Insight into reaction path and mechanism of catalytic cracking of n-hexane in HZSM-5 zeolites
ZHAI Peng ,ZHENG Jian ,ZHANG Jin-yan ,WANG Huan, ,QIN Yu-cai,* ,LIU Hong-hai ,SONG Li-juan,
(1. Key Laboratory of Petrochemical Catalytic Science and Technology, Liaoning Petrochemical University, Fushun 113001, China;2. College of Chemistry and Chemical Engineering, China University of Petroleum (East China), Qingdao 266555, China;3. PetroChina Petrochemical Research Institute Lanzhou Petrochemical Research Centre, Lanzhou 730060, China)
Abstract: The n-hexane was used as a model compound to study the catalytic cracking behavior of light hydrocarbon in HZSM-5 zeolites, and the law of product selectivity of real acid-catalyzed reaction was investigated by analyzing the product distributions. The results showed that no pyrolysis reaction was found at 300 ℃. Only the acid catalytic reaction took place by the mechanism of carbocation, whose activity was positively correlated to the amount of Br?nsted (B) acid sites. The selectivity of ethane, ethylene and propane was negatively correlated, while that of propylene was positively correlated with the Si/Al ratios and catalyst to oil ratios, suggesting that low acid density might be more favorable for monomolecular cracking reactions. It was worth nothing that the total selectivity of C4 products was much higher than that of C2 products.Combined with the quantum chemistry calculation results, it could be confirmed that the super-stability of C 2H+5 carbenium ion from the monomolecular cracking of n-hexane made it difficult to produce ethylene and ethane through hydrogen transfer reaction. It’s easier to form a C8 carbenium ion (C 8H+19) with another n-hexane molecule, and then to generate more C4 products. These results revealed the nature of the low selectivity of ethylene in light hydrocarbon catalytic cracking products.It could be concluded that the product selectivity of catalytic cracking of light hydrocarbons could be modulated by controlling reaction paths depending on the catalyst acid properties and the catalyst to oil ratios. This work will provide important theoretical support for the catalyst design and process development of naphtha catalytic cracking.
Key words: HZSM-5;catalytic cracking;Br?nsted acid sites;selectivity;carbenium ion
Light olefins, especially ethylene and propylene,are important industrial organic chemicals.Traditionally, thermal cracking of naphtha is the main source of light olefins production. However, the drawbacks of the process, such as high reaction temperatures, high energy consumption and high CO2emission make it insufficient to meet the increasing demand. Catalytic cracking technology has been expected to be a potential technology for light olefin production in the future due to its lower reaction temperature and adjustable product selectivity[1,2].
Zeolite catalyst is the core technology of catalytic cracking process. Due to the rich acidic sites and unique structure, zeolite possesses excellent catalytic activity and product selectivity. ZSM-5 zeolites are the most widely reported material and have been industrially applied in the field of catalytic cracking for olefin production[3]. Related researches mainly focus on the synthesis and modification of zeolite materials[4?7].In addition, the catalytic reaction mechanism is also a research hotspot[8?10].
The traditional catalytic cracking process consists of thermal cracking and catalytic cracking reactions based on free radical and carbenium ion mechanisms,respectively. Besides, catalytic cracking on zeolite acidic site can be further divided into monomolecular reaction mechanism and bimolecular reaction mechanism[11?14]. Hou et al[15]explored the role of free radical and carbenium ion mechanism in alkane cracking reaction. Based on the analysis of product distribution, it was suggested that the cracking of alkanes at above 700 °C followed the free radical and carbenium ion mechanisms. While at below 700 °C, the carbenium ion mechanism was dominate. The lower the temperature, the higher the percentage of the carbenium ions mechanism. It was suggested that carbenium ion mechanism was the main reason for the high selectivity of propylene. Afroukhteh et al[16]studied the catalytic cracking behavior ofn-hexane andn-heptane on ZSM-5 zeolites at 500/550/600 °C. Based on the product distributions, it was found that methane was formed in the products even at 500 °C, indicating that the alkane molecules underwent thermal cracking based on the free radical mechanism. Similar product distributions had been observed in other literature under 500 °C[17,18].
