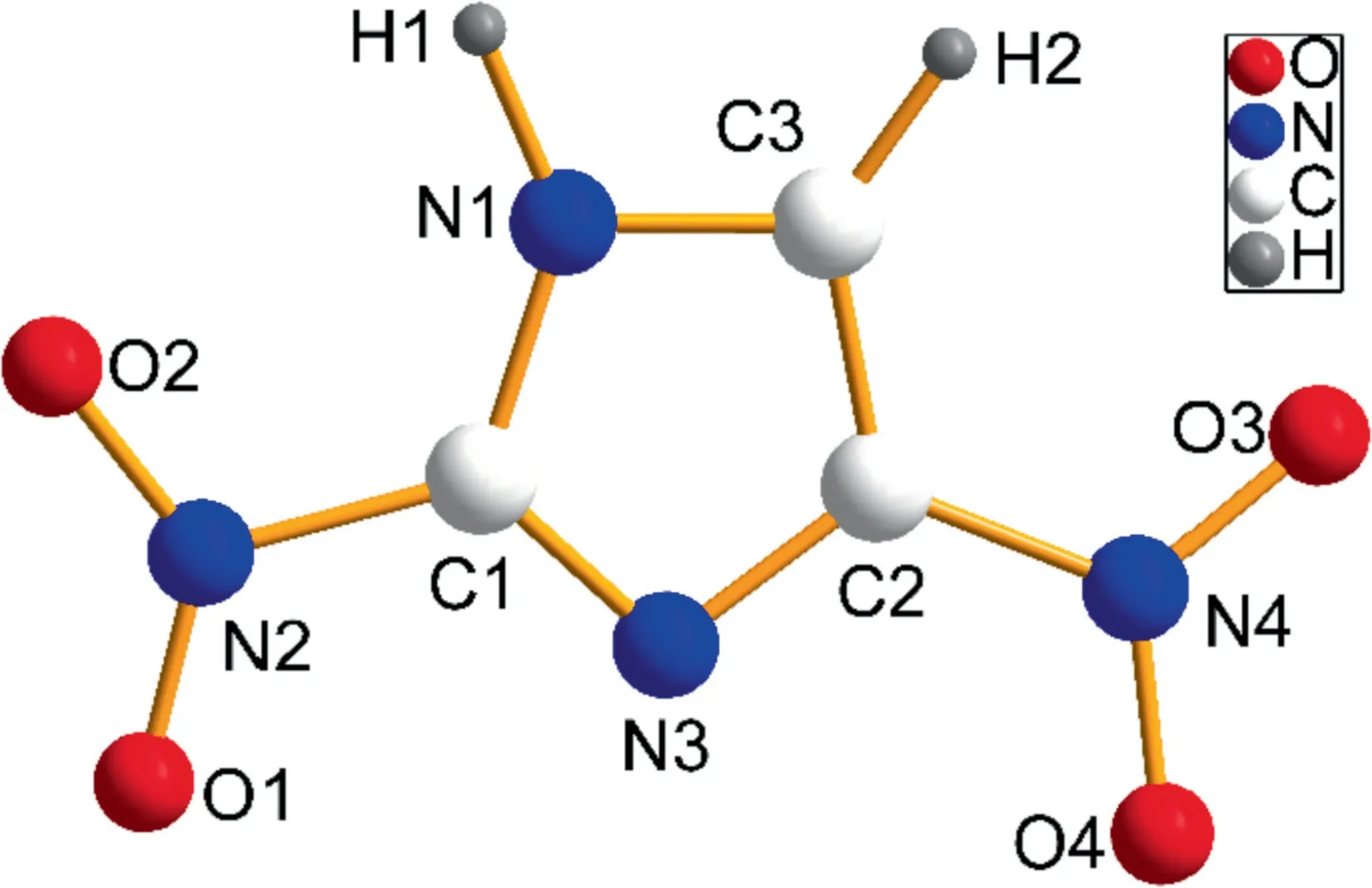
Fig.1.The structure of 2,4(5)-DNI.
2.Experimental
2.1.Materials
2,4(5)-DNI was prepared according to the procedures reported in the previous literature [8],and its purity was measured to be greater than 99.5%by using a high performance liquid chromatography.All solvents were analytical grade and purchased from Sinopharm Chemical Reagent Co.,Ltd.,China,which were analyzed by gas chromatography without further purification.
2.2.Apparatus and methods
The solubility test device of 2,4(5)-DNI was shown in Fig.2.It was widely used in our previous researches [22,25-29,31],which means that its reliability can be guaranteed.The experimental device consisted of a constant temperature water bath (error of ± 0.05 K,type SYP,China),a jacketed glass container (100 ml),a condenser tube,a magnetic stirrer,and a laser monitoring equipment (JDW-3,Department of Physics,Peking University).The model of the analytical balance was Mettler Toledo ML-T(Switzerland),and its accuracy is 0.0001 g.Pipette (a standard error of 0.1 ul)was used to add solvent,which was manufactured by DLAB Scientific Co.,Ltd.,China.
Before the start of the experiment,turn on and preheat the solubility test device for 0.5 h.The excess of 2,4(5)-DNI and a certain volume of solvent were added to the jacket glass bottle.At a constant temperature,the solution was stirred for 2 h.Then,using a pipette to add solvent until the solute was completely dissolved at a rate of 1 ml·h-1,which was supervised by the laser monitoring equipment.At the beginning of the experiment,the laser beam was blocked by many undissolved 2,4(5)-DNI particles.As the solvent increases,the 2,4(5)-DNI particles gradually decrease,and the intensity of the laser beam passing through the jacketed glass container also increases.When the 2,4(5)-DNI particles were completely dissolved,the intensity of the laser beam passing through the jacketed glass bottle reached the maximum value.Finally,the volume of solvent was recorded,and the solubility of 2,4(5)-DNI was calculated.The solubility experiments of each solvent were implemented three times at a constant temperature,and the abso-lute standard uncertainty of the experimental solubility data was not more than 0.3%.
