Is bicarbonate directly used as substrate to participate in photosynthetic oxygen evolution
Yanyou Wu
Abstract If the photosynthetic organisms assimilated only CO2 in the Archean atmosphere,hydroxide ion in the Archean seawater would not increase.If plants would not consume bicarbonate as a direct substrate during photosynthesis,it is difficult to explain the evolution of Earth’s environment.To date,it is generally accepted that photosynthetic O2 evolution of plants come from water photolysis.However,it should be debated by evaluating the effect of bicarbonate in photosynthetic O2 evolution,analyzing the role of carbonic anhydrase (CA) in photosynthetic O2 evolution,and the relationship between thylakoid CA and photosynthetic O2 evolution.In the paper,I propose that bicarbonate is directly used as substrate to participate in photosynthetic O2 evolution.The rationality of bicarbonate photolysis of plants is discussed from the thermodynamics and evolution of Earth’s environment.The isotopic evidence that bicarbonate is not the direct substrate of photosynthetic O2 release is reexamined,and the new explanation of bicarbonate photolysis in photosynthetic O2 evolution is proposed.
Keywords Bicarbonate photolysis · Carbonic anhydrase ·Photosystem II · Photosynthesis · Water photolysis
Seemingly final verdict
Carbon dioxide as the substrate of photosynthesis has been generally accepted,but bicarbonate as the substrate of photosynthesis has been controversial.At present,the role of bicarbonate in photosynthesis and oxygen evolution is specious,even contradictory.Some researchers believe that bicarbonate can stimulate the oxygen release of plants,which depends on either the acceptor side of Photosystem II(Van Rensen&Xu 1999;Van Rensen 2002)or the donor side of Photosystem II to stable O2evolving complex(Klimov et al.1995a,b;Baranov et al.2000).However,some researchers argue that bicarbonate play a direct role in oxygen evolution of plants (Stemler 1980,2002),or no evidence provide for that it coupled and bound in the O2evolving complex of Photosystem II (Clausen et al.2005;Aoyama et al.2008;Shevela et al.2008;Ulas et al.2008).Meanwhile,some isotopic evidence presents that bicarbonate does not work as a direct substrate in photosynthesis(Stemler &Radmer 1975;Metzner et al.1979;Radmer &Ollinger 1980;Clausen et al.2005),which makes the majority of people reach a consensus that oxygen released by plants must come from water in photosynthesis.
Bicarbonate effect in photosynthetic O2 evolution
In 1958,Otto Warburg and Günter Krippahl firstly discovered that bicarbonate can stimulate theChlorellarelease oxygen under the action of artificial reductant(Warburg &Krippahl 1958).In the following 60 years,many scientists studied the ‘‘bicarbonate effect’’ from different aspects of photosynthetic O2evolution,and got their own views and opinions,which are obtained from different works on different research materials under different conditions by using different means and methods.
Effect of bicarbonate on both the acceptor and the donor side of Photosystem II
The first evidence for an effect of bicarbonate on the acceptor side of Photosystem II was provided by Wydrzynski and Govindjee (1975),who measured chlorophyllafluorescence induction kinetics in spinach chloroplasts after CO2depletion in the presence of various artificial electron donors.They found that the variable fluorescence yield measured as a function of decreasing bicarbonate concentrations are qualitatively similar to those observed with increasing concentrations of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) which is known to block the reducing side in Photosystem II (Wydrzynski &Govindjee 1975).Afterwards,Govindjee et al.(1976) found that the chlorophyllafluorescence decayed after the third flash in bicarbonate-depleted chloroplasts,and the decay was faster in the chloroplasts to which bicarbonate was added (Govindjee et al.1976).
