一例基于5-((萘-1-基甲基)氨基)間苯二甲酸配體的Cd(Ⅱ)金屬有機骨架的合成、晶體結構及對酸性氨基酸的檢測
張齡文 劉淑芹 張佩佩 倪愛云 張建軍
(大連理工大學化工學院,大連 116024)
0 Introduction
Amino acids are important substances that constitute peptides and proteins and have an important role in metabolic and neurotransmitter transmission processes in living organisms[1].Among them,aspartic acid(Asp)and glutamic acid(Glu)are also known as acidic amino acids because they both have two carboxylate groups.Asp is an excitatory neurotransmitter in the central nervous system.When Asp is in excess in the body,it may cause many diseases such as stroke,epilepsy,and even Lou Gehrig′s disease[2].In contrast,Glu is the most abundant and strongest excitatory neurotransmitter in the central nervous system.Abnormal concentrations of Glu in the body can lead to amyotrophic lateral sclerosis(ALS)and Parkinson′s disease,etc[3].Therefore,fast and accurate detection of Asp and Glu is very important.Current methods for their detection include spectrophotometric,chromatographic,and electrochemistry[4],but these methods have the disad-vantages of time-consuming,cumbersome,and high cost.Therefore,it is urgent to find a fast,convenient,and low-cost method to detect the two acidic amino acids.
Luminescent metal-organic frameworks(LMOFs)are a new type of functional luminescent materials,which are self-assembled by metal ion/cluster nodes and organic linkers[5-7].LMOFs have the advantages of abundant excited energy levels,diverse weak interactions,and tunable structures,and have potential applications in sensing[8-9].Up to now,many LMOFs have been used as probes to detect various analytes,but most of them are“turn-off”type probes.In contrast,the number of“turn-on”type probes that exhibit enhanced luminescence response to analytes remains limited.In addition,many LMOFs are unstable in water and can only work in non-aqueous systems when used as probes,thus limiting their further development.So far,some MOFs have been used as probes for the detection of amino acids[10-11].However,MOF probes for Asp or Glu detection are still rare[12].Therefore,it is of great significance to develop water-stable“turn-on”type LMOF probes for Asp and Glu detection.
Herein,a water-stable 2D LMOF[Cd(L)(H2O)]·H2O(1)withkgdtopology was synthesized through the reaction of Cd2+and ligand 5-((naphthalen-1-ylmethyl)amino)isophthalic acid(H2L,Scheme 1).Photoluminescence(PL)studies show that the luminescence of 1 can be significantly enhanced in a pH range of 3-4 due to the protonation of the—NH— group of the ligand.This interesting property allows 1 to be used as a“turnon”probe for the detection of acidic amino acids in an aqueous solution with limits of detection(LODs)of 3.88 and 5.43 μmol·L-1for Asp and Glu,respectively.The detailed structure and detection behavior are reported.
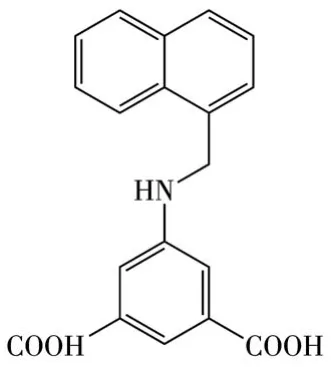
Scheme 1 Structure of H2L
1 Experimental
1.1 Material and methods
H2L was synthesized according to the literature method[13].All other chemicals employed were commercially available reagents of analytical grade and were used as received.FT-IR spectra were recorded(650-4 000 cm-1)from a Nicolet-20DXB spectrometer via a KBr pellet pressing method.Thermogravimetric analyses(TGA)were carried out on a TA-Q50 thermogravimetric analyzer with a heating rate of 10 ℃·min-1under N2protection.Elemental analyses of C,H,and N were performed on a Vario EL Ⅲ elemental analyzer.Powder X-ray diffraction(PXRD)patterns were collected on a D/MAX-2400 X-ray Diffractometer with CuKαradiation(λ=0.154 060 nm)at a scan rate of 10(°)·min-1(V=40kV,I=25mA,2θrange:5°-50°).Thesteadystate emission spectra were obtained on a Hitachi F-7000 FL spectrophotometer.
1.2 Synthesis of[Cd(L)(H2O)]·H2O(1)
A mixture containing 32.1 mg of H2L(0.10 mmol)and 31 mg of Cd(NO3)2·4H2O(0.1 mmol)was dissolved inN,N-dimethylformamide(DMF)/H2O(2 and 1 mL,respectively)in a 20 mL glass vial.Then the vial was sealed and placed in an oven at 105℃for one day,cooled naturally to room temperature.The brown lumpy crystals could be obtained,washed with DMF several times and then air-dried at room temperature.Element analysis Calcd.for C19H17NO6Cd(%):C,48.79;H,3.66;N,2.99.Found(%):C,48.83;H,3.79;N,3.11.IR(KBr,cm-1):3 386(m),1 693(w),1 609(w),1 532(s),1 419(s),1 355(w),1 314(w),1 242(s),1 244(m),1 140(w),1 037(w),931(w),908(m),771(s),732(m),684(m).