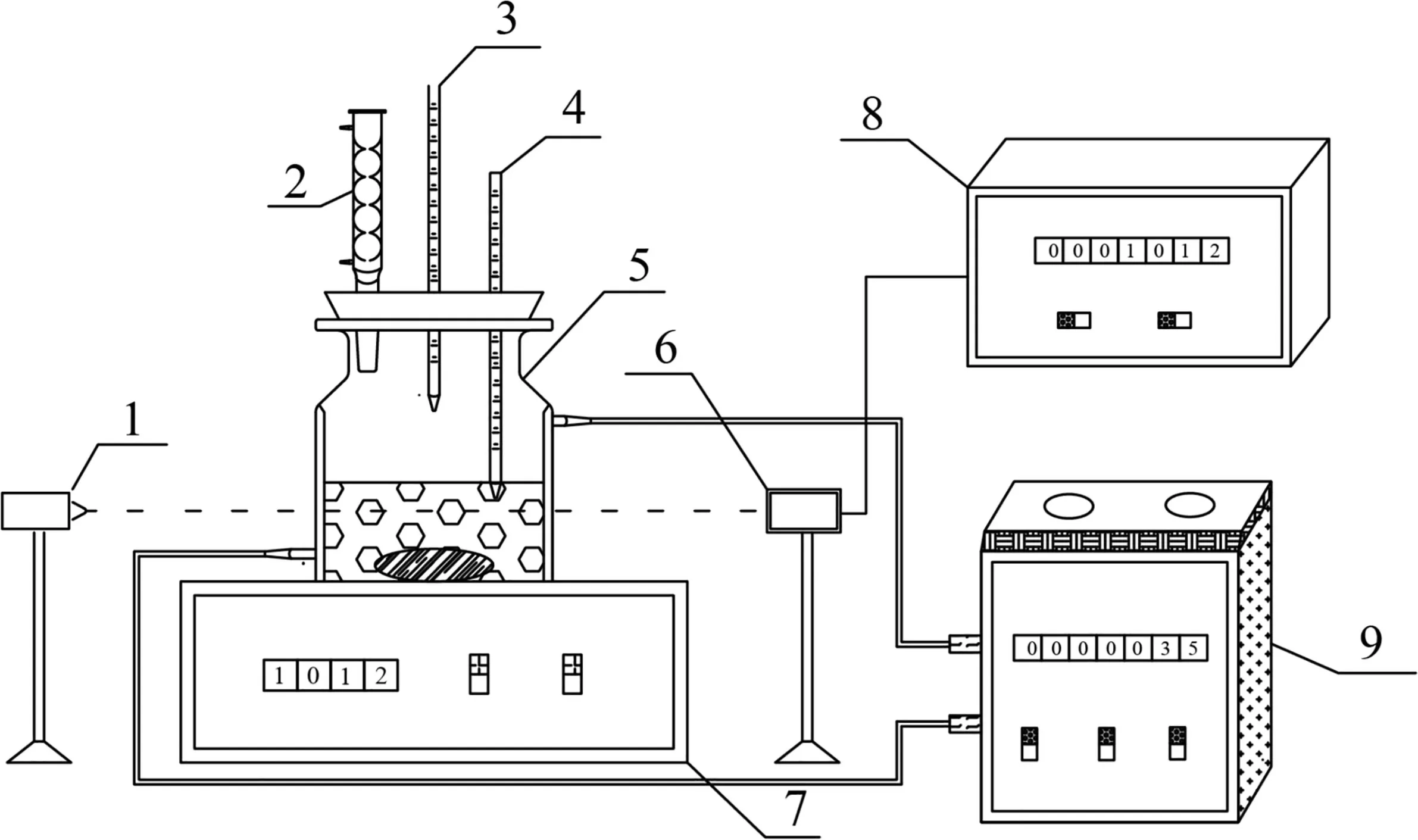
Fig.2.Device for solubility test.1—laser generator;2—condenser tube;3—burette;4—mercury thermometer;5—glass vessel;6—photoelectric switch;7—magnetic stirrer;8—digital display;9—thermostatic bath.
Eq.(1) was used to calculate the saturated solution mole fraction solubility of 2,4(5)-DNI (xT).

Here,xTstands for the saturated solution mole fraction solubility of 2,4(5)-DNI in pure solvent at absolute temperature;m1is the mass of 2,4(5)-DNI;M1andM2signify the molar mass of 2,4(5)-DNI and solvent,respectively;ρ represents the density of solvent at 293.15 K;andV,the volume of solvent.
2.3.Fourier transform infrared spectroscopy analysis
In order to analyze whether 2,4(5)-DNI has changed (transformation of crystal form,formation of solvent compounds,reaction,etc.) before and after solubility measurement,Fourier transform infrared spectroscopy(FT-IR)(Vertex 80 FTS spectrometer,Bruker,Germany) was used for analysis the raw material and the equilibrium solid phases obtained from the solubility measurement.After the solubility test of 2,4(5)-DNI at 323.15 K,the system was cooled down and the solids were precipitated.Then the equilibrium solid phases could be obtained by drying at 40 °C.
2.4.Solubility models
2.4.1.Ideal model
The solution was assumed to be in an ideal state (activity coefficient γ=1),then the ideal model (Eq.(2)) can be used to fit the solubility of 2,4(5)-DNI [32],which describes the relationship between the natural logarithm of saturated solution mole fraction solubility and the reciprocal of the absolute temperature:

whereAandBsignify parameters for the ideal model;Tstands for the absolute temperature.
2.4.2.Modified Apelblat equation
The modified Apelblat equation(Eq.(3))was proposed by Apelblat [33],which can be used to correlate the relationship between the solubility of 2,4(5)-DNI and the absolute temperature.

HereA,B,andCrepresent parameters for the modified Apelblat equation.