The acid properties of zeolites, especially the Si/Al ratios, are the key factors affecting the conversion and product selectivity[19,20]. Wang et al[1]found that then-butane catalytic cracking was dominated by bimolecular reactions occurring on the two adjacent Br?nsted acidic sites and concluded that the density of Br?nsted acidic sites was the most important factor affecting the performance ofn-butane catalytic cracking followed by the framework topology of zeolite. Liu et al[15]found that the Si/Al ratios of zeolites affected the product selectivity of alkane catalytic cracking by influencing the distribution of Br?nsted acidic sites.
In summary, the acidic catalytic cracking reaction depending on the carbenium ion mechanism is the most important factor affecting the product selectivity. It is also influenced by the reaction conditions such as temperature, pressure and space velocity. Since the existing reports on the catalytic cracking mechanism were mostly at high temperature (usually > 500 °C),under which the thermal cracking influenced the overall reaction process, especially the carbenium ion mechanism on the zeolites. By comparing the cracking reactions at different temperatures, it could be found that the effect of thermal cracking reaction can be basically eliminated at 300 °C. Therefore, in this work,the catalytic cracking behavior of hexane in ZSM-5 zeolite were tested at 300 °C in a Micro-Activity Test system (MAT) unit to investigate the effect of the mechanism of catalytic cracking reaction on the product selectivity by systematically revealing the relationship between zeolite Si/Al ratios and space velocity.
1 Experimental
1.1 Materials
HZSM-5 zeolites with different Si/Al ratios purchased from Nankai University were calcined at 550 °C for 8 h to remove the template and denoted as HZSM-5(A), HZSM-5(B) and HZSM-5(C),respectively. Hexane (analytical purity) was obtained from Tianjin Zhiyuan Chemical Reagent Co.. The quartz sand was purchased from Tianjin Damao Chemical Reagent Factory.
1.2 Catalyst characterization
The physical and chemical properties of the HZSM-5 zeolites were determined on an X-ray diffractometer manufactured by RIKEN, Japan, an Auto Chem 2920 chemisorption instrument from Micromeritics, USA, and an ASAP 2020 physisorption instrument from Micromeritics, USA, respectively, and the procedures were described in references[23,24].Pyridine was used as the probe molecule to characterize the acid amount and intensity of Br?nsted acid and Lewis acids in the samples byin situFourier transform infrared spectroscopy (Py-FTIR). The amount of the total acid was defined as the amount derived from the desorption peaks at 150 and 400 °C.The amount between the two peaks was the amount of the weak acid.
1.3 DFT calculations
All calculations were performed with the commercial software CASTEP code in Materials Studio 5.5 of Accelrys Inc, by using the first-principles calculations based on density functional theory (DFT).The optimized geometry, structural, adsorption properties and partial electronic properties were calculated by CASTEP module. The exchangecorrelation energy was calculated within the generalized gradient approximation (GGA) using the form of the functional proposed by the Perdew-Burke-Ernzerhof (PBE). The wave functions of the valence electrons were expanded using a plane-wave basis set within a specified cutoff energy of 400 eV. Electronic interactions were described by the ultra-soft pseudopotential, with valence electron configurations of Si 3s23p2, Al 3s23p1, O 2s22p4, C 2s22p2and H 1s1.The following convergence criteria for the structure optimization and energy calculation were set as follows: (a) self consistent field (SCF) tolerance of 5.0×10?7eV per atom; (b) total energy difference tolerance of 5.0×10?6eV per atom; (c) maximum force tolerance of 1.0×10?2eV·??1; (d) maximum displacement tolerance of 5.0×10?4?.
1.4 Catalytic tests
The performance ofn-hexane cracking on HZSM-5 zeolites was tested by using a fixed-bed Micro-Activity Test system (MAT) unit. The samples were firstly activated at 300 °C for four hours under an atmosphere of nitrogen. Then, the hexane was introduced into the reactor by nitrogen. The amount ofn-hexane feed were kept constant. The agent-oil ratios was changed by varying the mass of loaded catalyst.1.5?2.0 g of quartz sand was added to ensure a consistent bed height. Blank experiments were performed in the reactor containing quartz sand (2 g)under the same reaction conditions to exclude possible thermal cracking of hexane. The product was collected after 10 min of injection for detection.
After reaction, the hydrocarbon products in the gas phase were determined by gas chromatography equipped with hydrogen flame ionization detector (GCFID) (Beijing Beifen Ruili Co., Ltd., SP2100). The chromatograph was operated according to the following conditions: PLOT-Q capillary column (30 m ×0.53 mm × 1.5 μm) was selected as the column. The initial column temperature of 50 °C was held for 5 min.The column was thus heated at a rate of 5 °C/min to 115 °C and kept for 20 min. The inlet temperature was 210 °C. High purity nitrogen was used as the carrier gas. The injection volume was 0.6 μL.
