Observational study of enzyme in instrument cleaning for sterile reprocessing and factors impacting enzyme activity
ZHANG Zhengtao,ZHONG Mingwei
(1.Hangzhou Hygen Health Biotechnology Co.Ltd.,Hangzhou,China,310018;2.Medtronic Plc.,Minneapolis,MN,USA,55432)
ABSTRACT:Objective To evaluate the application of enzyme in instrument cleaning for hospital sterile reprocessing and different factors that impact the enzyme activity.Methods Standard soil objects for instrument cleaning quality evaluation,as testing coupons,were identified and used to evaluate different cleaning processes designed with varied conditions.Between testing groups using enzymatic detergent versus non-enzymatic detergent,the amount of residual protein on the testing coupons were quantified and compared at different soaking time(10min,20min,30min,45min and 60min).Then,within the enzymatic detergent group,different testing conditions were further explored by adjusting factors,in?cluding the soaking temperature(25°C,30°C and 45°C),use solution pH(7.0,8.0),and enzyme dosing(1/80,1/40,1/20,3/40,1/10 and 3/20 in v/v).Then,through an observational comparative study for each testing condition,the time needed to achieve a complete soil removal through visual inspection of the testing coupons was documented for analyses.Results In the test of enzymatic detergent versus non-enzymatic detergent,the non-enzymatic group did not show an obvious decline in the residual protein amount(1069 μg at 10 min vs.1042 μg at 60 min),whereas the enzymatic group showed significant decrease in residual protein quantity(947 μg at 10 min vs.620 μg at 60 min).Meanwhile,the amounts of the residual protein at different time points in the enzyme group(947 μg at 10 min,864 μg at 20 min,812 μg at 30 min,69 1μg at 45 min,and 620 μg at 60 min)were consistently lower than those at the same time schedule in the non-enzyme group(1069 μg at 10 min,1069 μg at 20 min,1067 μg at 30 min,1059 μg at 45 min,and 1042 μg at 60 min).Furthermore,within the enzymatic group,the soaking temperature,use solution pH and enzyme dosing factors all appear to impact the enzyme activity and significantly contribute to the cleaning outcomes.Specifi?cally,the higher soaking temperature,higher use solution pH and higher enzyme dosing showed 58.4%,20.0% and 34.4% time reduction to completely remove the soil on the testing coupons,respectively.Conclusion Enzyme seems to play a significant role in the instrument cleaning process for hospital sterile reprocessing.The soaking temperature,cleaning use solution pH,and enzyme dosing all appear to be critical factors impacting the enzyme activity and thus the overall cleaning outcomes.In practice,cleaning process verification should be considered to ensure the optimal useconditions for enzyme cleaning performance are well-understood and consistently achieved at the facility level.
KEY WORDS:sterile reprocessing;enzymatic cleaning;factors;process
1 Introduction
The sterile reprocessing of surgical instruments is vital to the quality and safety of patient care,be?cause invasive procedures involve direct contact be?tween the surgical instruments and the patient’s sterile tissues or mucous membranes[1].Without thorough cleaning,the sterilization and/or high-level disin?fection process could be a failure,especially in complicated devices like endoscopes that have been reported to cause infection transmission[2-3].
Enzyme,as an important additive to deter?gents that catalyzes the chemical reactions to break?down the organic soils,has been widely used in multiple specialty cleaning practices across differ?ent industries[4-5].However,in the instrument cleaning for the hospital sterile reprocessing,the use of enzyme and its contributions to enhance the cleaning process and outcome have been in debate,especially in areas where resources are in more con?straints[6].In addition,when enzymatic detergent is used,there is lack of data to better understand the factors that impact the enzyme activity to achieve its optimal performance.As a result,in hospital sterile reprocessing practice,there has been little awareness on process verification,in order to effec?tively monitor and control these critical factors and ensure the optimal use conditions for the enzyme performance in cleaning.This issue is more severe in manual cleaning,because it is typically em?ployed for complicated,hard-to-clean instruments cleaning,e.g.endoscopes,and is more suscepti?ble to process variations due to human factors[7-8].
This study is aimed to provide evidence to sup?port the use of enzyme in surgical instrument clean?ing and contribute data to better understand the fac?tors impacting the enzyme activity,in order to shed light on the need for cleaning process verifica?tion to optimize the cleaning processes for hospital sterile reprocessing.
2 Methods
The observational study was conducted in the central sterile reprocessing department in a 484-bed medical center that has an average of 27 surgi?cal procedures on a daily basis.The TOSI cleaning indicator(Healthmark Industries,Fraser,MI)was identified as the appropriate standard testing soil object or“testing coupon”to evaluate the effective?ness of the cleaning process.In the study,the test?ing coupons,from the same manufacturing lot,are fully immersed in the cleaning use solution,stand?ing against the wall of the use solution container in a pre-specified location in the same fashion across groups to allow direct comparison.A 2.5cm stir bar with 300 RPM was employed to provide agita?tion in the cleaning use solution.After each experi?ment,the testing coupon is retrieved and directly evaluated through visual inspection,per the TOSI product instructions for use.Whenever a result in?terpretation is hard to compare across testing cou?pons,they were stained with Coomassie Blue stain,which is a commonly used stain that binds to protein and make the protein appear blue in color to aid further visual inspection for comparison.