In 1977,Fred Crane and Rita Barr found that bicarbonate inhibited the DCMU-insensitive silicomolybdate reduction by Photosystem II but stimulated the O2evolution associated with ferricyanide reduction in presence of dibromothymoquinone (DBMIB) (Crane &Barr 1977).Paul Jursinic and Alan Stemler (1986) found that the photosynthetic electron flow between QA(the primary quinone acceptors) and QB(the secondary quinone acceptors) and QBand the plastoquinone pool was the slowest when formate is bound,but the highest when bicarbonate is bound (Jursinic &Stemler 1986).Meanwhile,van Rensen and Vermaas (1981) also found that the Hill reaction with ferricyanide does not require bicarbonate in trypsin-treated isolated broken pea in which ferricyanide accepts electrons directly at the QAsite (van Rensen &Vermaas 1981).All the above findings proved that the site of the bicarbonate effect was between QAand the plastoquinone pool.
Where are in specific sites of the bicarbonate effect?Many scientists have explored to reveal that some herbicides such as atrazine decrease the apparent affinity of the thylakoid membrane for bicarbonate when they studied the effects of bicarbonate and herbicides on electron transport in isolated chloroplasts,and suggested that the binding sites of bicarbonate are located on that of herbicide action (D1 protein)in Photosystem II(van Rensen and Vermaas 1981;Khanna et al.1981;Snel and van Rensen 1983,1984;Stemler and Murphy 1983).
Meanwhile,scientists demonstrate that bicarbonate is a bidentate ligand of the nonheme iron through measuring the electron paramagnetic resonance (EPR) spectra,and Mossbauer spectrum,examining Fourier transform infrared(FTIR)difference spectroscopy(Bowden et al.1991;Diner and Petrouleas 1987;Semin et al.1990;Hienerwadel and Berthomieu 1995).Afterwards,scientists have also observed this bidentate ligand of the nonheme iron between two quinone acceptors in higher plants,algae,and cyanobacteria from crystal structure information (Guskov et al.2010;Umena et al.2011;Ago et al.2016;Wei et al.2016).On the one hand,bicarbonate in Photosystem II was deduced to stabilize the QA-Fe-QBstructure,keep a fit distance between QAand QB,accelerate the electron transfer between these two quinone acceptors by facilitating the protonation of reduced QB(van Rensen et al.1999;Shevela et al.2012).On the other hand,the release of bicarbonate from the ligand can downregulate Photosystem II and oxygen evolution,thereby protect Photosystem II against photodamage (Brinkert et al.2016).Besides,Tikhonov et al.(2017) quantified the amount of bicarbonate-bind per Photosystem II reaction center in spinach Photosystem II membrane fragments.The value of 1.1 ± 0.1 in bicarbonate-bind per Photosystem II reaction center demonstrated that Photosystem II binds one bicarbonate molecule as ligand to the non-heme iron(Tikhonov et al.2018).
In the early 1970s,Stemler and Govindjee had observed the effect of bicarbonate on the donor side of Photosystem II,who found that bicarbonate increasing 4 to fivefold the rate of dichlorophenol indophenol reduction by isolated maize chloroplasts,and act close to the oxygen-evolving site (Stemler and Govindjee 1973).They deduced that one of the sites was before at or near the oxygen-evolving mechanism itself from chlorophyllafluorescence information (Stemler and Govindjee 1974).
Afterwards,many evidences demonstrated that bicarbonate plays roles in the donor side of Photosystem II.Assembly of the inorganic core of the oxygen-evolving center is an important role.Klimov et al.demonstrated that bicarbonate participates in the formation of the Mn-cluster,is essential for water oxidation in subchloroplast membrane fragments (Klimov et al.1995a,b 1997).Allakhverdiev et al.(1997) demonstrated that bicarbonate is an essential constituent of the oxygen-evolving center of photosystem II (Allakhverdiev et al.1997).Baranov et al.(2000,2004)demonstrated that bicarbonate as a cofactor accelerates assembly of the manganese cluster of the photosynthetic water oxidizing complex in photosystem II (Baranov et al.2000,2004).Kozlov et al.(2010) demonstrated that the composition and stability of Mn3+-bicarbonate complexes is involved in photoinduced electron transfer from Mn2+to reaction centers of photosystem II (Kozlov et al.2010).
Mobile bicarbonate has a more interesting role.Shevela et al.(2013) found that free or weakly bound bicarbonate had a possible function at the water-splitting side of Photosystem II (Shevela et al.2013).Koroidov et al.(2014)demonstrate that bicarbonate acts as a mobile proton acceptor during water oxidation (Koroidov et al.2014).