The hydrocarbon components in the liquid phase products were determined by gas chromatography equipped with hydrogen flame ionization detector (GCFID) (Beijing Beifen Ruili Co., Ltd., SP2100) under the following conditions: the column was KB-1 capillary column (30 m × 0.53 mm × 1.5 μm). The column temperature was 50 °C and the inlet temperature was 290 °C. High purity nitrogen was used as the carrier gas. The injection volume was 0.6 μL.
Then-hexane conversion (x), molar selectivity(s(M)i), mass selectivity (si) and mass yield (yi) of the product componentiwere calculated as follows:
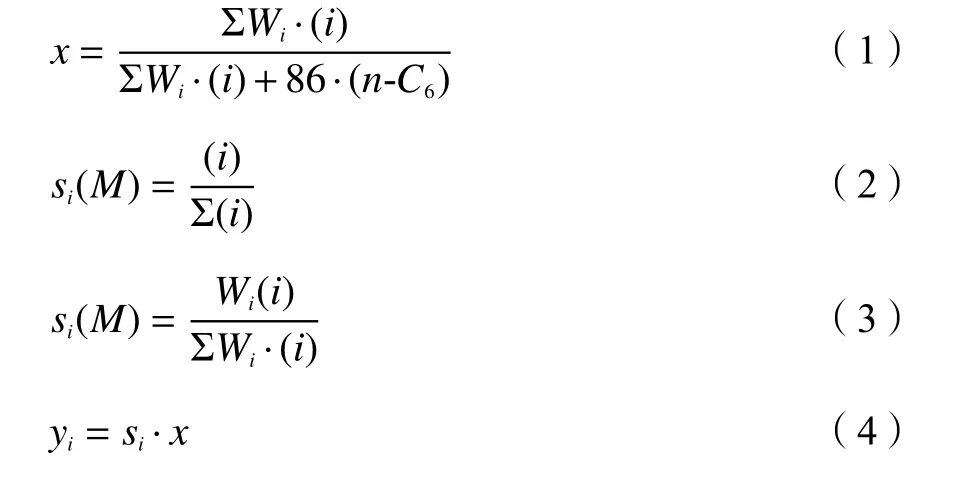
whereWiwas the molecular weight of species i in the product, (i) was the corresponding molar fraction in the product, and (n-C6) was the molar fraction ofnhexane in the product.
2 Results and discussion
2.1 Characterization of HZSM-5 zeolite physical and chemical properties
Figure 1 showed the XRD patterns of the three HZSM-5 zeolites with different silica-alumina ratio. By comparison of the intensity of each diffraction peak of the three samples, it was found that all samples had a typical MFI structure with good crystallinity.
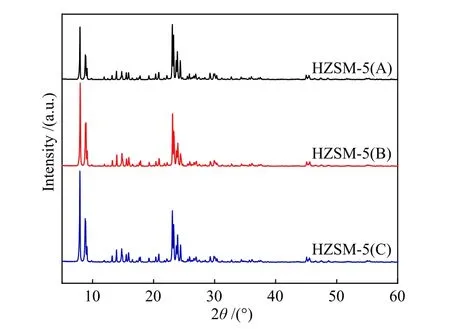
Figure 1 XRD patterns of HZSM-5 zeolites with different Si/Al ratio
Figure 2 showed the nitrogen adsorptiondesorption isotherms of HZSM-5 zeolites with different Si/Al ratio. As shown in the figure, the nitrogen adsorption of the three samples increased sharply when the relative pressure (p/p0) was < 0.1, indicating that all samples had good microporous structures. At the relative pressure (p/p0) range of 0.4?1.0, all samples had a small hysteresis loop, suggesting the small amount of mesoporous structures. The structural parameters of the three zeolites were listed in Table 1.It could be seen that the three samples have relatively similar structure parameters such as specific surface area and pore volume.
