Simvastatin inhibits HIF-1α and VEGF expression in RPE cells under hypoxia conditions
Xia Li1*, Yi Wang2*, Bing-Hui Wu3, Hao-Yuan Chen1, Jun-Hui Du
Abstract
?KEYWORDS:retinal pigment epithelial(RPE)cells; hypoxia; Simvastatin; choroidal neovascularization
INTRODUCTION
Age-related macular degeneration(ARMD)is the highest blinding disease in developed countries[1].85% of patients present a dry ARMD, while the wet(new blood vessel)form affects the remaining 15% and usually develops from the dry form of ARMD.Retinal pigmented epithelial(RPE)cells located between the neuroretina and choroid are monolayer pigmented epithelial cells.It is known that RPE dysfunction plays an significant role in the pathogenesis of ARMD[2-4].Statins or HMG CoA(3-hydroxy-3-methylglutaric coenzyme A)reductase inhibitors blocking cholesterol biosynthesis and up-regulating LDL receptor expression through methylovalerate pathways has been widely used to reduce serum cholesterol levels.Since cardiovascular risk factors are also related to ARMD, interventions to reduce cardiovascular risk factors, such as statins, maybe also contribute to the treatment of ARMD.Many studies have focused on the relationship between statins and ARMD, but the conclusion remains controversial[5-8].Therefore, more evidence is needed to verify the effect of statins on ARMD.It is known that VEGF is a key cytokine to promote angiogenesis[9].It promotes the division and proliferation of endothelial cells and increases vascular permeability.Clinical studies have found that VEGF can effectively inhibit choroidal neovascularization(CNV).Increasing evidence has shown that hypoxia contributes to the development of CNV, and RPE cells played a causative role in VEGF production during the formation of CNV[10].It has been reported that simvastatin(Sim)up-regulates the expression of VEGF in vascular endothelial cells through the HIF-1α signaling pathway and promotes neovascularization[11].While another study showed that atorvastatin inhibits VEGF expression and finally inhibits the CNV development in mice[12].Thus we conducted this study to confirm the effects of statins on HIF-1α and VEGF in RPE cells and tried to reveal the possible mechanisms.
MATERIALS AND METHODS
CellCultureRPE-19 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C, indoor air humidity of 95% and carbon dioxide concentration of 5%.The medium was changed after 24h of culture.RPE-19 cells were randomly divided into different groups: control group and hypoxia group(the final concentration of CoCl2in the cultured medium was 125 μmol/L), and Sim group(the final concentration of Sim in the cultured medium was 3 μmol/L).At the time of 24h, group of cells were observed and photographed with the inverted microscope.Western blotting and Enzyme-linked immunosorbent assay(purchased from Ex-Cell Biology Inc.Shanghai, China, EH015-96)were used to detect HIF-1α and VEGF protein expression(purchased from BD Biosciences, cat:555036)and secretion level.
MTTCellProliferationAssayThe logarithmic growth phase RPE-19 cells were digested and added to the cell culture mediums.Cells were then adjusted to a suspension with 5×104cells/mL and inoculated on 96 well plate(100 μL per well).According to group design, relevant factors were added and seven replicate wells were designed for each group.The culture plate was transferred to a CO2incubator for cultivation.After 24h, 10 μL MTT solution was added to each well and incubated for 4h.After centrifugation, remove the supernatant, remove 100 μL of solution and shaked for 10min.The absorbance(A)value was measured at 490 nm with an automatic microplate spectrophotometer.The experiment was repeated three times(n=3).
WesternBlottingRPE-19 cells were collected and treated to detect HIF-1α and VEGF.After treatment, the separated proteins were transferred to nitrocellulose membranes and then blocked with Tris-buffered saline(TBS)-T buffer containing 5% non fat emulsion β-actin(1∶500, Santa Cruz Biotech, CA, USA), HIF-1α(1∶500, Santa Cruz Biotech, CA, USA), VEGF(1∶500, Santa Cruz Biotech, CA, USA), LC3B(1∶500, Santa Cruz Biotech, CA, USA), Beclin-1(1∶500, Bioword technology, MN, USA), p62(1∶500, Santa Cruz Biotech, CA, USA), and then incubated with horseradish peroxidase(HRP)-bound secondary antibody at room temperature for 1h.The labeled bands were visualized and quantified using a chemiluminescence imaging system(CliNX, Shanghai, China).Use CliNX analysis software to scan and count the gray value.The ratio of target protein gray value/actin was expressed as the relative expression level of target protein.
