Syntheses, Crystal Structures and Property of Pyridine Zinc(II) Complexes Based on Halogenated Salicylaldehyde Schiff Base①
JIANG Wu-Jiu NI Peng-Hui MAO Fang-Fang TAN Yu-Xing
(Key Laboratory of Functional Metal-organic Compounds of Hunan Province, Key Laboratory of Organometallic New Materials,College of Hunan Province, Hunan Provincial Engineering Research Center for Monitoring and Treatment of Heavy Metals Pollution in the Upper Reaches of Xiangjiang River, College of Chemistry and Materials Science,Hengyang Normal University, Hengyang, Hunan 421008, China)
ABSTRACT Schiff base pyridine zinc(II) complexes 1~4 were synthesized by the reaction of the 2-((2-hydroxybenzylidene)amino)phenol Schiff base with appended donor functionality, zinc acetate, and pyridine.The results of the structural characterization of the complex show that they have the same coordination mode and similar steric structure. Complexes 1 and 3 form a one-dimensional chain structure and two-dimensional grid structure by lots of hydrogen bonds, respectively. Thermogravimetric analysis shows complexes 1~4 can exist stably below 150 ℃. The results of the fluorescence quenching experiments between the complexes and DNA-EB show that the interaction between them is intercalation, and the effect of complex 1 is the most obvious. It is speculated that the steric hindrance of complex 1 is relatively small, and the aromatic ring on the ligand is more likely to inserted into the base pair of DNA.
Keywords: zinc complex, Schiff base, synthesis, crystal structure, property;
1 INTRODUCTION
Zinc is a very important trace element in the normal growth and development of the human body[1]..The content of zinc ions in the living body is the second only to iron, and it ranks second in the total trace elements. Zinc is known as the‘spark of life’. Zinc is a constituent of more than 200 enzymes in the human body, mainly in the form of metalloenzymes, or as an activator of enzymes. Therefore, zinc has a wide range of distribution and action in the body. It is also a component of insulin in the body, which can prolong the physiological effect of insulin and have different effects on the metabolism of body cells[2]. Therefore, the intelligent design of Zn(II) complex metal drugs has important research significance for human health. Zinc is a transition metal element, which is well coordinated with a molecule containing lone-pair electron to form a complex[3-5]. Zinc ions have the following characteristics: good Lewis acidity; no redox activity; good solubility; low toxicity, and so on[6,7]. Since the coordination bonds have a certain directionality and intensity,the researchers can regulate molecular structure through a coordination bond, so the ligand and zinc ion unit having a specific structure and function are designed to obtain a new zinc complex. In recent years, more and more zinc complexes have been synthesized, and their structures have been continuously published[8,9]. These zinc complexes exhibit diverse properties due to the different ligand structures and coordination configuration, such as catalysis[10,11], fluorescence[12-15],biological activity[16,17], and so on. In this paper, four pyridine zinc(II) complexes based on halogenated salicylaldehyde Schiff base are synthesized (Scheme 1), and the interaction between complexes and ct-DNA were studied to screen the biologically active drug structure, which can provide an important theoretical basis for developing new metal drugs.

Scheme 1. Syntheses of complexes 1~4
2 EXPERIMENTAL
2. 1 Instruments
Infrared spectrum (KBr) was recorded by the Prestige-21 infrared spectrometer (Japan Shimadzu, 4000~400 cm-1).1H NMR spectra were measured with a Bruker AVANCE-400 NMR spectrometer. The elemental analysis was determined by PE-2400(II) elemental analyzer. Crystallographic data of the complexes were collected on a Bruker SMART APEX II CCD diffractometer. Melting points were determined using an X4 digital microscopic melting point apparatus without correction (Beijing Tektronix Instrument Co. Ltd.). Thermogravimetric analyses (TGA) were recorded on a NETZSCH TG 209 F3 instrument at a heating rate of 20 ℃?min-1from 40 to 800 ℃ under air. The UV spectra were determined with the UV-2550 spectrophotometer (Shimazu). Fluorescence spectra were obtained with a Hitachi F-7000 spectrophotometer with quartz cuvette (path length = 1 cm).
The reagents used in experiment were all analytical reagent,and used directly without further purification.
2. 2 Synthesis
2. 2. 1 Syntheses of ligands L1~L4
A mixture of 4-NAP (2-amino-4-nitrophenol) (10 mmol),halogenated salicylaldehyde (10 mmol) and CH3OH (50 mL)was added in a round-bottomed flask (100 mL), and refluxed with agitating for 4.0 h. The solution was cooled, and then the insoluble matter is removed by filtration. Four ligands L1~L4 were obtained.