2.4.3.Polynomial empirical equation
The polynomial empirical equation (Eq.(4)) describes the relationship between the mole fraction solubility of 2,4(5)-DNI and cubic curve of absolute temperature at atmospheric pressure [23].

HereA,B,CandDdenote constants for the polynomial empirical equation.
2.4.4.λh equation
λhequation (Eq.(5)) was first used to describe the solid-liquid equilibrium system by Buchowski [34],which describes the relationship between saturated solution mole fraction solubility,absolute temperature,and melting point of solid compound.WhereTm=502.18 K denote the melting temperature of 2,4(5)-DNI;λ andhstand for the association number of solute molecules in the non-ideal solution and the excess enthalpy of solution,respectively.
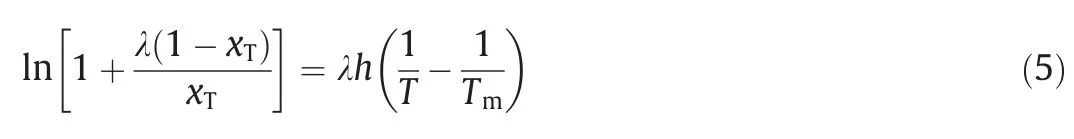
3.Results and Discussion
3.1.Fourier transform infrared spectroscopy analysis
The equilibrium solid phases obtained from the solubility measurement were compared with the raw material by FT-IR,and the results were shown in the Fig.3.It can be seen that the infrared spectrum of equilibrium solid phases are consistent with that of the raw material,and there is no peak change.Which shows that 2,4(5)-DNI did not change before and after the solubility measurement.
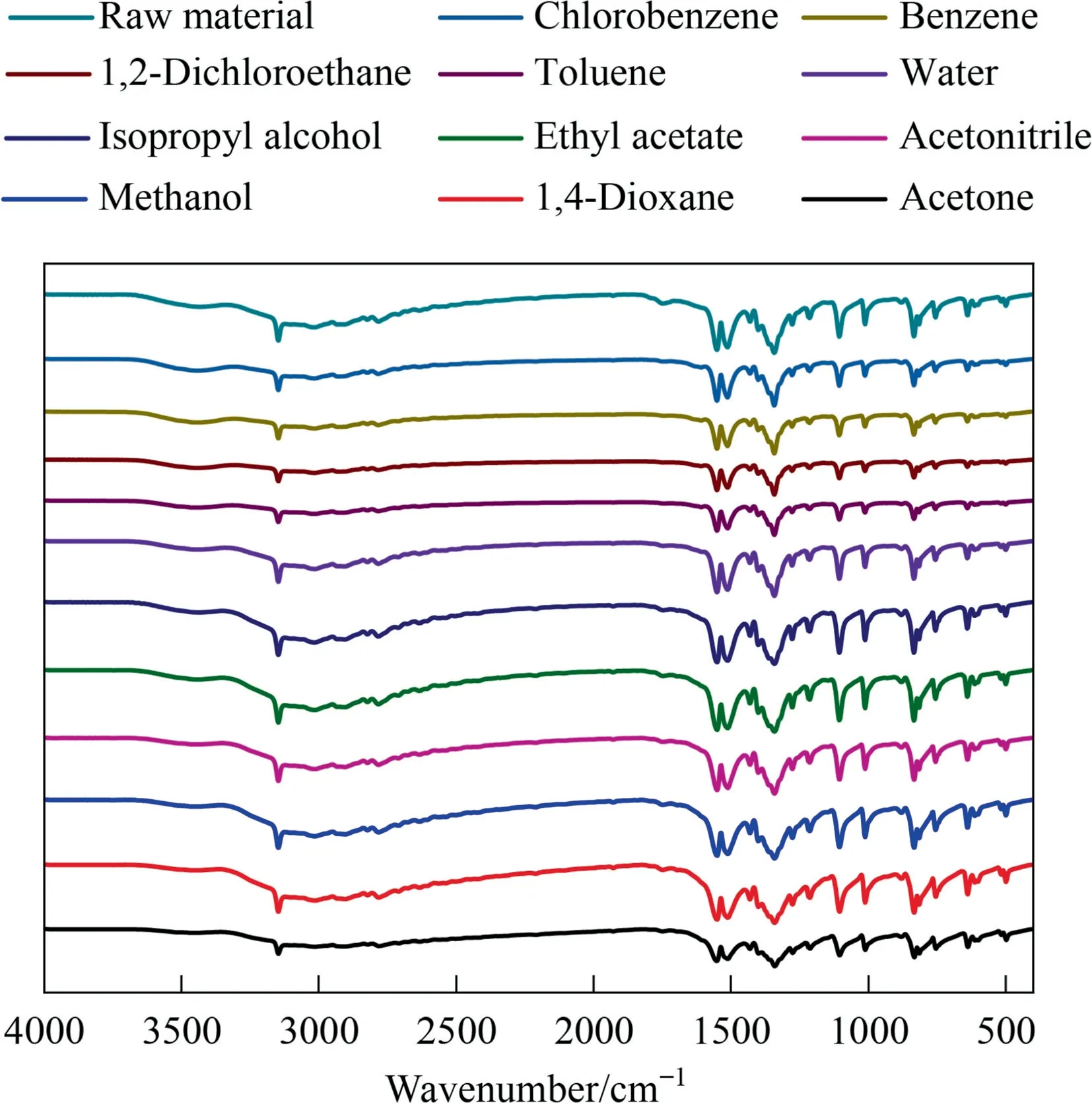
Fig.3.The infrared spectrum of raw material and equilibrium solid phases of 2,4(5)-DNI in pure solvents.