1.3 Structure determination
The intensity data from the single crystal of 1 was collected at 150 K on a Bruker SMART APEX Ⅱ CCD area detector system with graphite-monochromated MoKα(λ=0.071 073 nm)radiation.Data reduction and unit cell refinement were performed with Smart-CCD software.The structure was solved by the direct method using SHELXS-2014 and refined by the full-matrix least-squares method using SHELXL-2014[14].All nonhydrogen atoms were refined anisotropically.The hydrogen atoms related to C and N atoms were generated geometrically.Hydrogen atoms attached to oxygen atoms were located from the difference Fourier map and refined with restrained O—H and H…H distances.The naphthyl group was found to be disordered.The site occupancy factors determined using the free variable were 0.486 and 0.514 for the two parts,respectively.A summary of crystal structure refinement data is given in Table 1.Selected bond lengths and angles of 1 are given in Table S1(Supporting information).

Table 1 Crystal data collection and structure refinement parameters for compound 1
CCDC:2158763.
2 Results and discussion
2.1 Crystal structure of 1
Single-crystal X-ray diffraction study reveals that 1 is a 2D neutral coordination polymer.As shown in Fig.1a,two neighboring Cd2+ions are linked by two carboxylate oxygen atoms to form a binuclear{Cd2(COO)4}second building unit(SBU)with Cd…Cd separation ofca.0.368 nm.Each Cd2+ion bears a distorted octahedral{NO5}coordination polyhedron and is coordinated by one oxygen atom from the coordination H2O molecule,one nitrogen,and four oxygen atoms from the ligand.The Cd—O bond lengths and ∠O—Cd—O angles range from 0.219 1(2)to 0.242 9(2)nm and from 55.01(5)°to 156.03(7)°,respectively.Fig.1b shows that each ligand is utilized to coordinate four metal ions,and the coordination mode may be described as
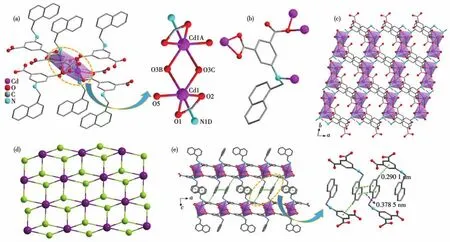
Fig.1 Structure of 1:(a)coordination environment of the Cd(Ⅱ) ion;(b)coordination mode of the ligand;(c)packing of the layers viewed along the c axis;(d)topology of the framework;(e)π-π interactions between the neighboring layers,where the interaction is represented as green dotted line
The ligand and{Cd2(COO)4}SBU act as 3-connected and 6-connected nodes respectively,which are interconnected in a 2∶1 ratio to form a 2D network withkgdtopology.The corresponding point symbol is(43)2(46,46,83).The packing of adjacent layers is so close that strongπ-πstacking interactions can occur between the aromatic rings of the ligands.As shown in Fig.1e,there is offset face-faceπ-πinteraction between naphthalene groups from different layers with ring-ring separations of about 0.379 nm.Furthermore,C—H…πinteractions are also observed between the naphthalene group and the benzene group.The corresponding separation between the carbon atom of the naphthalene group to the benzene ring isca.0.290 nm.These interactions connect the layers into a 3D supramolecular structure,thus improving the structural stability.
2.2 Characterization of the compound
To demonstrate the purity and crystallinity of the bulk sample,a PXRD test was performed.As shown in Fig.S1,the peak positions in the experimental pattern were in good agreement with those in the calculated pattern,indicating that the sample has good phase purity and crystallinity.Furthermore,1 retained its original framework after one month of immersion in water,indicating its excellent stability in an aqueous solution.TGA of 1(Fig.S2)reveals that 1 lost all solvent water molecules between 100 and 150℃(Obsd.7.59%;Calcd.7.70%),followed by a relatively stable plateau in a range of 150-388℃.After 388℃,1 lost weight significantly,indicating the destruction and collapse of the framework.
The solid-state luminescence spectra of H2L and 1 were obtained at room temperature(Fig.S3).Under 375 nm excitation,the ligand had a strong blue emission peaking at 435 nm.In contrast,1 had an emission peak at 425 nm under the same excitation,which can be attributed to ligand-based emission.