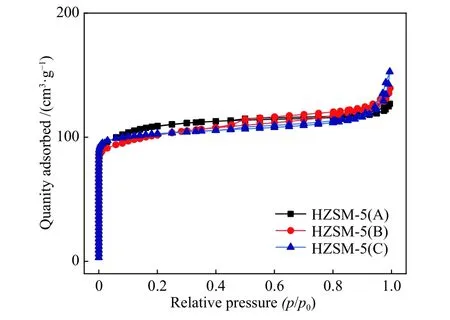
Figure 2 N2 adsorption-desorption isotherms of HZSM-5 zeolites with different Si/Al ratio
Figure 3 showed the NH3-TPD profiles of the HZSM-5 zeolites with different Si/Al ratios. Two kinds of acid sites with different acid intensities could be observed. The acidic content gradually decreased, and the acid strength of both acid sites were weakened with the increase of Si/Al ratio of zeolites. The enhancement of the acid strength could be attributed to the synergy between the increased interaction of acid sites and the ammonia molecules[25]. The Py-FTIR spectra of HZSM-5 zeolites with different Si/Al ratios were shown in Figure 4. The spectra of all samples presented absorption peaks at wave numbers of 1445 and 1545 cm?1, which were attributed to pyridine adsorption at the L acid site (PyL) and B acid site (PyH+),respectively[26,27]. As shown in the figure, the number of B acid sites decreased with the increase of the Si/Al ratio. In addition, there were some differences for the L acid sites of the samples with different Si/Al ratios, in which the strong L acid site (1455 cm?1) was dominant on HZSM-5(A), while the weak L-acid site (1455 cm?1)was dominant for the other two samples with high Si/Al ratios. The acid properties of three HZSM-5 zeolites were listed in Table 2. In summary, the most significant difference for the three zeolite samples was the number of strong B acid sites. Armaroli et al[28]investigated the acidity of HZSM-5 zeolite using 2,2-dimethylpropionitrile as the probe and confirmed that strong B acid sites distributed within the zeolite pore channels were the active centers for the catalytic cracking of alkane molecules. Therefore, the variability in acid density and acid strength caused by different zeolite Si/Al ratios might lead to different reaction pathways for alkane catalytic cracking.
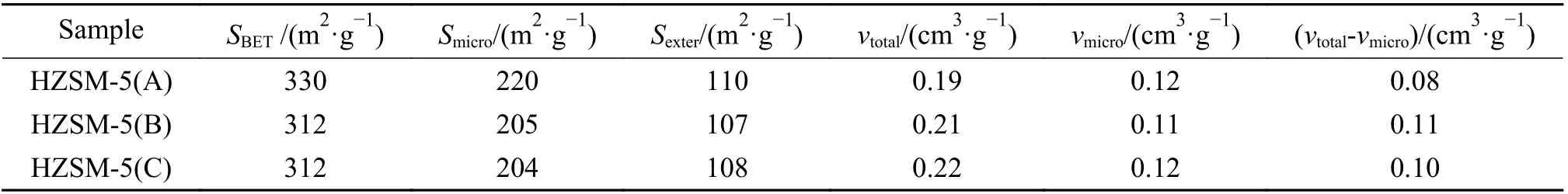
Table 1 Structure properties of HZSM-5 zeolites with Si/Al ratios
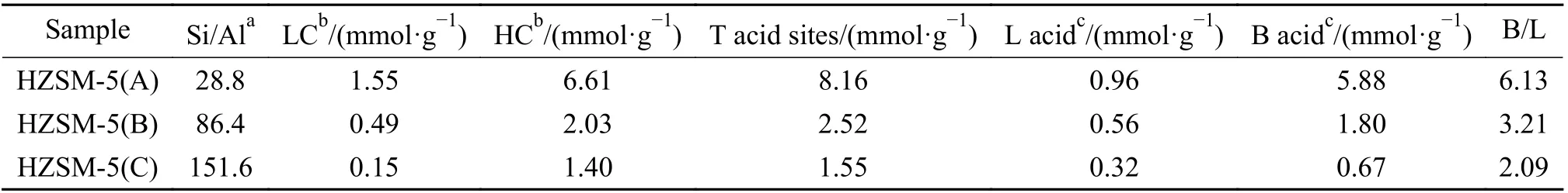
Table 2 Acidic properties of HZSM-5 zeolites with different Si/Al ratios
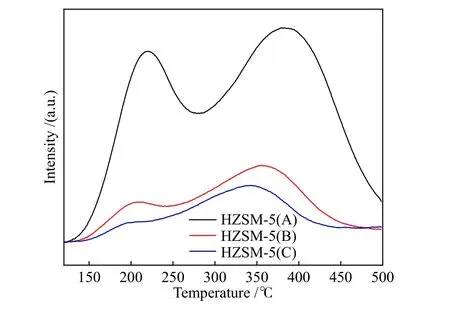
Figure 3 NH3-TPD profiles of HZSM-5 zeolites with different Si/Al ratios
2.2 Performance evaluation of n-hexane catalytic cracking and product distribution analysis
From the results of the gas phase products of the cracking reaction ofn-hexane at different temperatures,it was found that thermal cracking products could be detected when the reaction temperature reached 350 °C.While almost no thermal cracking products were detected at 300 °C. Therefore, the acid catalytic cracking reaction ofn-hexane was conducted at 300 °C to eliminate the effect of thermal cracking reaction.