3.2.Solubility data and discussion
Table 2 listed the experimental mole fraction solubility values() and calculated solubility data () by the λhequation of 2,4(5)-DNI in eleven pure solvents.Meanwhile,the experimental data points and the curves fitted by the calculated data points were shown in Fig.4.From Table 2 and Fig.4,the following conclusions can be obtained:
(1) At the same temperature,the solubility of 2,4(5)-DNI increases in the order of chlorobenzene,benzene,1,2-dichloroethane,toluene,water,isopropyl alcohol,ethyl acetate,(acetonitrile or methanol),1,4-dioxane,acetone.It can be seen that the solubility of 2,4(5)-DNI is not only affected by the temperature,but also by the solvent at the same time.Generally,the solubility of substances conforms to the ‘‘similar compatibility” principle,which means that solubility is closely related to polarity.Table 3 listed the solvent property parameters.2,4(5)-DNI has an asymmetric structure and a certain polarity.Meanwhile,the nitrogen atom of the imidazole ring of 2,4(5)-DNI contains a lone pair ofelectrons,which may form hydrogen bonds with some solvents.However,the order of solubility is different from the order of solvent polarity and summation of the hydrogen bond acceptor propensities of the solvent (∑β).Therefore,the solubility of 2,4(5)-DNI may be jointly determined by these two factors.Usually,it is difficult to explain solubility for one reason,which is affected by many factors,such as solute-solvent interactions and the shape and size of molecules,especially in the nonpolar atomic interactions,hydrogen bonding interactions,van der Waals force,cohesive energy density and so on.
Table 2 Experimental mole fraction solubility()and calculated solubility values()by the λh equation of 2,4(5)-DNI in eleven pure solvents at the temperature from 278.15 K to 323.15 K under 101.1 kPa.
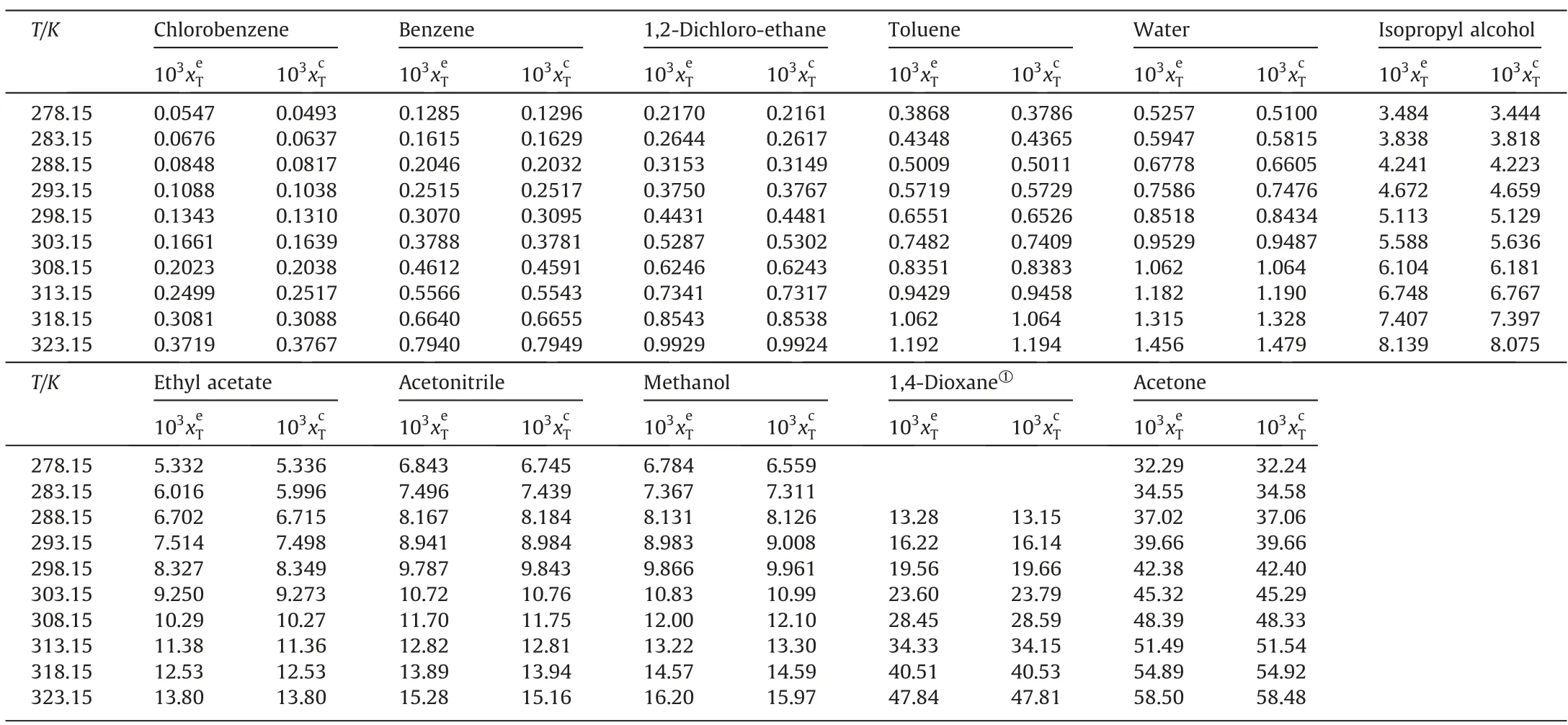
Table 2 Experimental mole fraction solubility()and calculated solubility values()by the λh equation of 2,4(5)-DNI in eleven pure solvents at the temperature from 278.15 K to 323.15 K under 101.1 kPa.
Note: The standard uncertainty u(T)=0.05 K, u(p)=0.2 kPa,Relative standard uncertainty ur(xT)≤2.26%.①Since the freezing point of 1,4-dioxane is 283.15 to 285.15 K,the solubility measurement temperature starts from 288.15K when it is used as a solvent.