2. 2. 2 Syntheses of complexes 1~4
1 mmol Schiff base ligand (L1/L2/L3/L4) and 1 mmol zinc acetate were added in CH3OH (50 mL) and refluxed with agitating for 4 h. Then, most of the methanol was evaporated, followed by adding pyridine dropwise until it just dissolved. The reaction solution continued to reflux for 2.5 h,filtered. Finally, the crystals were precipitated by controlling solvent volatilization.
2. 3 Crystal test
The crystals of complexes were mounted on a diffractometer equipped with graphite-monochromated MoKα radiation at a ?-ωmode. All the data were corrected byLpfactors and empirical absorbance. Structure has been solved by direct methods. All non-hydrogen atoms were defined in successive difference Fourier synthesis, and H atoms were added according to theoretical models or located from the Fouriermaps. Non-hydrogen atoms were refined by their isotropic and anisotropic thermal parameters. All calculations were completed by the SHELXTL-97[18]program. Crystallographic data are listed in Table S1, and the bond data are summarized in Table S2.
2. 4 Interaction with DNA
ct-DNA (30 μM), EB (3 μM) and different concentration complex solution (0~50 μM) were placed in a 5 mL volumetric flask in tris-HCl (0.01 mol·L-1) buffer solution[19].After 3 h, the fluorescence spectra were acquired at 25 ℃.The excitation wavelength was 258 nm, and the emission wavelength is shown in the spectrum. The slit scanning width of emission and excitation is 5.0 nm.
3 RESULTS AND DISCUSSION
3. 1 Synthesis
In the synthesis of ligands L1~L4, their solubility in CH3OH is relatively small. After cooling, they will be precipitated from the reaction mixture. The raw material will be dissolved in CH3OH, so the crude products of L1~L4 have been obtained by Vacuum filtration. After that, these ligands were recrystallized with CH3OH to obtain pure product with the yields of 68%~78%. This is a general way to synthesize Schiff base compounds. Complexes 1~4 have been obtained by self-assembly reactions. Pyridine participates in coordination as an electron donor. Finally, the complex crystals were obtained by solvent evaporation method. All complexes are orange yellow transparent crystals with their yields all around 80%.
3. 2 Spectral analyses
The infrared peak shapes of the four ligands are the same,and the infrared peak shapes of the four complexes are also very similar, which preliminarily shows that they all have similar main structures. By comparing the infrared-spectra of ligands L1~L4 and complexes 1~4, we can see that the latter do not show O-H bond stretching vibration in the highfrequency region, indicating that the ligand may participate in coordination of new complexes through hydrogen protons[20-24]. The characteristic absorption peaks of -C=N and-NO2are basically the same in ligands L1~L4 and complexes 1~4.
In the1H NMR spectrum, the peak positions and integrated areas appearing in the spectrum match the number of hydrogen protons of the predicted complexes[25]. The other peaks are all in the low-field position, and no peak signal is found in the high-field position, indicating that all the complexes contain unsaturated hydrogen atoms without saturated ones.In the complex, the two -OH hydrogen proton peaks disappeared, indicating that the metal atom is coordinated with the Schiff base ligand. The characterization results of the complexes are consistent with those of X-ray single-crystal diffraction.

Fig. 1. Molecular structures of 1~4
3. 3 Structure description
The structures of complexes 1~4 in solid state are shown in Fig. 1. The complexes are all single-metal nuclear molecules,and the coordination mode of the central Zn atom is similar.Take complex 1 as an example, Zn(1) forms a five-coordinated triangular bipyramidal configuration by two oxygen atoms (O(1) or O(2)) and one imino nitrogen atom (N(1))from the ligand, and N(3) or N(4) from the coordination pyridine, respectively. N(1), N(3), and N(4) occupy three positions on the equatorial plane, on both sides of which the two oxygen atoms O(1) and O(2) are located. The axial angle O(1)-Zn(1)-O(2) is 167.08°, which obviously deviates from a line (180°). The angle and distance of three atoms of complex 1 in the equatorial-plane and Zn(1) atom are different:dZn(1)-N(1)= 2.0846(17) ?,dZn(1)-N(3)= 2.1060(17) ?,dZn(1)-N(4)= 2.0711(17) ?, bond angle N(4)-Zn(1)-N(3) = 100.87(7)°,bond angle N(1)-Zn(1)-N(3) = 134.23(7)°, and bond angle N(4)-Zn(1)-N(1) = 124.77(6)°. Therefore, the center zinc atom adopts a distorted five-coordinate triangular bipyramidalconfiguration. 2~4 are similar to 1 in crystal structure, but the bond parameters are different, and the center Zn atom also shows a distorted five-coordinate triangular bipyramidal configuration.