Figure 5 showed the effect of Si/Al ratios and catalyst to oil ratios on the activity and product distribution ofn-hexane catalytic cracking on HZSM-5 zeolite at 300 ℃. The overall increasing trend ofnhexane cracking conversion was observed with decreased Si/Al ratio and increased catalyst to oil ratios, indicating that the reaction activity mainly depended on the amount of acidic sites and the amount of reactants contacting with the acidic site per unit time. In other words, the increase of catalyst acid density and catalyst dosage was beneficial to enhance the catalytic activity, which was consistent with the conventional knowledge.
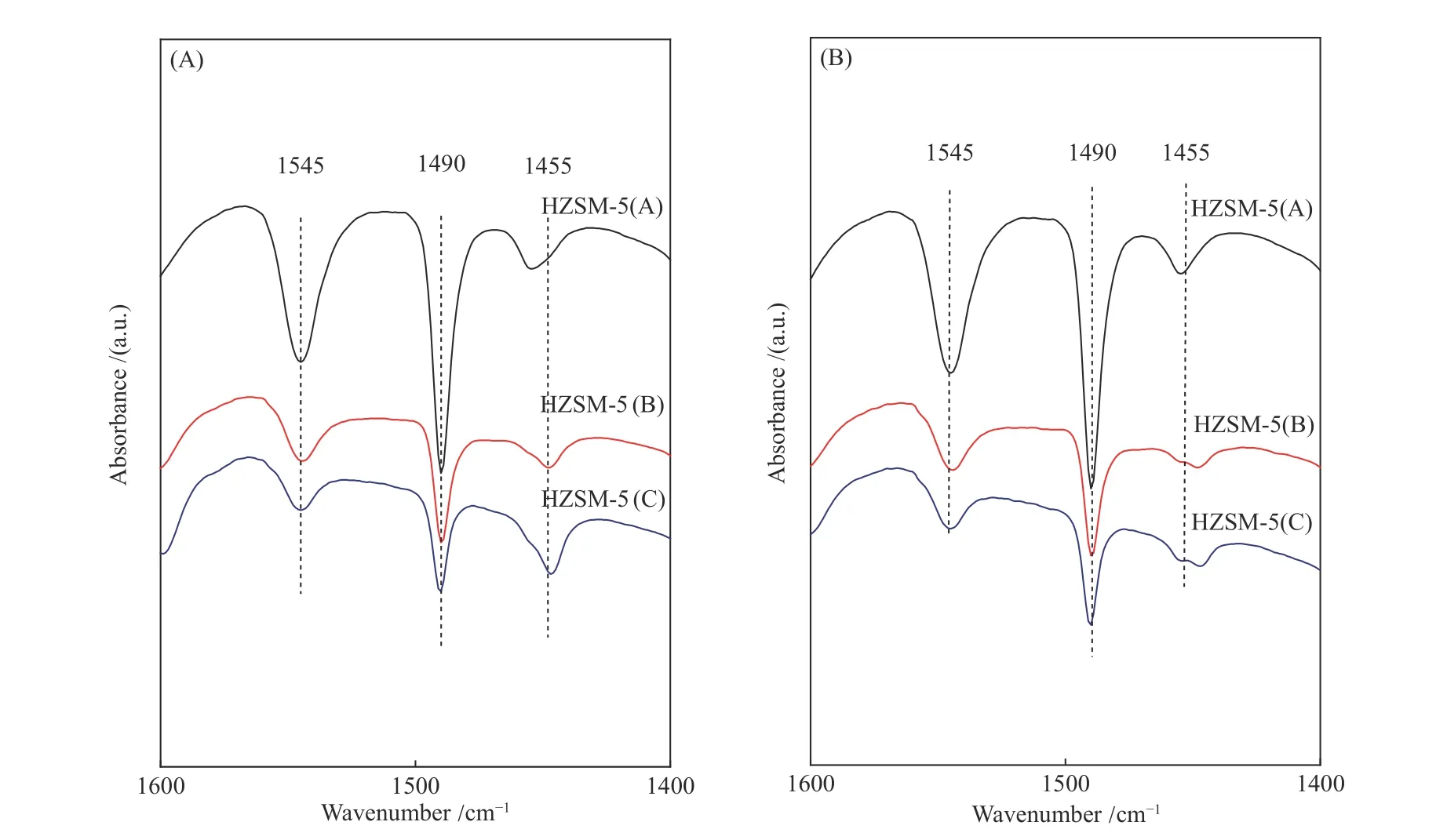
Figure 4 Py-FTIR spectra of HZSM-5 zeolites with different Si/Al ratios (A: desorption at 150 ℃, B: desorption at 400 ℃)
However, it was noteworthy that the correlation between product selectivity and Si/Al ratios and catalyst to oil ratios changed significantly. Combining the products and the results of thermogravimetric analysis, the following information could be obtained:(1) The carbon number of then-hexane catalytic cracking products was 2?6. (2) Only the HZSM-5 zeolites with high Si/Al ratio (> 150) could produce ethylene and ethane which decreased with the increase of catalyst to oil ratio. (3) Propane selectivity decreased with the decreasing of the Si/Al ratios of the HZSM-5 catalysts while the increasing of catalyst to oil ratios.(4) Then-hexane catalytic cracking reactions displayed high selectivity toward C4products that were mainly composed ofn-alkanes andn-olefins, with almost no C4isomers. (5) The amount of coke on the catalyst surface after reaction was very low.
