Efficacy and safety of argatroban in treatment of acute ischemic stroke:A meta-analysis
INTRODUCTION
Acute ischemic stroke (AIS) is the most common type of cerebrovascular disease.Ischemic stroke (IS) is the leading cause of adult disability and has the second-highest fatality rate in the world[1].Still,morbidity and mortality have shown a growing trend in recent years[2].Evidence suggests that only aspirin and recombinant tissue-type plasminogen activator (r-tPA) have a definite curative effect on the acute phase of IS(Class A evidence,level I recommendation).The efficacy of other drugs is still lacking evidence-based support.Anticoagulant therapy has always been a focus in the field of AIS,but the results were controversial.Although anticoagulant therapy can reduce the recurrence of IS and the incidence of pulmonary embolism and deep vein thrombosis,its effect on the mortality and disability rate of IS is still unknown[3].Also,anticoagulation increases the incidence of intracranial hemorrhage (ICH)[4].So,traditionally used anticoagulant drugs,such as heparin,low molecular weight heparin,and warfarin are not recommended for AIS treatment.Argatroban is a novel,smallmolecule,direct thrombin inhibitor.It exerts its anticoagulant function by binding with thrombin,not only in the state of dissolution but also in blood clotting[5].It has been mainly proved for the treatment of thrombosis caused by heparin-induced thrombocytopenia.There is a growing body of evidence on the safety and efficiency of argatroban therapy for AIS[6-8].In Japan and South Korea,argatroban therapy is also used in ischemic diseases including myocardial and cerebral ischemia[9].However,there is still a lack of evidence for its efficacy and safety.To provide more reliable evidence for clinical practice,we conducted a Cochrane Collaboration systematic review that included the randomized controlled studies on AIS treatment using argatroban.
MATERIALS AND METHODS
Search strategy
A search of PubMed,Embase,Science Citation Index,Medline,and Cochrane Library was performed up to October 2020.The search was conducted using medical subject headings and keywords including “argatroban”,“4-methyl-1-(N(2)-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl)-2-piperidinecarboxylic acid”,“cerebral infarction”,“ischemic stroke”,“cerebrovascular disorder”,and “cerebrovascular accident”.Meanwhile,we retrieved references listed in studies and reviews researched from the online databases to obtain relevant data.
Selection criteria
We only enrolled randomized controlled studies that assessed the efficacy and safety of argatroban in treating AIS.All the studies were in English and published as full articles.Case reports,reviews,commentaries,editorials,and studies written in abstract form or published repeatedly were excluded to prevent homogeneity.The methodological quality of the included studies was assessed using the risk assessment tool for RCT bias in the Cochrane Systematic Reviewers’ Handbook.
If only I had a duchy! What! He wants to marry me? said the scissors, and she was so angry that she gave the collar a sharp snip9, so that it had to be cast aside as good for nothing
Outcome index
The outcome and adverse effects were calculated from the data provided by the researchers.Validity and adverse effect assessment were based on the information synthesized from the studies.Validity mainly referred to therapeutic effect,assessed by neurological function scores.Adverse effects mainly referred to bleeding.
The efficiency and safety of argatroban in treating cardiogenic and non-cardioembolic stroke have differed among studies.A retrospective study in Japan analyzed the efficacy and safety of argatroban in the treatment of cardiogenic stroke.The study divided 2529 eligible patients into heparin,argatroban,and control (not receiving anticoagulant or antiplatelet therapy) groups,and the results showed that both heparin and argatroban decreased the risk of death from stroke,but the risk of bleeding was not increased in the argatroban group[28].However,for noncardioembolic stroke,the efficiency of argatroban was indefinite.A study including 2289 pairs of patients with atherothrombotic stroke was performed in 2016.The results showed that,despite its safety,argatroban yielded no additional benefit for acute atherothrombotic stroke[29].Another study found that argatroban was not superior to control therapy in non-cardioembolic AIS[30].On the contrary,a recent retrospective study of 1325 patients found that argatroban was safe and effective for improving short and long-term outcomes in patients with non-cardioembolic AIS[31].The results of the studies above indicate that argatroban might have a better therapeutic effect in treating cardiogenic stroke than non-cardioembolic stroke.In Japan,argatroban has already been recommended for patients without embolic IS within 48 h of onset in their 2013 guidelines for management of IS[32].However,for acute non-cardiogenic stroke,the benefit of argatroban is not definite,and the drug has not been recommended in any treatment guidelines.Most of the recent studies were performed in Japan and were retrospective.More high-quality studies from other regions are needed to support the advantages of argatroban in treating cardiogenic stroke.