Synthesizing evidences from the above two aspects as well as other works,and now it is currently acceptable that bicarbonate affect photosynthetic oxygen evolution on both the acceptor and the donor side of photosystem II (van Rensen et al.1999;Stemler 2002,Shevela et al.2012;Tikhonov et al.2018).
Bicarbonate effect neither in the acceptor side nor the donor side of Photosystem II
In fact,it seems to has inadequate evidences to prove whether the acceptor or the donor or both side of Photosystem II during photosynthetic O2evolution influenced by bicarbonate.In donor side of photosystem II,Shevela et al.(2008) found that bicarbonate is not a tightly bound constituent,has only ‘indirect’ effects on the oxygen-evolving center in Photosystem II using membrane-inlet mass spectrometry (MIMS) and isotope labelling techniques(Shevela et al.2008).Aoyama et al.(2008) suggested that bicarbonate is neither a ligand nor a cofactor closely coupled to the oxygen-evolving Mn cluster in Photosystem II using Fourier transform infrared (FTIR) difference spectroscopy(Aoyama et al.2008).Ulas et al.(2008)observed no tightly bound bicarbonate ions from the active site using mass spectrometry(Ulas et al.2008).Clausen et al.(2005)was in contradiction with Koroidov et al.(2014)to exclude that exchangeable bicarbonate is the substrate for photosynthetic oxygen evolution (Clausen et al.2005).
Similarly,bicarbonate affect photosynthetic O2evolution on the acceptor side of Photosystem II was also questioned.Bowden et al.(1991) found that bound bicarbonate is absent at pH 6.0,but present at pH 7.5,and bicarbonate depleted isolated chloroplasts with displacement by another anion is not suitable to study bicarbonate effect as well as the status of bicarbonate binding(Bowden et al.1991).van Rensen and Vermaas (1981) found the binding site for bicarbonate between quinone and plastoquinone in isolated thylakoid membranes ofSynechococcus leopoliensisis absent,but this blue-green alga can release O2,indicating bound bicarbonate in the acceptor side of Photosystem II is not necessary for photosynthetic O2evolution (van Rensen and Vermaas 1981).Fundamentally,bound bicarbonate to stabilize the QA-Fe-QBdoes not match the decay of the fluorescence intensity after three or more flashes.Crystal structure of oxygen-evolving Photosystem II does not display the bidentate ligand bound bicarbonate (Umena et al.2011).Therefore,a more reasonable theoretical explanation is needed to settle the dispute under the considering all data and evidence on‘‘bicarbonate effect’’.
Bicarbonate as a direct substrate involving carbonic anhydraseCarbonic anhydrase(CA,EC 4.2.1.1)is ubiquitously found in most cells from all kingdoms of life,sometimes several forms exist in the same cell.CA catalyzed the reversible conversion of bicarbonate to carbon dioxide,which is one of the fastest enzymatic reactions.CA plays many physiological functions in photosynthesis,respiration,pH homeostasis and ion transport,etc.
The proportions of algal inorganic carbon sources or inorganic carbon utilization pathway was quantified using bidirectional isotope labeling tracer technique whenChlamydomonas reinhardtiiandChlorella pyrenoidosawas cultured in medium under different concentration of bicarbonate added (Wu et al.2015).BothC.reinhardtiiandC.pyrenoidosahave a great proportion of bicarbonate utilization pathway,larger than 76%,or even close to 100%.However,the proportion of bicarbonate added as algal inorganic carbon sources,which increases with the increasing concentration of bicarbonate added,is small(Table 1).It demonstrates thatC.reinhardtiiorC.pyrenoidosaassimilate bicarbonate not only as a direct substrate,but also as most dominant way for inorganic carbon assimilation.However,CA is completely inhibited when the culture medium adding 10.0 mmol/L acetazolamide,an inhibitor of extracellular CA.In this time,the pathway of utilization bicarbonate by algae,which increases with the increasing concentration of bicarbonate added,decreases dramatically (Table 2).It suggests that CA is convenient to that photosynthetic organisms use bicarbonate as a direct substrate (Wu et al.2015).