Complexes 1 and 3 form respectively a one-dimensional chain structure and a two-dimensional grid structure by lots of hydrogen bonds (Figs. 2 and 3). In 1 and 3, the C-H···O hydrogen bonds are also mainly constructed by hydrogen atoms from phenyl group and oxygen atoms from -NO2. Interestingly, despite all complexes have similar structures, the hydrogen bonds are different. There are no hydrogen bonds in complexes 2 and 4. In a basic unit, the number of hydrogen bonds in 1 and 3 is also 2 maybe due to the too large steric hindrance of the phenyl group in complexes 2 and 4. For 1,dC(21)-H(21)...O(3)= 2.410 ?,dC(22)-H(22)...O(4)= 2.535 ?,C(21)-H(21)···O(3) = 176.21°, C(22)-H(22)···O(4) = 161.46°; For 3,dC(16)-H(16)...O(3)= 2.670 ?,dC(22)-H(22)...O(4)= 2.594 ?,C(16)-H(16)···O(3) = 150.65°, C(22)-H(22)···O(4) = 125.90°. The weak action of the complexes is listed in Table S3 (SI).
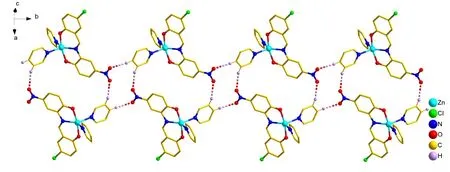
Fig. 2. Chain structure of complex 1

Fig. 3. Two-dimensional grid structure of complex 3
3. 4 Thermal stability
Thermal stability was performed under air atmosphere with the gas-flow rate of 20.0 mL·min-1from 40 to 800 ℃ at a heating rate of 20 ℃·min-1. As depicted in Fig. 4, complexes 1~4 show a similar weight loss process, which can be roughly divided into two weight loss stages. All four complexes display weight loss at 150~250 ℃, corresponding to the departure of pyridine molecule. In Fig. 4, there is a short and small platform at the first weight loss stage (150~250 ℃) in complexes 2 and 4, while 1 and 3 don not have, so the two pyridine molecules in 1 and 3 are lost at the same time, while in 2 and 4 they are lost one by one. In the next stages, complexes 1~4 undergo the second decomposition until 800 ℃, which corresponds to the loss of Schiff base. The remaining weight indicates the final products are ZnO[26-29]. In summary, complexes 1~4 can exist stably below 150 ℃.

Fig. 4. TG-DTG curves for 1~4
3. 5 Interaction with DNA
Fig. 5 shows the fluorescent curve of complexes 1~4 in EB-DNA system with different concentrations. With increasing the concentration, the fluorescence intensity of EB-DNAcomplex declines. Therefore, it speculated the complexes can coordinate with the bases of DNA, resulting in the extrusion of EB out of DNA. To analyze quantificationally the binding capacity of complexes and DNA, we employed Stern-Volmer equation[30]I0/I= 1 +KSVccomplexto obtain the quenching constantsKSVof complexes replacing EB and DNA with 6.27× 104L·mol-1(1), 4.69 × 104L·mol-1(2), 5.95 × 104L·mol-1(3), and 2.99 × 104L·mol-1(4). This value suggests the complexes insert in DNA to a certain degree. Furthermore, the quenching constant is consistent with the reported zinc complex. For example, in 2020, Marzieh Daryanavard’ groups have reported a new mononuclear Zn(II) complex, with itsKSVvalue to be 7.57 × 104[31]. Tapan Kumar Mondal’ groups have reported a Zn(II) complex with hex-adentate N4S2donor thioether ligand, and theKSVvalue of Zn(II) complexes is 2.6× 104[32].