Statistical analysis
The drug safety of argatroban has been proved by many studies.However,the results of present studies on evaluating curative effect of argatroban on AIS were quite controversial,which has puzzled us in confirming the role of argatroban in AIS treatment.Therefore,it is necessary to do such an analysis to further evaluate the efficiency and safety of argatroban in treating AIS.
RESULTS
Description of the studies
A total of 412 relevant studies were retrieved,and 408 were excluded because of duplication or failure to meet the inclusion criteria.Finally,four trials were included in our study[6,10-12].The studies included 354 cases with 213 in the argatroban group and 141 in the control group.The literature screening process and results are showed in Figure 1.Two of the studies were conducted in North America and two in Japan.Three studies were multicenter and one was single center.The main characteristics of the included studies are presented in Table 1.

All four studies used improvement of neurological deficits to assess the efficiency of argatroban.The National Institutes of Health Stroke Scale,Modified Rankin Scale,Barthel Index,and activity in daily living were used in three studies.The evaluation method was not described in the other study[10].Although there was no uniform standard,all the enrolled studies reported the effective rate of nerve function improvement,which was used to assess the efficacy of argatroban in the treatment of AIS.Three studies[6,11,12] ICH or major bleeding as an adverse reaction,which was not found in the fourth study[10].
Main analysis
Acute ischemic stroke (AIS) has been a global health challenge.And new treatments have been explored.Argatroban as a novel direct thrombin inhibitor has been used in treating AIS.However,the exact efficiency and safety remain unclear.
When I picked out the fish which were chosen by him from the pond and put them in a plastic bag, I always liked to show them to the customers. So when I showed the gold fish to them, the gentleman said.

Argatroban is a small-molecule thrombin inhibitor that was first synthesized by Japanese scientists[15].It can inhibit coagulation by interacting with the catalytic site of thrombin reversibly[16].Compared with other anticoagulant drugs,argatroban has some advantages.First,argatroban can penetrate and inhibit thrombin effectively despite the fibrin barrier,benefiting from its small molecular size.That means that argatroban has a therapeutic effect on more organized thrombi[5].Second,argatroban acts quickly.Normally,it can reach steady-state plasma levels in 1-3 h after intravenous administration.Besides,the dose-response curve of argatroban is steady and predictable,which means that it has a wide margin of safety of dose titration[17,18].Third,argatroban is metabolized rapidly in the liver.The elimination half-life is 39-51 min and is mostly affected by hepatic function,despite age,gender,and renal function[19].Although there is no specific antidote,the coagulation parameters generally return to normal within 2-4 h after withdrawal of argatroban,as long as liver function is normal[20].Also,its pharmacological mechanism is selective and it hardly influences other serine proteases.

DISCUSSION
Although the results of this meta-analysis suggested that argatroban did not increase the risk of ICH in the acute phase of cerebral infarction,it failed to show the advantage of argatroban over other drugs in the treatment of AIS.Anticoagulants have been used to treat AIS for>70 years[13].The use of anticoagulants for prevention and treatment of IS is still controversial.Anticoagulants are effective in preventing recurrence of cerebral infarction but can also cause bleeding.So far,there is no evidence to support short or long-term benefit of anticoagulants for patients with AIS[14],and more evidence-based data are needed.