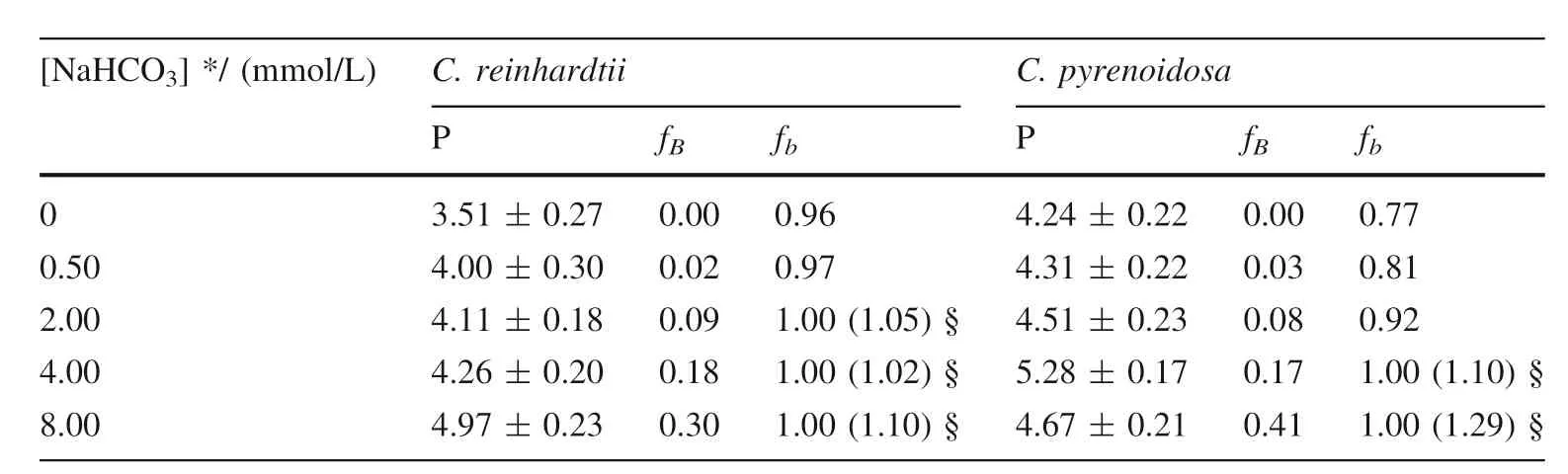
Table 1 The proportion of carbon sources and utilization pathway when C.reinhardtii and C.pyrenoidosa was cultured in medium under different concentration of bicarbonate added (Wu et al.2015)
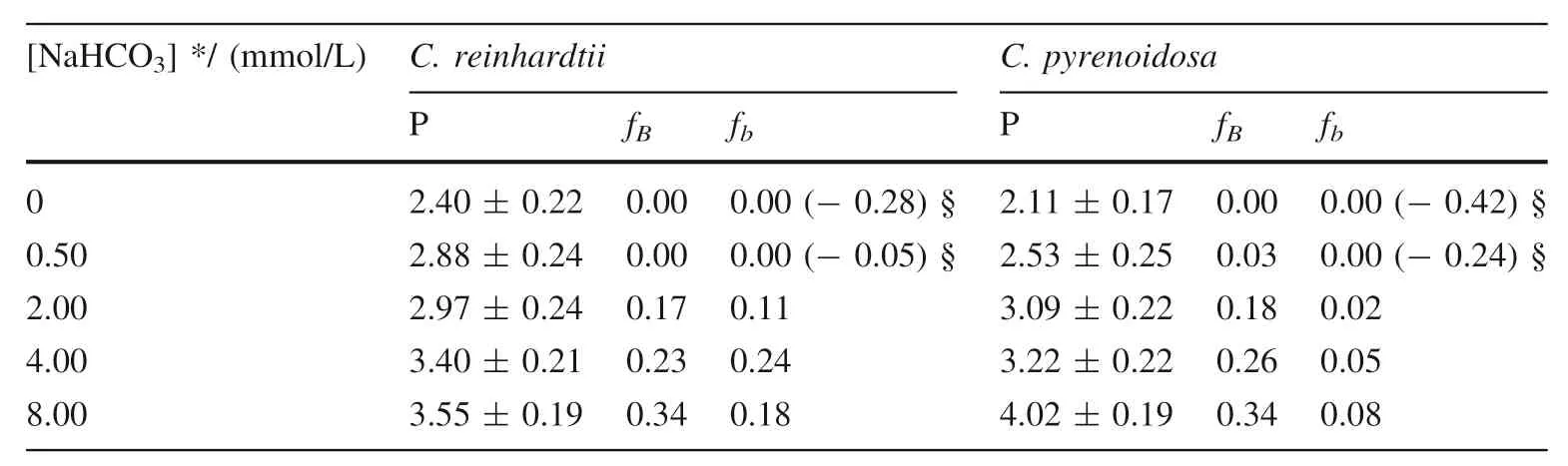
Table 2 The proportion of carbon sources and utilization pathway when C.reinhardtii and C.pyrenoidosa was cultured in medium with 10.0 mmol/L acetazolamide under different concentration of bicarbonate added (Wu et al.2015)
Thylakoid CA versus photosynthetic oxygen evolution
Photosystem II indeed possessed CA activity
There are two types of CA in the chloroplast,one was a soluble CA located in the stroma,a second was tightly bound to thylakoids membranes,called thylakoid CA(tCA)(Komarova et al.1982;Pronina et al.2002;Moskvin et al.2004;Rudenko et al.2006,2007).Many studies of tCA from maize (Stemler 1986),wheat (Khristin et al.2004),pea (Moskvin et al.1995) and Arabidopsis (Ignatova et al.2011) chloroplasts showed the association of enzymatic activity with Photosystem II.A lot of evidence showed that Photosystem II indeed possessed CA activity.
Acetazolamide inhibits photosynthetic electron transfer in Photosystem II and the acetazolamide-induced inhibition is totally reversed by the addition of bicarbonate (Shitovet al.2011).Meanwhile,acetazolamide and imidazole suppressed the the photoinduced yield of chlorophyll fluorescence (Pronina et al.2002).Formate,an anionic inhibitors,similarly decreased tCA activity,and the inhibition of other anionic inhibitors,such as bicarbonate,I-,is same for both Photosystem II and tCA activity (Stemler 1980,1986).