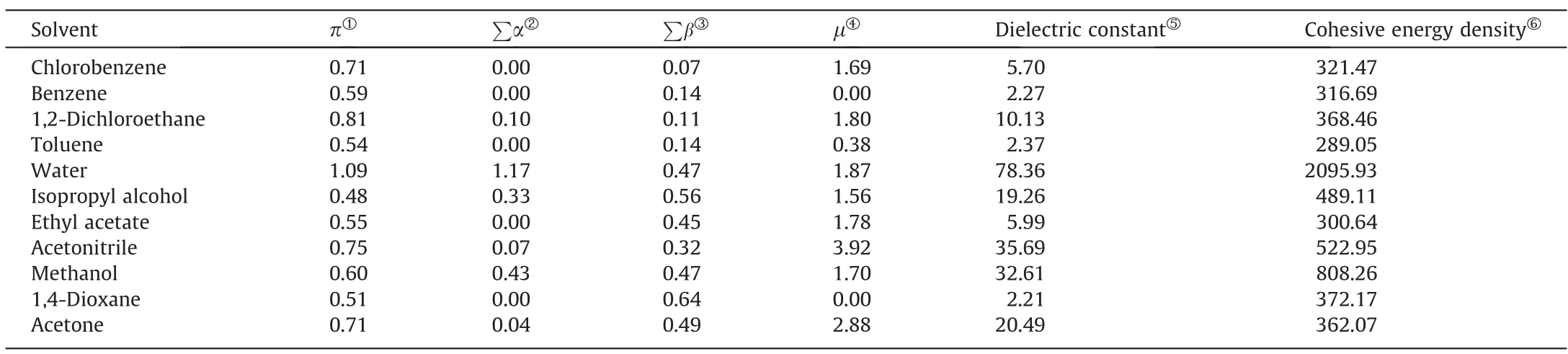
Table 3 Solvent property parameters of selected solvents [35].
(2) In the same solvent,the solubility of 2,4(5)-DNI increases with increasing temperature,which shows that its dissolution process is an endothermic process.Meanwhile,it can be obtained that the solubility of 2,4(5)-DNI has an intersection at 291.77 K in acetonitrile and methanol.The solubility of 2,4(5)-DNI in acetonitrile is greater than that in methanol,when the temperature is lower than 291.77 K;however,the opposite is true if the temperature is greater than 291.77 K.This means that the solubility of 2,4(5)-DNI is different in sensitivity to temperature in methanol and acetonitrile.With the increase of temperature (278.15-323.15 K),the solubility increase(Δ103xT)of 2,4(5)-DNI in different pure solvents were listed in Table 4.Due to the larger Δ103xTfor 2,4(5)-DNI and the smaller Δ103xTfor 4(5)-nitroimidazole are more favorable to obtain 2,4(5)-DNI by cooling crystallization purification,thus acetone and 1,4-dioxane can be used as preferred solvents for cooling crystallization (Table 4).However,compared with acetone,1,4-dioxane has stronger environmental and human hazards and a higher freezing point.Therefore,acetone was selected as the best solvent for cooling crystallization in this study.In the same solvent,the solubility of 2,4(5)-DNI is greater than that of 4(5)-nitroimidazole.The possible reason is that 2,4(5)-DNI has one more nitro group than 4(5)-nitroimidazole,and its acidic increase makes the -NH of the imidazole ring easier to ionize,and the increase of nitro groups increases the chance of forming intermolecular hydrogen bonds with solvent molecules.
3.3.Correlation of the solubility data
The ideal model,modified Apelblat equation,polynomial empirical equation,and λhequation were used to fit the solubility of 2,4(5)-DNI.Meanwhile,in order to evaluate the error of calculation results of four models,relative average deviation(RAD,Eq.(6))and root-mean-square deviation (RMSD,Eq.(7)) were engaged.
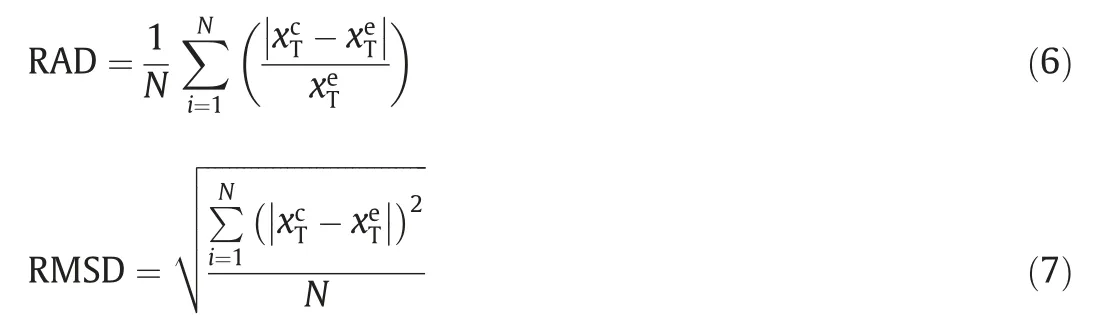
HereNdenotes the number of experimental points.
Table 5 listed the fitted values of the parameters,and the RAD and RMSD values of the fitted results for the four solubility models.The maximum values of RAD and RMSD did not exceed 3.07×10-2and 22.3 × 10-5,which shows that the error between the fitting value of the solubility model and the experimental value is very small.Meanwhile,the RAD and RMSD values of modified Apelblat equation and polynomial empirical equation are relatively close,and smaller than those of ideal model and λhequation.Thus,the modified Apelblat equation and polynomial empirical equation can better match the experimental data in this work.
3.4.Calculation of thermodynamic properties
Three thermodynamic parameters,such as dissolution enthalpy(ΔdisH),dissolution entropy(ΔdisS)and Gibbs energy(ΔdisG)can be calculated,when the solubility curves of 2,4(5)-DNI have known.Eq.(8) [36] can be employed to calculate ΔdisHand ΔdisS.

Here,R=8.3145 J·K-1·mol-1,the gas constant.The solid lines were the relationship between the mole fraction solubilityof 2,4(5)-DNI and absolute temperature in Fig.5,which were fitted by polynomial empirical equation.The slope and intercept obtained from Fig.5,which can be used to calculate ΔdisHand ΔdisS,respectively.The relationship between ΔdisH,ΔdisSand ΔdisGcan be described by Eq.(9).