2.3 pH-modulated luminescence enhancement of LMOF 1
Considering the excellent stability of 1 in water,the PL properties of the suspensions of 1 under different acid-base conditions(pH was adjusted by 0.1 mol·L-1HCl or NaOH aqueous solution)were firstly investigated.As shown in Fig.2a and Fig.S4,the suspension of 1 had an emission peak at 421 nm at pH=7.The luminescence intensity remained stable in a pH range of 5-10.However,the intensity gradually increased when the acidity gradually increased from pH=5,increased sharply in a pH range of 3-4,and reached a maximum at pH=2,accompanied by an obvious red shift of the peak position.When the acidity continued to increase to pH=1,there was a certain degree of weakening of the luminescence.Furthermore,there was a relatively small luminescence enhancement in a pH range of 10-13 as the alkalinity of the system increased.The above results indicate that 1 has good sensitivity to the pH range of 3-4 and can be used as a good luminescence sensor for detecting pH in a small range.To further explore the effect of pH on the PL of 1,PXRD tests were performed on the samples immersed in aqueous solutions of different pH values for one day.As shown in Fig.S1,the framework was stable in a pH range of 2-13,indicating that the enhanced PL of 1 at a low pH value is not due to the collapse of the framework.

Fig.2 Luminescence response of the suspensions of 1 to pH and amino acids:(a)line chart of luminescence intensity in a pH range of 1-13;(b)variation in the luminescence intensity toward different amino acids
We speculate that the above pH-dependent luminescence changes may be due to the fact that the secondary amine group in the ligand is easily protonated under acidic conditions,resulting in the weakening of the coordination of the Cd2+ion with the N atom.This ultimately leads to enhanced luminescence.
2.4 Sensing of acidic amino acids
The excellent response of 1 to a small range of pH prompts us to further explore the possibility of using 1 to detect common amino acids.When different amino acids were added to the aqueous suspension of 1,only two acidic amino acids,Asp and Glu,significantly enhanced the luminescence of the suspension.As shown in Fig.2b and S5,the luminescence of 1 was enhanced by 14.4-fold and 9.0-fold after the addition of Asp and Glu,respectively,accompanied by an obvious red shift of the emission peak(429 nm).In contrast,lysine(Lys)could only lead to a 2.8-fold enhancement,while other amino acids did not affect the luminescence of 1.Therefore,1 can effectively distinguish acidic amino acids from other amino acids by the difference in luminescence intensity.
Further titration experiments were performed to investigate the possibility of 1 as a probe.The luminescence intensity of 1 was gradually enhanced as the amount of the two acidic amino acids increased(Fig.3a and 4a).For Asp,a linear relationship was followed in a wide concentration range of0-0.67 mmol·L-1(Fig.3b),and the linear correlation could be fitted asI=4 412.63c+321.17.The corresponding LOD was calculated to be 3.88 μmol·L-1based on the signal-noise ratio of 3σ/k.Similarly,a linear range of 0-0.58 mmol·L-1was also observed in the Glu case(Fig.4b)with the fitted equation ofI=3 151.37c+298.57,and the corresponding LOD was 5.43 μmol·L-1.The correlation coefficients(R2)were calculated to be 0.996 and 0.995 for the two fits respectively,indicating that 1 can be a good probe for the quantitative detection of Asp and Glu in the concentration range.

Fig.3 Detection of Asp by compound 1:(a)emission spectra of the aqueous dispersion of 1 after incremental addition of Asp solution(λex=375 nm);(b)corresponding linear fitting plot

Fig.4 Detection of Glu by compound 1:(a)emission spectra of the aqueous dispersion of 1 after incremental addition of Glu solution(λex=375 nm);(b)corresponding linear fitting plot
In addition,after adding 1.0 mmol·L-1Asp or Glu solution to the suspension of 1,the luminescence intensity of the suspension tended to stabilize within 300 s(Fig.S6),indicating that 1 has a fast response speed as an acidic amino acid probe.Anti-interference experiments were also carried out in the presence of other amino acids at the same concentration(Fig.S7 and S8).Except for the most basic arginine which can cause some interference,the addition of other amino acids did not affect the luminescence of the system,indicating that 1 has good selectivity for acidic amino acids.
So far,several MOF-related composite probes prepared by post-synthetic modification have been used to detect Asp or Glu[15],but they suffer from the drawbacks of the leaching of the loaded metal ions from the composites as well as the possibility of channel blockage.Several MOFs have also been directly used to detect Asp or Glu[10],but their LODs are relatively high.In addition,the preparation of some of the probes more or less requires the use of expensive rare earth metal ions,either Tb3+or Eu3+[2,11].In contrast,the probe we report here is a non-composite probe,which uses transition metal ions as nodes,has excellent stability in water,and has a low LOD.Therefore,it has certain advantages.
3 Conclusions
To sum up,a water-stable MOF(1)with 2Dkgdtopology was prepared.The interesting luminescence enhancement of 1 under acidic conditions allows 1 to be used for the detection of acidic amino acids in an aqueous solution with LODs of 3.88 and 5.43 μmol·L-1for Asp and Glu,respectively.The work provides a good example of the preparation of an LMOF-based probe for amino acid detection.
Acknowledgments:This research is supported by the National Natural Science Foundation of China (Grant No.21871038).
Supporting information is available at http://www.wjhxxb.cn