Both tCA and Photosystem II photosynthetic activities are inhibited by Zn2+(Tripathy and Mohanty 1980;Rashid et al.1991;Stemler 1997).The effects of Cl-on tCA was similar to that on Photosystem II activities (Stemler 1986,1997;Lu and Stemler 2007).The effects of Ca2+and Mn2+on tCA was also similar to that on Photosystem II activities (Stemler 1986,1997;Lu and Stemler 2007).
Photosystem II inhibitors,3-(3,4-dichlorophenyl)-1,1’-dimethylurea (DCMU) and hydroxylamine,similarly inhibited the activity of tCA(Rudenko et al.2015).Strong light inhibited both tCA activity and electron transport of Photosystem II,induced photoinhibition,and Photosystem II modifiers hydroxylamine and atrazine prevented this‘‘photoinhibition’’ (Stemler 1986;Kyle et al.1984).
The unique characteristics of tCA among known CA types was that tCA activity was sensitive to the surrounding redox-potential,and was similar with that of Photosystem II (Bearden and Malkin 1973;Stemler and Jursinic 1993;Moubarak-Milad and Stemler 1994).Far-red light also stimulates both the tCA activity and Hill reaction of Photosystem II (Govindjee et al.1960;Stemler 1997).In addition,maize mesophyll chloroplasts were found that an antibody produced againstChlamydomonas reinhardtii’s thylakoid lumen CA(Cah3) reacts with a protein in enriched Photosystem II membranes (Lu and Stemler 2002).