Enzyme-linkedImmunosorbentAssayAdd sample diluent 100 μL to blank well, standard or test sample 100 μL to the rest well.The plate was coated and incubated at 37℃ for 90min.Dispose the liquid, add chromogen solutions A and B 100 μL to each well(prepared within 15min before use), add film covering to the HR-labeled plate, incubate at 37℃ for 1h.Wash the plate 3 times, add HRP conjugate working solution(prepared within 15min before use)100 μL to each well, add film mulching, incubate at 37℃ for 30min.Discard the liquid in the hole, shake dry and wash the plate for 5 times, using the same method as Step 1.Add substrate solution 90 μL to each well, and incubate with HR-labeled plate coated with film at 37℃ for 15min.Add Stop Solution 50 μL to each well, stop the reaction, the blue color turns yellow.Immediately measure the optical density(OD value)of each hole at 450 nm with a microplate analyzer.
TUNELAssayApoptosis was analyzed using the One Step TUNEL Apoptosis Assay kit(C1088, Beyotime Institute of Biotechnology), according to the manufacturer’s instructions.RPE-19 cells were placed on a glass cover and fixed with 4% paraformaldehyde and 70% ethanol for 30min at room temperature.The slides were permeated with Triton X-100 at room temperature for 5min and washed with PBS for 3 times.The slides were then sealed in 3% H2O2for 10min at room temperature and washed 3 times with PBS.Add 50 μL TDT enzyme reaction solution, keep it at 37℃ for 60min, and wash it with PBS solution for 5min, three times.Add 50 μL streptomycin horseradish peroxidase solution, keep it at 37℃ for 30min, and wash it with PBS solution for 5min, three times.50 μL DAB working solution was dripped and kept at room temperature for 10min.The number of apoptotic cells was obtained.It is used to reflect the severity of apoptosis in RPE-19 cells.
StatisticalAnalysisStatistical analyses were used Statistical Products and Services Solutions(SPSS)26.0 software program.All quantitative data were represented at least three independent experiments.The data were expressed as the Mean of the Standard Deviation.The differences between groups were tested by analysis of variance(ANOVA).The paired LSD-T test was used for comparison among groups.The two tailedP-value withP<0.05 was considered significant.
RESULTS
RPE-19Cells’MorphologyObservedUnderInvertedPhaseContrastMicroscopeAfter cultured for 12h, the morphology of RPE-19 cells in each group was observed.RPE-19 cells in the control group were typical polygonal.Irregular round cells increased in hypoxia group.After treated with Sim under hypoxia, cells number were decreased compared with the hypoxia group(Figure 1).

Figure 1 After 12h of cell culture, the morphology of RPE-19 cells in CoCl2 and CoCl2+Sim group was observed under hypoxia Sim is short of simvastatin.
SimInhibitsRPE-19CellsProliferationUnderHypoxiaConditionsThe effect of Sim on the RPE-19 cells’ proliferation was investigated by MTT assay(Figure 2).Our results showed that cell proliferation was significantly increased under hypoxia(P<0.05).When exposed to Sim under hypoxia, RPE-19 cell proliferation was reduced obviously.
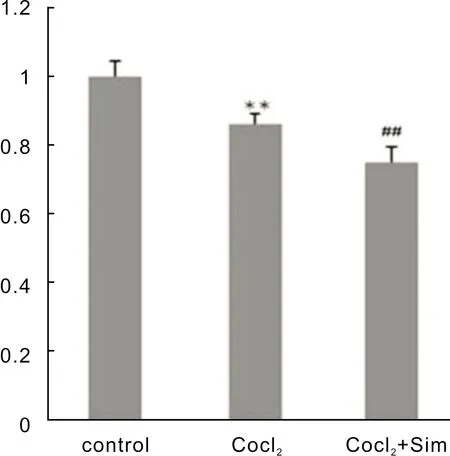
Figure 2 Effects of Sim on the proliferation of RPE-19 cells **P<0.05 vs control; ##P<0.05 vs CoCl2 group.Sim is short of simvastatin.