The 3D plots of the selectivity of ethane, ethylene,propane, and propylene with the variation of Si/Al ratios and catalyst to oil ratios (Figure 6) clearly showed that the selectivity of ethane, ethylene, and propane, significantly exhibited a negative correlation with the total acid amount, while the selectivity of propylene was positively correlated.
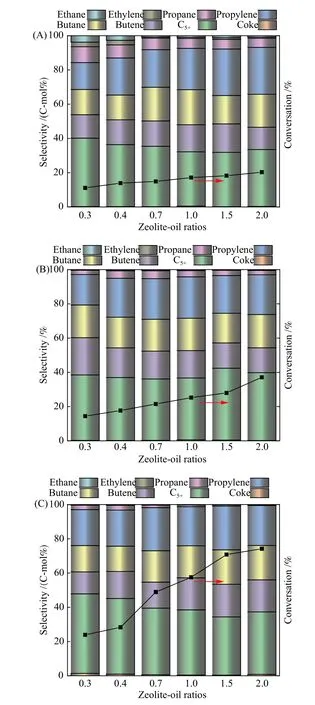
Figure 5 Products distribution of n-hexane catalytic cracking at 300 °C for different catalyst to oil ratios on HZSM-5 zeolites with different Si/Al ratios (A: HZSM-5(C) B: HZSM-5(B) C:HZSM-5(A); the bar graph shows the molar product selectivity,and the line graph shows the n-hexane conversion)
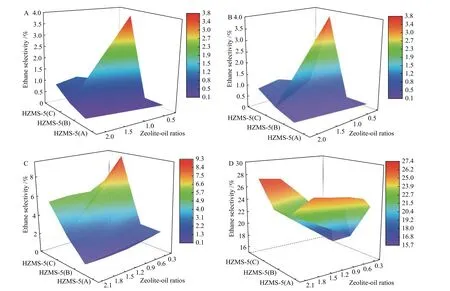
Figure 6 Change of the distribution of typical products of n-hexane catalytic cracking at 300 °C as a function of Si/Al ratios and catalyst to oil ratio (A: ethane, B: ethylene, C: propane, D: propylene)
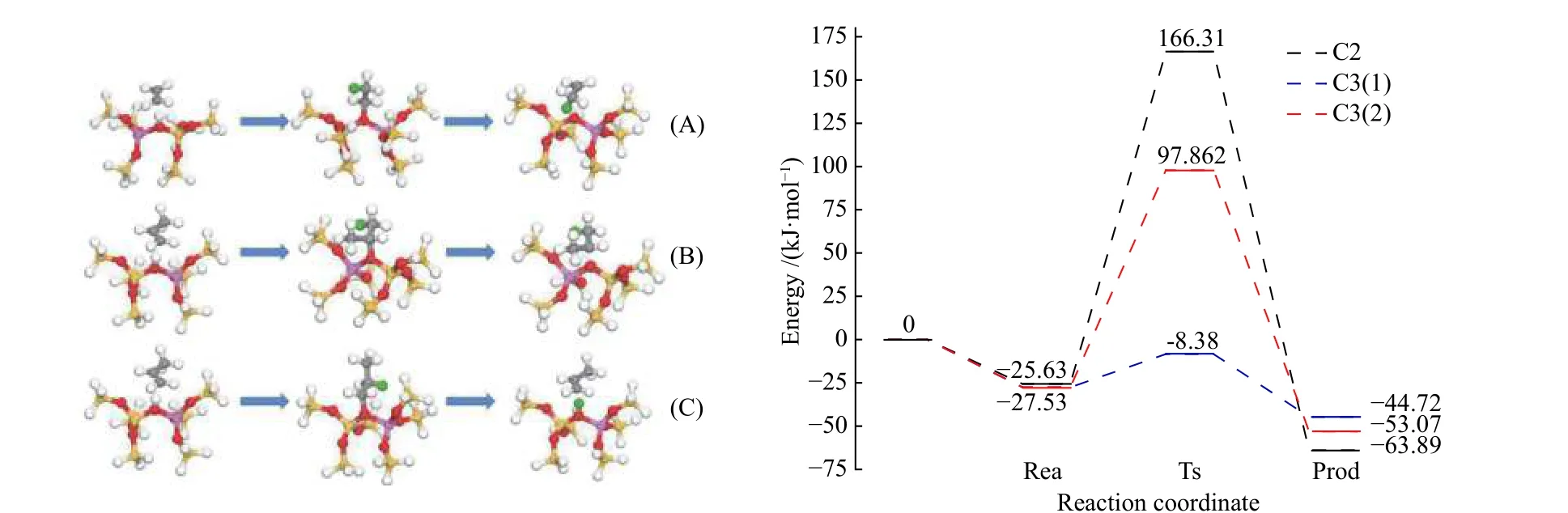
Figure 7 Structure diagram of model process of adsorption and protonation of ethylene and propylene on B acidic site of HZSM-5 zeolite (top) and process energy barrier diagram (bottom)
Therefore, the product distribution indicated that the acidic catalytic reaction paths based on the carbenium ion mechanism, such as cracking,isomerization and hydrogen transfer reactions showed remarkable differences in all reaction systems. While side reactions such as polymerization accounted for a small percentage and the thermal cracking reactions could be neglected.