Actually,in the last two decades,it is widely accepted this view that Photosystem II possessed CA activity.Numerous studies on the nature of tCA have shown that Photosystem II-membranes of plants,such as maize,pea,wheat,spinach,Chlamydomonas reinhardtii,Thermosynechococcus elongatus,Arthrospira maxima,contained two CAs,one was called extrinsic CA,is removable by washing PSII membrane fragments with 1 M CaCl2,the other tightly associated with the core Photosystem II complex called as intrinsic CA (Dai et al.2001;Lu and Stemler 2002;Villarejo et al.2002;Khristin et al.2004;Moskvin et al.2004;Ignatova et al.2006;Hillier et al.2006;Rudenko et al.2006,2007;Enami et al.2008;Shitov et al.2009).
According to the known data,there are great differences in the characteristics between the extrinsic CA and the intrinsic CA of Photosystem II,such as Table 3.
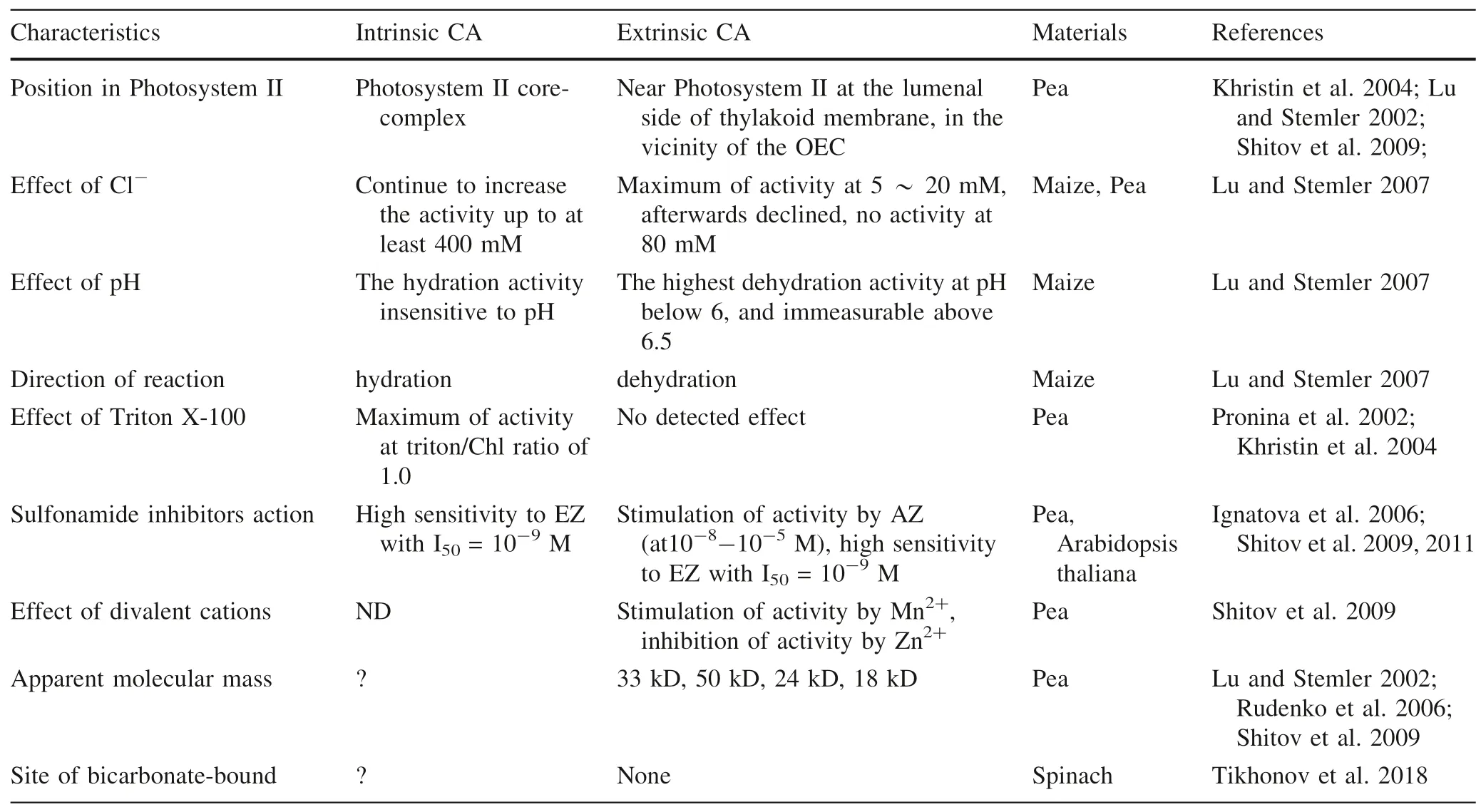
Table 3 The difference of the characteristics of CAs in Photosystem II-membranes
Photosystem II core-complex with the carrier of photosynthetic O2 evolution and CA activity not CA in the conventional sense
According to Table 3,there are quite different properties among the extrinsic CA,the intrinsic CA in Photosystem II and the soluble CA,and the functions they perform are also quite different.So far,the intrinsic CA of Photosystem II has not been isolated.Some inhibitors exhibit good inhibitory effect on CA in Photosystem II,but do not affect much photosynthetic activity (Rodionova et al.2017).Although some inhibitors can inhibit the activity both photosynthetic and CA in Photosystem II,the degree of inhibition is different.For example,one of the Cu(II)-phenyl sulfonylhydrazone complexes inhabits CA activity of Photosystem II by 100%,but the Photosystem II photosynthetic activity by only 66.2% (Karacan et al.2014).The activity of oxygen evolution was suppressed,but that of the intrinsic CA in Photosystem II was unchanged after removing manganese clusters,which demonstrated that CA activity was not directly correlated with O2evolution activity,and is independent of the presence of manganese clusters (Dai et al.2001).The CA (CA[Mn]) manganesesubstituted the active-site zinc functions as peroxidase,and produces O2in the presence of hydrogenperoxide and bicarbonate (Okrasa &Kazlauskas 2006).It can be seen that Photosystem