SimInhibitedtheProteinExpressionofHIF-1α,VEGF,andLC3BUnderHypoxiaTo study the effect of statins on HIF-1α and VEGF in RPE cells under hypoxia conditions, we detected HIF-1α and VEGF by western blotting, we also detected the expression of autophagy-related protein LC3B(Figure 3).Our results showed that when RPE-19 cells were treated with CoCl2,the level of HIF-1α, VEGF, and LC3B protein expression were increased and then decreased after Sim treatment.
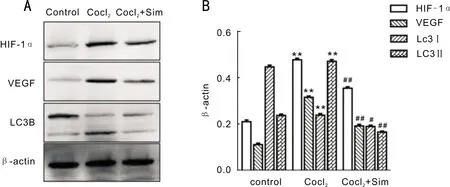
Figure 3 Effects of Sim on the expression of HIF-1α, VEGF, and LC3B in RPE-19 cells A: Western blot was used to detect the expression in each group(control group, CoCl2 group and CoCl2+Sim group); B: The grayscale ratios of HIF-1α, VEGF and LC3B(LC3II/LC3I)to the internal reference β-actin in 3 replicates.**P<0.01 vs control; ##P<0.01 vs CoCl2 group; #P<0.05 vs CoCl2 group.Sim is short of simvastatin.
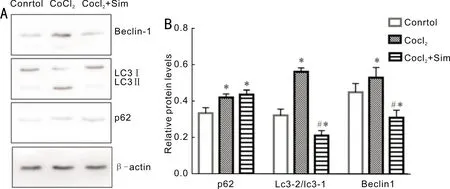
Figure 4 Effects of Sim on the autophagy of RPE-19 cells A: The effect of Sim on autophagy was detected by Western blot assay; B: The grayscale ratios of autophagy-related proteins to the internal reference β-actin in 3 replicates.*P<0.05 vs control; #P<0.05 vs CoCl2 group.Sim is short of simvastatin.
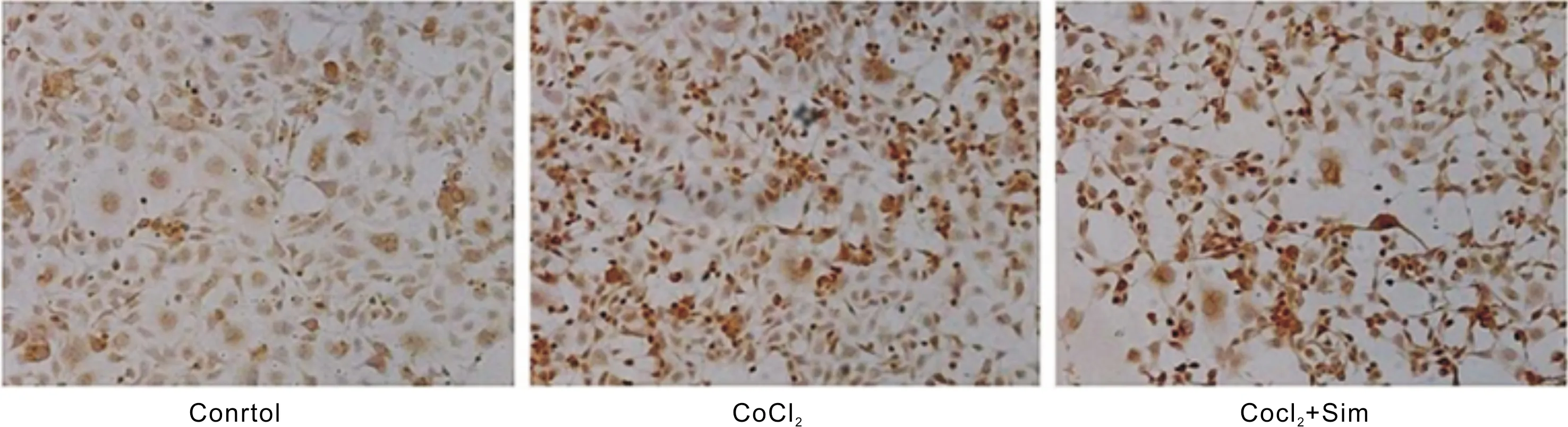
Figure 5 Comparison of apoptotic cells in each group(Tunel dyeing)Sim is short of simvastatin.