Here,Tmeanstands for the mean temperature of the experiment,which can be obtained from Eq.(10):
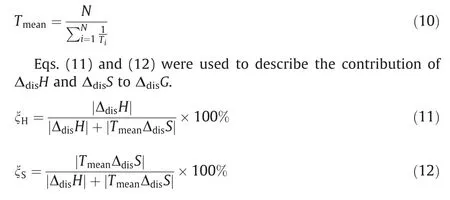
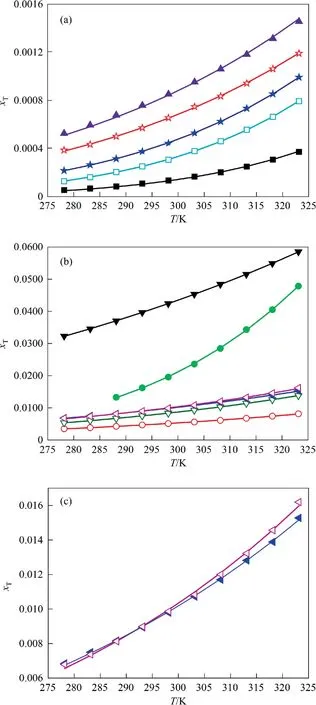
Fig.4.The saturated solution mole fraction solubility (xT) of 2,4(5)-DNI in eleven pure solvents at the temperature from 278.15 K to 323.15 K under 101.1 kPa.(a)■,Chlorobenzene;□,Benzene;★,1,2-Dichloroethane;☆,Toluene;▲,Water;(b) ○,Isopropyl alcohol;▽,Ethyl acetate;?,Acetonitrile;?,Methanol;1,4-Dioxane;▼,Acetone;(c)the diagram of ethyl acetate and methanol,in order to clearly observe their intersection;solid lines are calculated curves by the λh equation.
The calculated values of ΔdisH,ΔdisS,ΔdisG,ξH,and ξSwere shown in Table 6.The dissolution process of 2,4(5)-DNI is an endothermic process in these eleven pure solvents,which can be proved by the positive value of ΔdisH.Due to ξHis always greater than ξSin eleven pure solvents,which means that the contribution of ΔdisHto ΔdisGis more than ΔdisS.However,the contributions ofΔdisHto ΔdisGvary greatly in different solvents,which contributes up to 93% in the three solvents of toluene,water and isopropyl alcohol,while only about 40%in benzene and 1,4-dioxane.ΔdisG>0,and the order of its decrease is basically the same as the order of solubility increase,which shows that the dissolution process is a non-spontaneous and favorable process.Except for water,the ΔdisSare all positive,indicating that the dissolution process in the remaining solvents in this study is an entropy-driven process.
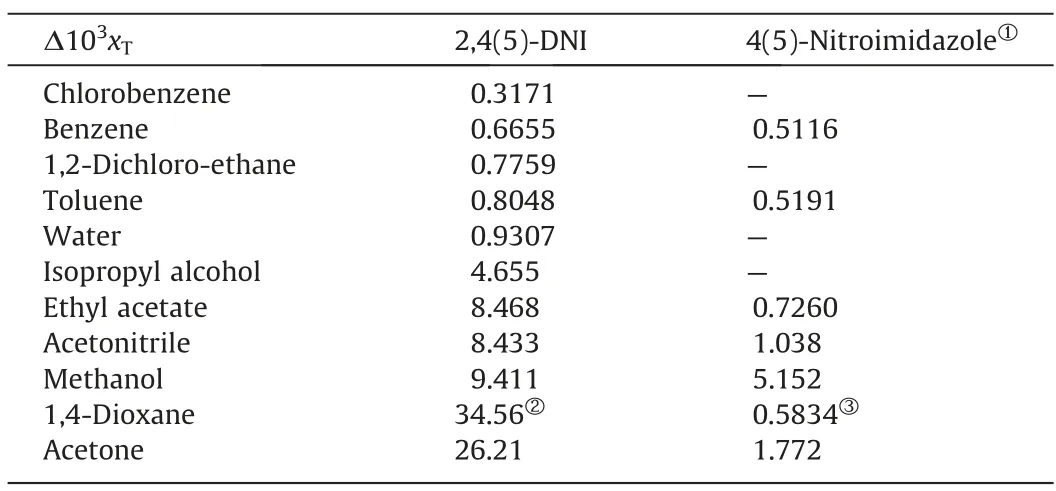
Table 4 The solubility increase (Δ103xT) of 2,4(5)-DNI and 4(5)-nitroimidazole [31] in different pure solvents.
