Protein composition of extracellular vesicles from skin secretions of the amphibian Bombina maxima
DEAR EDITOR,
Extracellular vesicles (EVs) are important for the transport of biologically active materials and for intercellular communication.As an exposed mucosa,amphibian skin participates in many essential physiological processes.To date,however,little is known about EVs in amphibian skin.Here,we successfully isolated EVs from the skin secretions ofBombina maxima,and characterized the EVs using nanoparticle tracking,western blotting,and electron microscopy.Proteomic analysis using liquid chromatographytandem mass spectrometry identified 131 proteins in the skin secretion-derived EVs,which were enriched in biological processes and molecular functions such as immune stress,metabolism,oxidoreduction,and transport.Non-EV skin secretions were also analyzed,with 313 proteins (not in EVs)identified in these samples.Comparative analysis revealed that 62 proteins were unique to EVs,244 proteins were unique to non-EVs,and 69 proteins were shared between EVs and non-EVs.Flow cytometry showed that the isolated EVs could be taken up byB.maximaskin-derived fibroblasts.These findings provide the first evidence that EVs containing bioactive molecules are secreted from amphibian skin,suggesting that EVs may play important physiological roles in amphibian skin.
EVs are lipid bilayer granules (30–1 000 nm in diameter)that are naturally released from cells under both physiological and pathological conditions (van Niel et al.,2018).They are broadly classified as microvesicles or exosomes,depending on their size,biogenesis,and content (van Niel et al.,2018).EVs are present in most body fluids and function in intercellular communication,allowing cells to exchange proteins,metabolites,lipids,and nucleic acids (Zhang et al.,2021).EVs are also found on mucosal surfaces and in secreted mucus (Sj?qvist et al.,2019),where they play important roles in wound healing and repair,inflammation,and immune responses (Sj?qvist et al.,2019).
Amphibians represent a transitional group of organisms from aquatic to terrestrial environments.They show a degree of similarity to each other in ecology,morphology,and behavior (Xu &Lai,2015;Zhang,2015).During their water-toland transition,amphibian skin evolved special adaptability,with common elements in composition,structure,and physiological functions,including maintaining water balance,respiration,immune defense,and oxidation resistance (Zhang,2015).Amphibian skins are characterized by a thin and moisturized epidermal layer,which allows fast gas exchange(cutaneous respiration) and water permeability (Xu &Lai,2015),as well as abundant glands that secrete diverse bioactive compounds,such as peptides (Xu &Lai,2015;Zhang,2015).These bioactive molecules have been implicated in the physiological functions of amphibian skin,including peptides with antimicrobial and antioxidant properties (Xu &Lai,2015).To the best of our knowledge,however,amphibian skin-derived EVs remain poorly studied.
Bombina maximatoads,which belong to the family Bombinatoridae,are found in Yunnan,China,and potentially in Myanmar (Zhang,2015).Similar to most amphibians,these toads contain many conserved skin glands,including mucous and granular glands (Zhao et al.,2021).In a previous study,we identified a large number of bioactive molecules inB.maximaskin secretions,including antimicrobial peptides,aerolysin family pore-forming proteins (af-PFPs;previously named aerolysin-like proteins (ALPs)),trefoil factors (TFFs),and oxidoreductase peroxiredoxin 6 (Zhang,2015).We also isolated the βγ-CAT protein complex,which is composed of an af-PFP subunit (βγ-crystallin domain fused with an aerolysin domain,BmALP1) and a TFF subunit (BmTFF3) (Zhang et al.,2021).The BmALP1 subunit can be reversibly switched between active and inactive forms,and its paralog BmALP3 is a negative regulator depending on environmental oxygen tension (Wang et al.,2020).By targeting gangliosides and sulfatides in cell membrane lipid rafts (Guo et al.,2019),βγ-CAT acts on both endocytic and exocytic pathways to form channels on endolysosomes and drive the import and export of cellular material (such as nutrients,water,ions,and antigens) via the endolysosomal pathways (Zhang et al.,2021).Thus,βγ-CAT and its regulatory network define a secretory endolysosomal channel (SELC) pathway,thus representing a novel PFP-driven cell vesicular delivery system(Zhang et al.,2021).The βγ-CAT pathway plays multiple roles in toad adaptation to environment change,dependent on the cellular and surrounding environment,including in immune defense and tissue repair (Zhang et al.,2021),water maintenance under osmotic stress (Zhao et al.,2021),and nutrient acquisition or delivery under starvation (Shi et al.,2022).