EffectsofSimontheSecretionLevelsofHIF-1αandVEGFinRPECellsUnderHypoxiaELISA results showed that hypoxia up-regulated the secretion levels of HIF-1α and VEGF expression.And they reduced when treated with Sim as shown in Table 1.Compared with the control group, HIF-1α and VEGF expression were up-regulated in the CoCl2group(P<0.05).HIF-1α and VEGF expression were down-regulated in the CoCl2+Sim group compared with the CoCl2group(P<0.05).
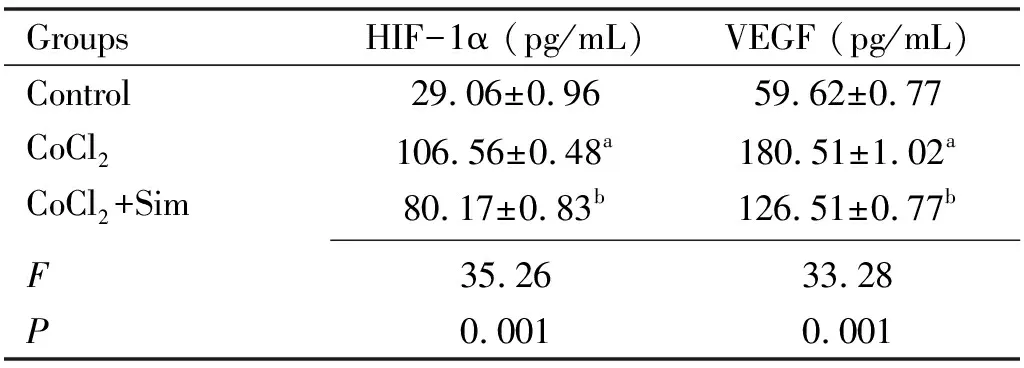
Table 1 The levels of HIF-1α and VEGF were determined by ELISA (24h)
SimInhibitedtheExpressionofAutophagyProteininCellsinRPECellsUnderHypoxiaAutophagy level was monitored by expression of autophagy related proteins Beclin-1, LC3B, and p62.RPE-19 cells were lysed and processed for determination of LC3B-II/LC3B-I ratio, p62, and Beclin-1 by Western blot method, and it was found that autophagy related proteins were expressed in all of the three group, but the expression level of P62, Beclin1, and LC3B proteins in the Sim group was lower than the CoCl2group but higher than control, and the difference was statistically significant(Figure 4).
EffectsofSimontheCellApoptosisinRPECellsUnderHypoxiaThe TUNEL assay was used to detect the effect of apoptosis on cell apoptosis: genomic DNA would be broken when the cell apoptosis occurred.The TUNEL assay could mark the exposed 3-0h, and the cell apoptosis could be observed with the light microscope.The results were shown in Figure 5.Under hypoxia, Sim promoted the apoptosis of RPE cells.
DISCUSSION
Hypoxia plays an important role in leading the progression of ARMD.In our study, we found that Sim restrained the proliferation of RPE cells and inhibited HIF-1α and VEGF expression under hypoxia.Our results suggested that Sim inhibited autophagy and promoted apoptosis in RPE cells under hypoxia.Activation of autophagy protects cells and reduces apoptosis under stress conditions.Our results suggested that the mechanism by which Sim promoted apoptosis in RPE cells may be related to its inhibition of autophagy.