In the enzymatic vs.non-enzymatic comparison testing,the same neutral pH generic base detergent was used with one having enzyme in formulation and the other not.Testing coupons were soaked for in?creasing amount of time,and the amount of the re?sidual protein on the testing coupon was measured for each by using a fluorescent assay based on the reac?tion of proteins with o-Phthaldialdehyde/sodium 2-mercaptoethanesulfonate.The soaking time observed were 10 min,20 min,30min,45min and 60min for both testing groups.
Within the enzymatic cleaning group,differ?ent testing conditions were further explored to eval?uate the factors that impact the enzyme activity in the cleaning use solution.Factors adjusted to cre?ate variations include the soaking temperature(25°C,30°C and 45°C),use solution pH(7.0,8.0),and enzyme dosing(1/80,1/40,1/20,3/40,1/10 and 3/20).For each experiment,the time needed to achieve a full removal of the standard soil on the testing coupon,as an indicator of the enzyme activ?ity,was recorded for comparison.
All data were tracked and analyzed by using Microsoft Excel(Microsoft Corporation,Red?mond,WA)and were recorded as their true values observed in the study.
3 Results
In the enzymatic vs.non-enzymatic compari?son testing,the non-enzymatic group did not show an obvious reduction in the amount of the residual protein over time,whereas the enzymatic group demonstrated a significant decrease in the residual protein quantity in the same time schedules.De?tails of the testing results are listed in Table 1.
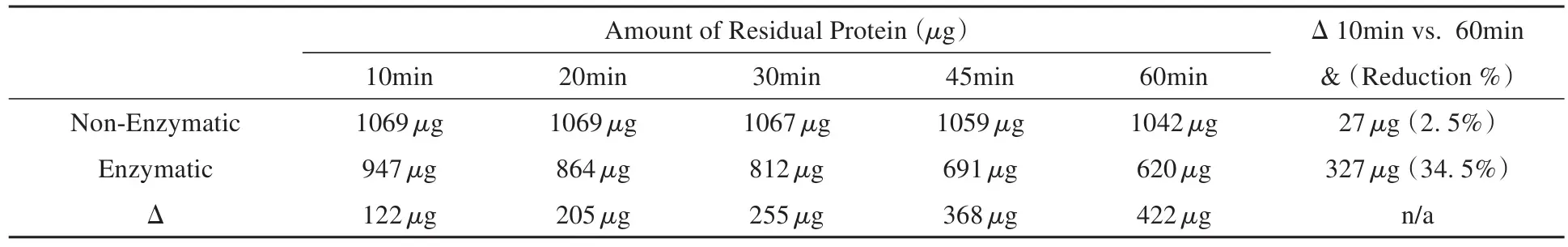
Table 1 Amount of Residual Protein in Increasing Soaking Time in Enzymatic and Non-Enzymatic Cleaning Groups
In the enzymatic group,the soaking tempera?ture,use solution pH,and enzyme dosing were ex?plored as the potential critical factors that would im?pact the enzyme activity.The time needed to achieve a full removal of the standard soil on the testing coupons was recorded correspondingly in Table 2,Table 3 and Table 4.

Table 2 Different Soaking Temperature and Time Needed for Complete Removal of Standard Soil in Enzymatic Cleaning Group

Table 3 Different Use Solution pH and Time Needed for Com?plete Removal of Standard Soil in Enzymatic Cleaning Group

Table 4 Different Enzyme Dosing and Time Needed for Complete Removal of Standard Soil in Enzymatic Cleaning Group
4 Discussions
Sterility Assurance Level(SAL)is a funda?mental concept defined as the probability of a sin?gle viable microorganism occurring on an item af?ter sterilization,and an SAL of 10–6(one in onemillion chance that a single viable microorganism present after sterilization)is typically used to de?fine the level of assurance in surgical instrument re?processing[9].However,such quality assurance lev?el and approach have been historically defined on the basis of the sterilization process,and there has been limit guidelines on the quality assurance level for cleaning,which is a critical step of the entire in?strument reprocessing lifecycle before sterilization.From research in recent years,inadequate cleaning can cause sterilization failure and the transmission of the healthcare-associated infections,particular?ly with the growingly complicated devices thanks to the advancement of medicine[10-11].Additional?ly,the cleaning failure poses a significant financial risk to the health care facility–in a reported caseof the contaminated instrument in a major medical center in the US in 2016,the cost incurred to the facility was estimated to be about$2 million[12].As such,in practice,the instrument cleaning is as crit?ically important as the sterilization and/or disinfec?tion and is essential to the sterile reprocessing quali?ty assurance.Yet,it is also a challenge in today’s practice,because the onus today is still on the enti?ty that is performing the sterile reprocessing to de?velop strategies,programs and processes,as a quality system in the specific facility,to increase the effectiveness of cleaning and enable optimal outcomes of sterile reprocessing.