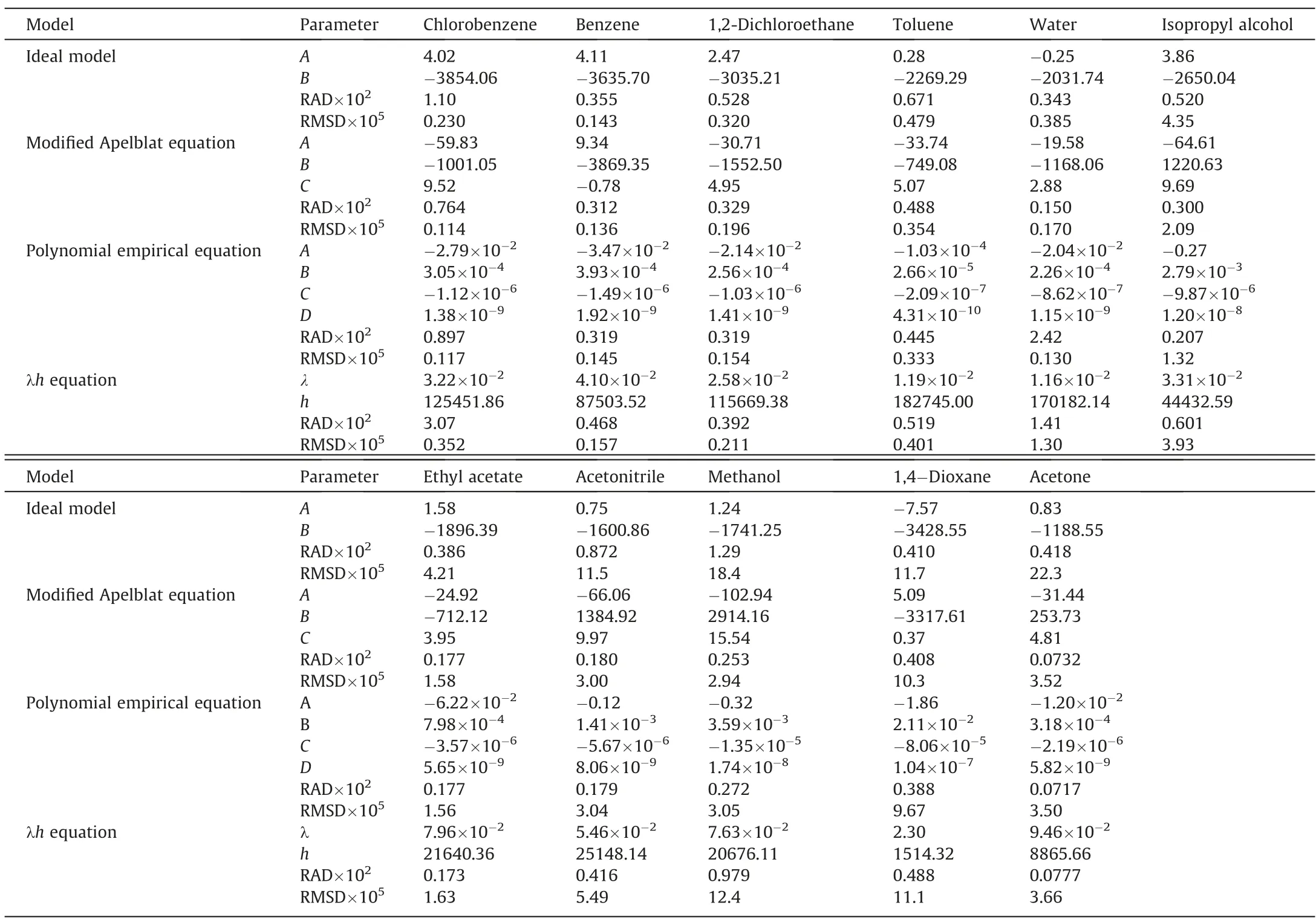
Table 5 Values of parameters obtained using four solubility models.
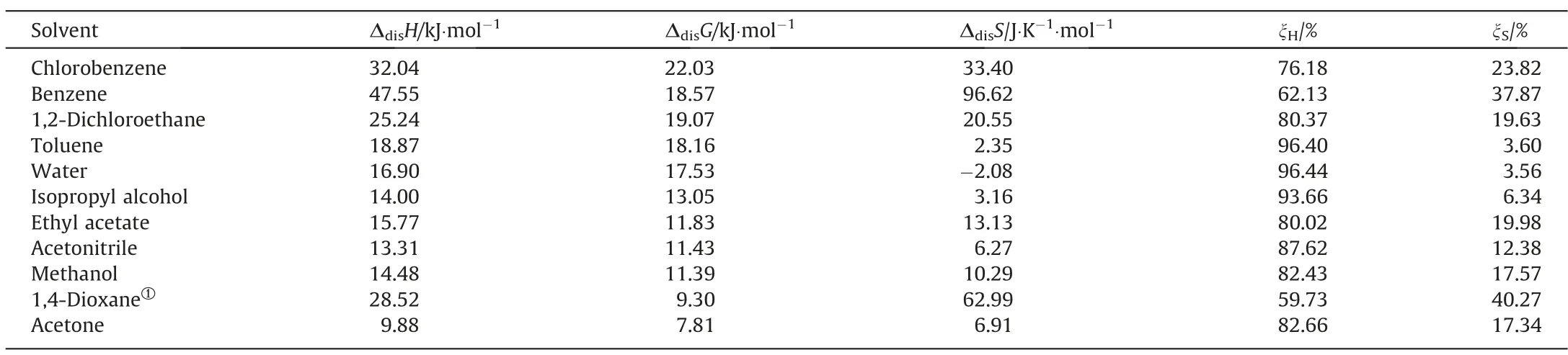
Table 6 The calculated of ΔdisH,ΔdisS,ΔdisG,ξH,and ξS for 2,4(5)-DNI in eleven pure solvents at 299.96 K.