It was also confirmed that the total amount of acidic sites of the catalyst had a significant effect on the catalytic cracking reaction ofn-hexane. It was necessary and important to deeply investigate the catalytic cracking reaction mechanism of the alkane.
2.3 Analysis of the relationship between the product selectivity of n-hexane catalytic cracking and reaction pathway

It was worth nothing that the total selectivity of the C4products (then-butane, 1-butene and 2-butene,then-alkanes) of all reaction systems was higher and less affected by the Si/Al ratios and the catalyst to oil ratios. It can be inferred that the C4products were obtained by direct cracking ofn-hexane. However, the selectivity of ethane and ethylene in the products was very small, which meant that then-hexane molecules did not directly crack to equal molar ratio of C2and C4products. This reaction characteristic should be the key factor affecting the selectivity of the ethylene.
3 Conclusions
- 燃料化學學報的其它文章
- 生物質氣再燃脫除流化床N2O 的機理研究
- Catalytic pyrolysis of sugarcane bagasse by zeolite catalyst for the production of multi-walled carbon nanotubes
- Nitrogen-doped porous carbon supported nickel nanoparticles as catalyst for catalytic hydroconversion of high-temperature coal tar
- Probing into the crystal plane effect on the reduction of α-Fe2O3 in CO by Operando Raman spectroscopy
- CoSOH/Co(OH)2 復合納米片的制備及其氧析出催化性能
- Effects of promoters on carburized fused iron catalysts in Fischer-Tropsch synthesis