In the three studies that assessed adverse reactions,none of them found that argatroban increased the risk of bleeding.Detailed data were not provided by Kobayashi et al[11],so only the studies of Barreto et al[6] and LaMonte et al[12] were included in our analysis.The heterogeneity of the two studies was insignificant (=0.54,=0%) and the fixed-effects model was used.The overall analysis showed that there was no significant difference between the argatroban and control groups (RR=1.34;95%CI:0.66-2.74;=0.42) (Figure 4).The results indicated that argatroban does not increase the risk of bleeding in AIS.In all the four studies,there was no gender difference between the argatroban and control groups (>0.05).The safety and efficacy of argatroban were not assessed according to gender.Therefore,the impact of gender on the safety and efficacy of argatroban cannot be evaluated.We analyzed the impact of patient age.In the studies of Barreto[6],Kari[10],and Kobayashi[11],the mean age of different groups was described but comparisons were not made.In LaMonte[12]’s study,there was an age difference between the arga-troban and control groups (=0.038),but the results were not grouped by age.Therefore,the variable of age cannot be analyzed.In Barreto[6]’s study,medical history was described,and patients may have had prior stroke,hypertension,coronary artery disease,diabetes mellitus,heart failure,or atrial fibrillation.However,the impact of comorbidity on the efficacy and safety of argatroban was not analyzed.None of the studies mentioned whether the patients had renal or liver disease.Therefore,the metabolism of argatroban cannot be evaluated.
A few years later, in a bid to rekindle2 their love, Smith tracked down her mother s address in Spain and sent a letter to her there. It was placed on the mantelpiece() , but slipped down behind the fireplace and was lost for over a decade.
About an hour after some one arrived on business, and the girl untied15 the dog and said, Go to the inn and call my father! The dog bounded off, but ran straight to the shoemaker
As far as we know,this study is the first systematic review of the safety and efficacy of argatroban for treatment of AIS.We only analyzed four studies.We found considerable heterogeneity among the studies.Clinical heterogeneity might occur for many reasons,such as geographic region,racial difference in severity of initial symptoms,and interference with other treatment.The four studies were not designed to the same standard,which may have caused heterogeneity.Also,subgroup analysis was not possible because of the absence of detailed data.Although we did not find evidence supporting the efficacy of argatroban for treatment of AIS,there were some shortcomings in our study.First,we found considerable heterogeneity in the data sources.We only analyzed four studies.The small sample size limits the credibility of the results and was the main source of the heterogeneity.The chronological span of the four studies was large.Kario[10]’s and Kobayashi[11]’s studies were performed in 1995 and 1997,respectively.The inclusion criteria were not fully described in these studies.Both reported clinical improvement,but the assessment tools and criteria for evaluation were not listed clearly.On the contrary,the studies of Barreto[6] and LaMonte[12] had unified standards.The heterogeneity of all four studies was large,but was smaller in the studies of Barreto[6] and LaMonte[12].Differences in inclusion and assessment criteria may have caused heterogeneity.Besides,the clinical characteristics of patients enrolled in the studies of Kario[10]and Kobayashi[11] were not described in detail.Thus,it was hard to perform subgroup analysis and meta-regression.We are not able to analyze the specific reason for the heterogeneity,and the heterogeneity made it hard to draw a significant conclusion.However,the quality of the other two recent studies was higher.The inclusion and assessment standards were unified and the heterogeneity of the studies was small.Although the two studies yielded different results on efficacy,the metaanalysis still found no evidence supporting the therapeutic effect of argatroban in AIS.However,the conclusion is debatable due to the limited amount of research and its quality.We might conclude that it is safe to use argatroban for treatment of AIS,but the efficacy needs verification.More high-quality surveys with a large sample are needed in the future for more reliable results.Therefore,our results need to be interpreted with caution.
CONCLUSION
Patients with AIS might not benefit from argatroban and combination therapy with argatroban does not increase bleeding tendency.
ARTICLE HIGHLIGHTS
Research background
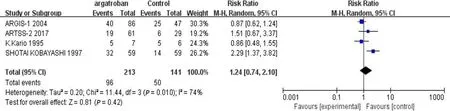
We performed a meta-analysis of the four studies mentioned above.The efficacy of argatroban was controversial.Two studies reported superior improvements in the argatroban group than in the control group[6,11].However,the other two studies did not find definitive effectiveness of argatroban in the treatment of AIS compared with the control groups[10,12].Thevalue of heterogeneity among the studies was significant (<0.05,=74%),so the random-effects model was used for the analysis.The result showed that the overall effect was not significant (RR=1.24;95%CI:0.74-2.10;=0.42) (Figure 2).Since there was considerable heterogeneity among the four studies,the result was not reliable.The nonconformity of enrollment criteria and result evaluation might have been the cause of the heterogeneity.We found that the inclusion and assessment criteria of two studies[6,12] performed in recent years were in good coincidence.Therefore,we only analyzed the results of these two studies.The results showed that the heterogeneity was insignificant (=0.21,=36%).And the fixed-effects model was used for the analysis.The overall effect was also not significant (RR=1.0;95%CI:0.72-1.39;=0.99) (Figure 3).The existing research results do not support the efficacy of argatroban in treating AIS.