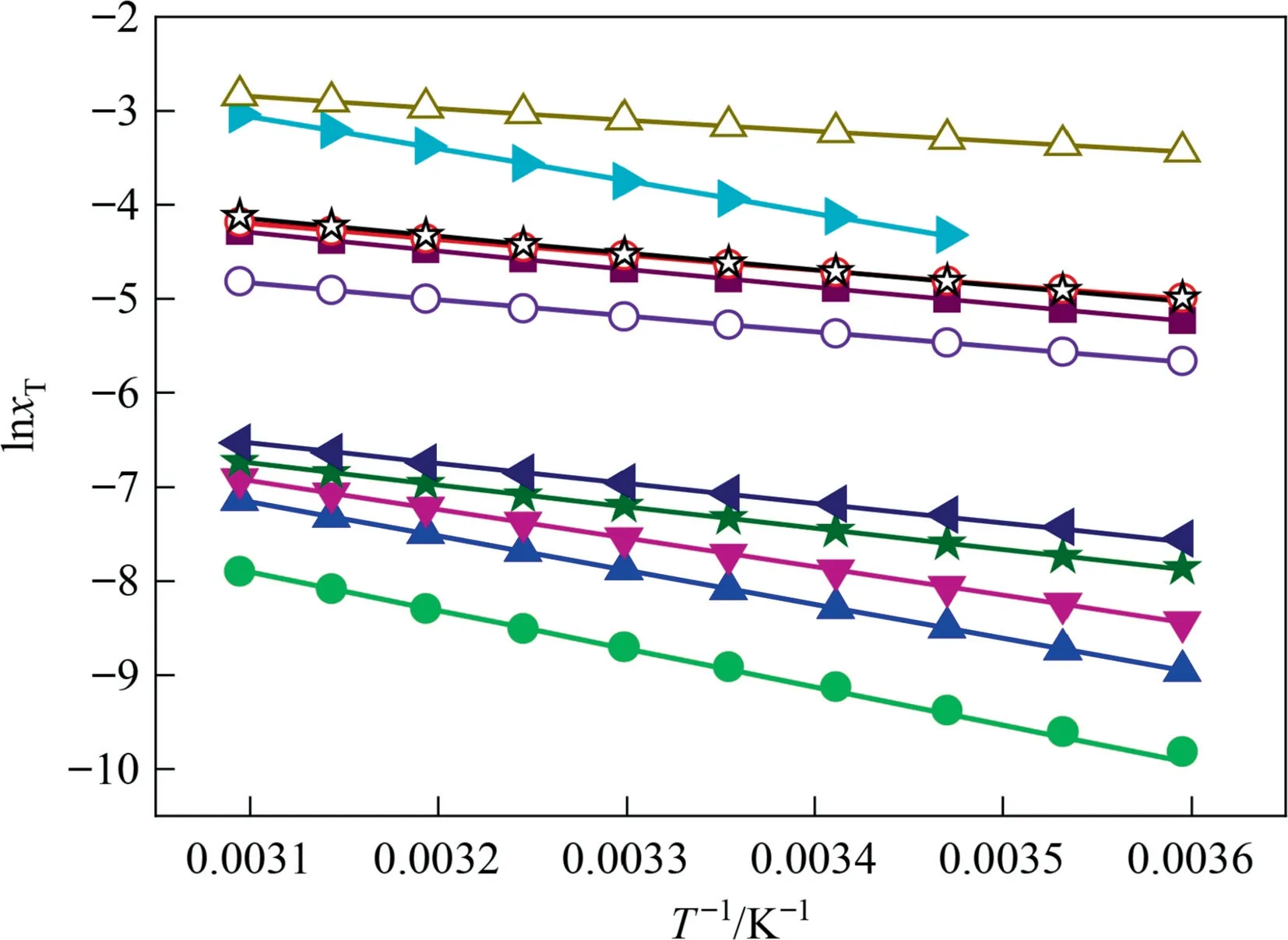
Fig.5.The relationship between lnx and 1/T of 2,4(5)-DNI in eleven pure solvents at temperature from 278.15 K to 323.15 K under 101.1 kPa: ●Chlorobenzene;▲Benzene;▼1,2-Dichloroethane;★Toluene;?Water;○Isopropyl alcohol;■Ethyl acetate;☉Acetonitrile;☆Methanol;?1,4-Dioxane;△Acetone;solid lines were calculated lines by polynomial empirical equation equation.
3.5.Analysis of crystallization products
Using acetone as crystallization solvent and starting temperature at 323.15 K,the crude product of 2,4(5)-DNI (purity,97.38%)was purified by cooling crystallization.It can be seen from Fig.6 that the purity of 2,4(5)-DNI was greater than 99.5% after once cooling crystallization,which can meet military standards.
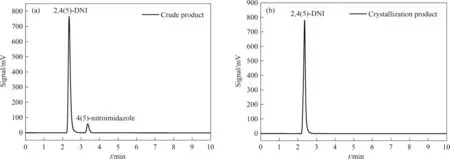
Fig.6.HPLC curves for crude and crystallization products of 2,4(5)-DNI,a) crude product,b) crystallization product.
4.Conclusions
In this paper,the solubility of 2,4(5)-DNI was determined by using a dynamic test method in eleven solvents.The solubility of 2,4(5)-DNI increases in the same solvent with increasing temperature.At the same temperature,the solubility of 2,4(5)-DNI increases in the order of chlorobenzene,benzene,1,2-dichloroethane,toluene,water,isopropyl alcohol,ethyl acetate,(acetonitrile or methanol),1,4-dioxane,acetone.Meanwhile,the solubility of 2,4(5)-DNI has an intersection at 291.77 K in acetonitrile and methanol,which means that the solubility of 2,4(5)-DNI in these two solvents are different in sensitivity to temperature.Four solubility models were employed to fit the experimental solubility values of 2,4(5)-DNI,and the calculated results matched the experimental values very well.The maximum values of RAD and RMSD did not exceed 3.07×10-2and 22.26×10-5.Three thermodynamic parameters were calculated in eleven solvents,and the dissolution enthalpy was all positive,indicating that the dissolution of 2,4(5)-DNI was an endothermic process.Meanwhile,using acetone as the solvent,the crude product of 2,4(5)-DNI was purified using the cooling crystallization method,and the purity of the product after crystallization was not less than 99.5%.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.07.031.
 Chinese Journal of Chemical Engineering2022年8期
Chinese Journal of Chemical Engineering2022年8期
- Chinese Journal of Chemical Engineering的其它文章
- Notes for Contributors
- Preparation and functional study of pH-sensitive amorphous calcium phosphate nanocarriers
- Chiral LVFFARK enantioselectively inhibits amyloid-β protein fibrillogenesis
- An international comprehensive benchmarking analysis of synthetic biology in China from 2015 to 2020
- Research on prediction model of formation temperature of ammonium bisulfate in air preheater of coal-fired power plant
- Insights into depolymerization pathways and mechanism of alkali lignin over a Ni1.2-ZrO2/WO3/γ-Al2O3 catalyst