It is known that multiple factors affected the progression of ARMD.The function of statins in the ARMD development has been researched in many clinical and epidemiological types of studies[5-8].Statins are well tolerated and severe side effects are rare[12].Previous studies have shown that statins are a potentially effective drug in ARMD[13].Statins are divided into two categories: lipophilic drugs and hydrophilic drugs[14].This classification is important because different lipophilic statins may have different effects.Lipophilic statins(such as atorvastatin)are more effective than hydrophilic drugs(such as pravastatin)in reducing apoB100 secretion and cholesterol level in cultured RPE cells by regulating RPE cholesterol levels[15].Statins have therapeutic effect on dry ARMD.A study found that high-dose atorvastatin can induce drusen degeneration, no atrophy or neovascularization, and improved the vision of high-risk subgroup of ARMD patients[16].However, the precise mechanism of the therapeutic effect of statins is still not clear.It was reported that lipotropic statins such as atorvastatin and Sim also promoted the phagocytic function of RPE cells[17].Statins have been reported to significantly reduce the risk of early ARMD by about 13%.This is mainly due to the reduction of serum LDL cholesterol levels by statins, which in turn reduces the deposition of LDL cholesterol in drusen[18].Statins also reduce the plasma concentration of C-reactive protein(CRP)by decreasing the number and function of inflammatory cells[19].As an inflammation maker, a high concentration of CRP inhibits the inactivation of the complement cascade, leading to dysfunctions in RPE cells and accumulation of drusen[20].Therefore, statins might play a more important role in the initiation of drusen and delay the onset and progression of early ARMD.
Some studies have shown that statins may be involved in the regression of pigment epithelial detachments and helped to improve vision[17].Atorvastatin has been proved to have multiple effects on regulating various biological behavior of human RPE cells.It was reported that statins inhibited cell proliferation and migration[21].It has been reported that atorvastatin and Sim reduced oxidative stress-induced RPE damage, which is a factor affecting the pathogenesis of ARMD and improving the activity of RPE cells[22-23].Besides, statins may also play a role in neovascularization, and participate in the pathogenesis in wet ARMD either.It is reported that atorvastatin inhibited laser-induced CNV effectively and decreased the inflammatory chemokine and VEGF[24], prompting statins may also prevent the progression from dry ARMD to wet ARMD.Other studies also found that atorvastatin inhibited CNV and down regulated VEGF expression[12].However, some other studies have shown that Sim promoted VEGF expression and neovascularization in vascular endothelial cells[11].In our study, we found that Sim inhibited the levels of HIF and VEGF protein, which might be the mechanism of Sim under wet ARMD treatment.
Statins were supposed to be participated in the regulation of HIF-1α.We have previously found that the inhibition of autophagy down-regulated the expression of VEGF[25].Therefore, we speculate that the inhibition of VEGF expression by Sim may be related to autophagy either.Researches have found that cholesterol loading in hepatocytes lead to the activation of HIF-1α.This is due to hypoxia and the excessive production of nitric oxide and mitochondrial reactive oxygen species[26].Hisadaetal[27]have revealed that fluvastatin attenuated HIF-1-dependent ET-1 gene expression in human vascular smooth muscle cells(VSMC)invitro.Our study also found that after treatment with Sim, the expression of HIF-1α was consistent with the reduction of VEGF protein expression in time.It is known that VEGF is a downstream regulator of HIF-1[28-29].Therefore, we speculated that Sim might downregulate VEGF protein by inhibiting the expression of HIF-1α.This may be one of the important mechanisms of statins preventing CNV progression.Huangetal[11]found that statins regulate the expression of HIF-1α through the RhoA pathway after vascular endothelial cell injury, thus increasing the expression of VEGF.Similar studies have also shown that statins can regulate the expression of HIF-1α, but some results displayed down-regulated while others were up-regulated.For example, in the vascular endothelial injury model, statins may up-regulate the expression of HIF-1α, reduce the oxidative stress response, and the expression of VEGF, Akt, and eNOS[30].Besides, statins can effectively inhibit the expression of HIF-1α, reduce the expression of VEGF and phosphorylated STAT3, and control the expression of ICAM-1 in vascular endothelium[27,31].All of these studies indicate that statins play a biological role by regulating the expression of HIF-1α.
In brief, we indicate that Sim involves in hypoxia signaling in cultured RPE cells.Sim effectively reduces cell proliferation and HIF-1α and VEGF protein expressioninvitro.It is reasonable to propose that Sim potentially provides a means to attenuate the damage of ARMD.Further studies still need to be conducted to reveal the exact molecular mechanism of Sim treatment for ARMDinvitroandinvivo.