At present,argatroban is mainly used to treat heparin-induced thrombocytopenia[21].In Japan and Korea,it has also been used to treat AIS[19].Several reports have shown that argatroban is effective in treating AIS.Compared with high-dose aspirin(300 mg daily),argatroban plus standard-dose aspirin (100 mg daily) was as effective and safe for the treatment of moderate AIS[22].Several single-center,nonrandomized,controlled studies have found that argatroban is effective for treating AIS[23-25].However,the number of patients enrolled in the published studies was small and the studies were all carried out in Asia.In addition to the anticoagulant effect,some studies have shown that argatroban can improve ischemic symptoms by ameliorating cerebral blood flow in patients with AIS[26,27].However,the number of studies is small and evidence-based medicine is insufficient.Therefore,these findings cannot be extrapolated to clinical application.
Research motivation
Relative risk ratio (RR) and 95% confidence interval (CI) were used as effect analysis statistics for categorical data.Efficiency and safety were calculated for all of the studies that were identified for the meta-analysis,and the results were combined using fixedor random-effects modeling.Statistical heterogeneity was assessed usingtests (<0.05 indicated statistical significance) andtests (<0.05,>50% indicated significant heterogeneity;>0.05,<50% indicated insignificant heterogeneity).The fixedeffects model was used if there was no statistical heterogeneity,otherwise,the random-effects model was used.Subgroup analyses were conducted for further investigation.Meta-analysis was conducted using RevMan version 5.4 (Cochrane collaboration),and<0.05 was defined as statistically significant.
They could all speak quite well when they were in the street, but as soon as they came inside the palace door, and saw the guards in silver, and upstairs the footmen in gold, and the great hall all lighted up, then their wits left them! And when they stood in front of the throne where the princess was sitting, then they could not think of anything to say except to repeat the last word she had spoken, and she did not much care to hear that again
Research objectives
The objective of this study was to evaluate the efficiency and safety of argatroban in treating AIS by extracting available data from existing studies.
If you like, however, I will go with you to my brother, the North Wind;55 he is the oldest and strongest of all of us, and if he does not know where it is no one in the whole world will be able to tell you
Research methods
We have searched database PubMed,Embase,Science,Medline,and Cochrane Library to retrieve all of the studies associated with argatroban and AIS.Only randomized controlled clinical studies were screened for this review.Meta-analysis methodology was used and the standard mean difference values and 95% confidence intervals were estimated to get final results.
Research results
Only four studies that met the criteria were included in our review,which contained a total of 354 cases with 213 cases in the argatroban group and 141 in the control group.The overall analysis showed that patients with AIS did not improve more with argatroban treatment.And argatroban did not increase the bleeding risk in AIS patients.
Research conclusions
Our study that integrated the existing data suggested that patients with AIS might not benefit more from argatroban and combination therapy with argatroban will not increase bleeding tendency.
Research perspectives
More high-quality studies are needed for further evaluation of the efficacy and safety of argatroban in treating AIS.
 World Journal of Clinical Cases2022年2期
World Journal of Clinical Cases2022年2期
- World Journal of Clinical Cases的其它文章
- Successful management of delirium with dexmedetomidine in a patient with haloperidol-induced neuroleptic malignant syndrome:A case report
- Using a fretsaw in treating chronic penial incarceration:A case report
- Occupational fibrotic hypersensitivity pneumonia in a halogen dishes manufacturer:A case report
- Accelerated Infliximab Induction for Severe Lower Gastrointestinal Bleeding in a Young Patient with Crohn’s Disease:A Case Report
- Tension pneumocephalus following endoscopic resection of a mediastinal thoracic spinal tumor:A case report
- Primary adrenal diffuse large B-cell lymphoma with normal adrenal cortex function:A case report