In the current study,we isolated and characterized EVs from skin secretions ofB.maximaand identified a large number of bioactive proteins.We compared protein compositions in EV and non-EV skin secretions.We also found that the isolated EVs could be taken up byB.maximaskin-derived fibroblasts.Our results should help elucidate the role of EVs in amphibian skin.
To investigate amphibian skin EVs,skin secretions fromB.maximawere first collected.EVs were isolated by differential centrifugation coupled with size exclusion chromatography(Supplementary Figure S1).The purified EVs were characterized by size exclusion using nanoparticle tracking analysis (NTA).Western blot analysis was performed using EV-specific markers CD63,CD9,CD81 and flotillin-1.Morphological analysis was conducted using transmission electron microscopy (TEM).NTA revealed a polydisperse particle population ranging from 30 to 450 nm in diameter with a modal of~157 nm (Figure 1A).Western blotting showed that the EVs were positive for the EV-specific markers (Figure 1B).Morphological analysis confirmed the polydisperse EV population (Figure 1C).
To determine the protein composition of EVs isolated from theB.maximaskin secretions,the EV proteins were first separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie blue staining,which indicated enrichment of proteins,such as BmALP1,BmALP3,and BmTFF3 (Figure 1D).Protein identification using liquid chromatography-tandem mass spectrometry (LCMS/MS) confirmed the presence of 131 proteins.These proteins were classified based on function and/or protein family,e.g.,stress and immune response proteins,oxidation reduction proteins,metabolic enzymes,proteasome,protease,and protease inhibitor proteins,transcription and translation proteins,and transport proteins (Figure 1E;Supplementary Tables S1,S2).To better understand the biological functions of the EV-identified proteins,Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed.Results showed that the proteins were enriched in 29 KEGG pathways,included “metabolic pathway”,“endocytosis”,“necroptosis”,“protein processing in endoplasmic reticulum”,and “phagosome” (Figure 1F).
Non-EV proteins were also detected in the skin secretions and LC-MS/MS identified 313 proteins (Figure 1E;Supplementary Tables S2,S3).Based on comparative analysis,we found that 62 proteins were unique to EVs,244 proteins were unique to non-EVs,and 69 proteins were found in both EVs and non-EVs (Figure 1E;Supplementary Tables S1,S2).The EV and non-EV skin secretions contained many common bioactive proteins,including βγ-CAT,redox proteins,immune and stress response proteins,as well as metabolic enzymes (Figure 1G;Supplementary Table S2).These proteins are closely related to the functions of amphibian skin,such as antioxidation,immune defense,and metabolism.In particular,as major skin secretion components,βγ-CAT and its regulators represent a novel PFP-driven cellular vesicle delivery system,and thus play essential roles in nutrient acquisition,water maintenance,and immune defense inB.maxima(Shi et al.,2022;Zhang et al.,2021;Zhao et al.,2021).
The EV-specific components included proteins related to stress,transcription,translation,protein-folding,and the skeleton (Figure 1G;Supplementary Table S1).Stress-related proteins,especially heat shock proteins,accounted for a large proportion (Supplementary Table S1).Heat shock proteins are molecular chaperones that help ensure proper synthesis and decomposition of proteins and maintain normal cellular functions in response to stressors,such as heat,oxidation,nitrite,and bacterial infection (Simoncelli et al.,2019).Amphibian skin is exposed to both water and land environments and is easily damaged by environmental stressors,such as microorganisms,ultraviolet radiation,and heavy metal ions (Xu &Lai,2015;Zhang,2015).Our findings showed that EVs were enriched in stress and heat shock proteins,suggesting that EVs in amphibian skin secretions may play important roles in maintaining protein synthesis and decomposition homeostasis in response to stress.
As observed by flow cytometry,the isolated EVs were successfully internalized by toad skin-derived fibroblast cells(Figure 1H).However,whether EVs can be taken up by fibroblasts or other cellsin vivois not clear.The detailed effects of EVs onB.maximain vitroandin vivo,such as on stress responses,endocytosis,metabolism,and antioxidation,will be a fascinating subject for future research.