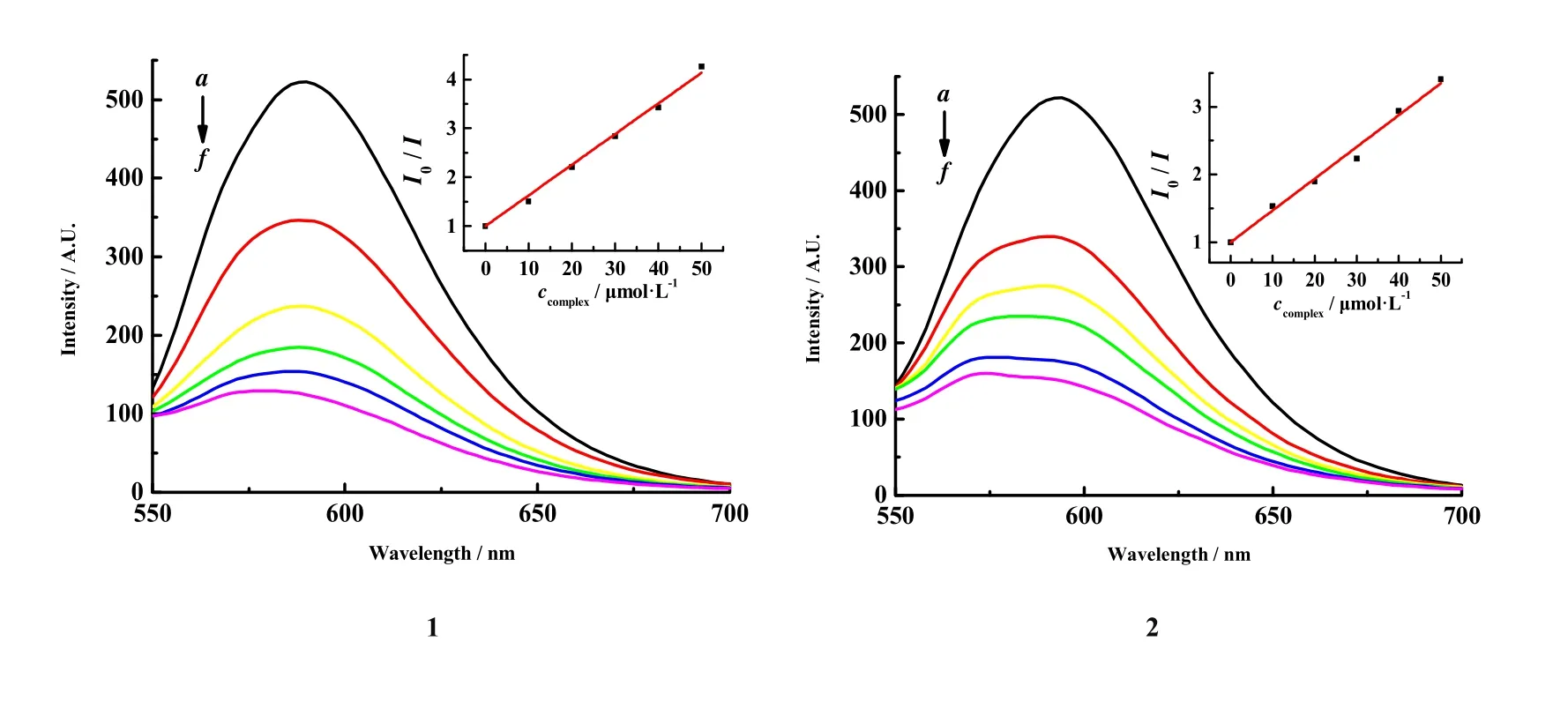

Fig. 5. Fluorescent curves of complexes 1~4 in the EB-DNA solution.cCT-DNA = 30 μmol·L-1; cEB = 3 μmol·L-1; from a to f, ccomplex = 0~50 μmol·L-1; λex = 258 nm
By comparing theKSVvalues of the four complexes, it is not difficult to find that they are all in the same order of magnitude, and the difference is not very large. In general,the interactions between complexes 1~4 and DNA are stronger than EB, and they all can squeeze EB from DNA,but complexes 1 and 3 are slightly stronger than 2 and 4. It is
4 CONCLUSION
Schiff base pyridine Zn(II) complexes 1~4 have been synthesized and characterized. Structural analysis results show that they have the same coordination mode and similar steric structures. Through intermolecular hydrogen bonds, complex 1 constitutes a one-dimensional chain structure, and complex speculated that the aromatic ring of the ligand in the complex is inserted into the base pair of DNA, which competes for the binding of EB to DNA, squeezing EB out of the base pair of the DNA, and the steric hindrance effect of 1 and 3 is relatively small, so that it is the relatively strongest interaction with DNA.3 constitutes a two-dimensional network structure, respectively. Thermogravimetric analysis shows complexes 1~4 can exist stably before 150 ℃. The result of the interaction with ct-DNA shows that it Is intercalation, and the insertion effect of complex 1 is the strongest. It will provide an important theoretical basis for developing new metal drugs.
- 結構化學的其它文章
- Two Polynuclear Fe Complexes with Boat-like Core:Syntheses, Structures and Magnetic Properties①
- A Robust Heterometallic Cd(II)/Ba(II)-Organic Framework with Exposed Amino Group and Active Sites Exhibiting Excellent CO2/CH4 and C2H2/CH4 Separation①
- Synthesis, Crystal Structure, Spectroscopy and Hirshfeld Analysis of 4,6-Diamino-2-cyclopropylaminopyrimidine-5-carbonitrile with Different Solvents: N,N-dimethylformamide, Methanol and Water①
- Syntheses, Crystal Structures and DNA-Binding Properties of Zn(II) and Mn(II) Complexes Based on Imidazole Derivatives and Carboxylic Acid
- CoMFA Study on Anti-proliferative Activity of Fluoroquinolone Amide Derivatives①
- Synthesis and Properties of Dinuclear Europium(III)Complex Containing 2-Benzoylbenzoic Acid①