Enzymes are a large group of proteins that ex?ist prevalently in all living entities,in which they play a critical role in many biological functions[13].With the aid of enzyme,specific reactions needed to breakdown organic molecules can occur in milli?seconds[13].The nature of the biological contamina?tion on surgical instruments is a mix of organic and inorganic materials.Body fluid,such as blood,can easily dry up while waiting for sterile reprocess?ing,resulting in the denatured protein sticking to the surface of the instruments.In this case,prote?ase,as a type of enzyme in detergent can help break down the protein soil and facilitate the surfac?tant for the cleaning process.For such reasons,in hospital sterile reprocessing,enzymatic detergent has been discussed in research and demonstrated improved performance over non-enzymatic deter?gents for cleaning soiled devices,including biofilm developed in hard-to-reach locations[14-15].Howev?er,there has also been conflicting evidence on the performance and applicability of the enzymatic de?tergent in sterile processing,showing non-enzy?matic detergents might perform better[6,16-17].In re?view of these researches,one of the limitations was the testing objects identified as a standard soil.Typically,the contaminated surgical instruments randomly selected from daily surgical procedures were directly recruited.Because the nature of the soil,the level of the contamination,and the varia?tion in terms of the soil location on the instrument surface can pose significantly different levels of challenges to the cleaning,making the testing ob?jects less comparable in the studies.In our study,we chose the industry widely accepted TOSI as the standard testing soil and strictly defined how it will be used,e.g.positioning in the use solution con?tainer,in each experiment to ensure the standard?ization and consistency.
With the intent to show that the enzymatic cleaning power can be impacted under the subopti?mal conditions,our study further explored the po?tential factors that might impact the enzyme activi?ty,based on scientific assumptions and existing un?derstanding from other industry applications.In our results,the soaking temperature,use solution pH and enzyme dosing were evaluated.Another major consideration of choosing these factors for evaluation is that they are highly variable in the ac?tual hospital sterile reprocessing practice.Particu?larly,in the manual cleaning process,the techni?cians are typically responsible for the control of these factors.If any compromise in the process happens,these factors are most likely to be impact?ed.In our study,we observed significant impact these factors had on the enzyme activity and thus the overall cleaning process and outcomes.
The limitations of our study include the small sample size data only being collected for one test?ing coupon for each experiment and only one lot of the TOSI cleaning indicators were used.Although we are confident that the results should be highly replicable in a larger scale of the study,there might be chances that the results might be biased.Addi?tionally,this is a single center study that some of the uncontrollable factors,e.g.water quality,might be different in other facilities,so that the re?sults might not be able to be directly generalized to other health care facilities.Finally,the results in?terpretation is based on visual inspection.While we strictly followed the manufacturer’s instruction for use,there is still inherent subjectivity in the re?sults interpretation,especially for the bordering re?sults hard to determine a pass or failure result.
The most significant value of our study is to develop evidence to contribute to the overall under?standing of the application of enzymes in instru?ment cleaning and contribute to the body of evi?dence that the performance of enzyme can be en?hanced by creating an optimal condition in clean?ing.On the flipside,our study also highlights the needs for the cleaning process verification,with standard soil testing object,to well understand what the facility specific optimal process is and how it can be consistently achieved in daily prac?tice in the facility.For future research,a larger scale of the study with more potential impacting factors might be considered for more evaluation.
5 Conclusion
Enzyme seems to play a significant role in the instrument cleaning process for hospital sterile re?processing.Soaking temperature,the pH of the cleaning use solution,and enzyme dosing appear to be important factors impacting the enzyme activi?ty and thus the overall cleaning outcomes.In hospi?tal sterile reprocessing practice,process verifica?tion might be needed to ensure the facility specific optimal use conditions is well understood and con?sistently achieved.
Peer-Review Coments:Adequate cleaning is essential to the sterilization assurance of surgical instruments.In recent years,developing and standardizing the cleaning process that aims to improving cleaning quality and efficiency through an evidence-based approach has been at the forefront of the research in the field of sterile reprocessing.The study led by Zhengtao Zhang is a significant contribution to the body of evi?dence and provides insightful data to support the appropriate use of enzyme in instrument clean?ing.Most importantly,by evaluating the critical factors impacting the enzyme performance and overall cleaning outcomes,the study makes an excellent and timely case about an industry im?perative for cleaning process verification,as the real-world hospital cleaning practice and process may pervasively be performed under suboptimal conditions without due diligence and rigorous quality system in place to make sure that the fa?cility-level variations are well understood,ef?fectively managed,and proactively monitored for adherence.Overall,the study contributes sig?nificant value to the hospital sterile reprocessing practice and serves as a great foundation for fur?ther research in this space.