Notably,we identified many antimicrobial peptides inB.maximasecretions in our previous study (Zhang,2015),whereas no such peptides were found in the EV or non-EV skin secretions in the current study.This may be due to the use of LC-MS/MS and trypsin treatment of samples for protein analysis (Zhang,2015).Identifying low-intensity signals from small (digested) antimicrobial peptides is difficult.
We demonstrated for the first time the presence of EVs in amphibian skin (Figure 1I).Although a considerable number of common bioactive proteins were identified in both EV and non-EV skin secretions,their protein compositions were distinct.Results also showed that the purified EVs could be taken up by toad skin-derived cells.The current study provides evidence for the roles of EVs in amphibian skin and improves our understanding of the biological functions of amphibian skin secretions.
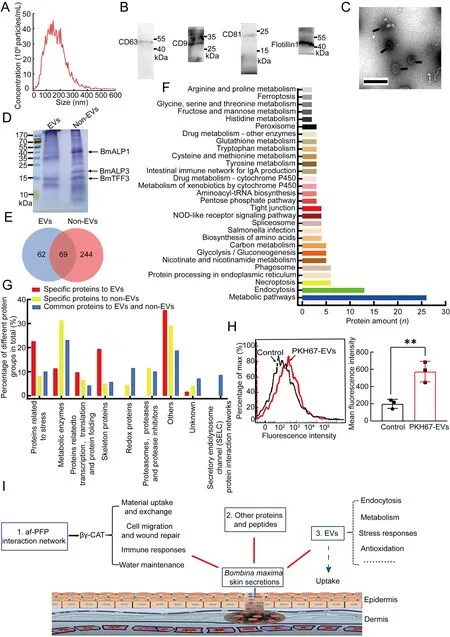
Figure 1 EVs in Bombina maxima skin secretions
DATA AVAILABILITY
All raw MS files are available at Zenodo (Accession No.6025387)and Science Data Bank (https://www.scidb.cn/en) database(DOI10.57760/sciencedb.j00139.0001).
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Q.Q.W.,Y.Z.,and L.L.conceptualized,drafted,and modified the manuscript.X.S.W.,L.Z.L.,L.Z.,X.L.B.,and W.H.L.performed and analyzed the experiments.Q.Q.W.and Y.Z.analyzed the data.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank the Core Technology Facility of the Kunming Institute of Zoology (KIZ),Chinese Academy of Sciences(CAS),for providing transmission electron microscopy.We are grateful to Ying-Qi Guo for help in making the TEM samples.
Xue-Song Wei2,3,Ling-Zhen Liu2,Xian-Ling Bian2,Lin Zeng2,5,Wen-Hui Lee2,Ling Lin3,*,Yun Zhang2,4,*,Qi-Quan Wang1,2,*
1Human Aging Research Institute and School of Life Science,Nanchang University,and Jiangxi Key Laboratory of Human Aging,Nanchang,Jiangxi330031,China
2Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences&Yunnan Province,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming,Yunnan650201,China
3College of Life Sciences,Anhui Normal University,Wuhu,Anhui241000,China
4Center for Excellence in Animal Evolution and Genetics,Chinese Academy of Sciences,Kunming,Yunnan650201,China
5Institutional Center for Shared Technologies and Facilities of the Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming,Yunnan650201,China
*Corresponding authors,Email:linling8@ahnu.edu.cn;zhangy@mail.kiz.ac.cn;wangqiquan@ncu.edu.cn
- Zoological Research的其它文章
- Towards a primate single-cell atlas
- Chromosome-level genome assembly of the freshwater snail Bellamya purificata (Caenogastropoda)
- Liobagrus chengduensis,a new species of torrent catfish (Teleostei:Siluriformes:Amblycipitidae) from the upper Changjiang River basin in southwest China
- A new seamoth species of Pegasus (Syngnathiformes:Pegasidae) from the East China Sea
- Allele-specific expression analyses reveal immune divergences between ibex and goat species
- Importance of genetic data from type specimens:The questionable type locality of southern white-cheeked gibbon,Nomascus siki (Delacour,1951)