II core-complex has not only the activity of photosynthetic O2evolution,but also that of CA.The manganese clusters (Mn4CaO5),which oxygen atoms as oxo bridges linking the manganese atoms may be catalyzed for dioxygen formation to produce oxygen (Umena et al.2011),were involved in photosynthetic O2evolution of Photosystem II.However,Photosystem II core-complex itself cannot be isolated,and its properties are obviously different from common zinc-contained CA.Therefore,it can be said that Photosystem II core-complex is the carrier of photosynthetic oxygen evolution and CA activity not CA in the conventional sense.
Dehydration and hydration of thylakoid CA depended on pH
Oxygen evolution ofChlorellailluminated in the presence of nitrate completely ceases when carbon dioxide is absorbed by alkali (Warburg et al.1965),it demonstrates that the premise of oxygen evolution should be in present of inorganic carbon.Moreover,Koroidov et al.(2014)proved that that bicarbonate functions not only as a mobile proton acceptor,but also results in a light-driven production of both O2and CO2(Koroidov et al.2014).Meanwhile,bicarbonate was found that it fit to act as a tridentate bridge between MnIVand Ca2+in architecture of the photosynthetic oxygen-evolving center (Ferreira 2004).These evidences seem to demonstrate that bicarbonate rather than water as direct substrate take part in photosynthetic O2evolution.Water incorporating CO2into bicarbonate was mistaken for direct substrate during photosynthetic O2evolution.
Whether on the acceptor or on the donor of side of Photosystem II,existence of bicarbonate binding is uncertainty,which depends on pH (Bowden et al.1991).Lu and Stemler (2007) showed that intrinsic CA in Photosystem II core-complex just had the hydration activity insensitive to pH,and extrinsic CA in the vicinity of the oxygen-evolving center just had the dehydration activity sensitive to pH,the highest dehydration activity was at pH below 6,and immeasurable above 6.5.Moreover,the hydration activity of intrinsic CA in Photosystem II corecomplex is greater 5 times than the dehydration activity of extrinsic CA (Lu and Stemler 2007).It suggests that the photosynthetic O2evolution of Photosystem II core-complex is accompanied by the hydrolysis of inorganic carbon.Similarly,it is demonstrated that that bicarbonate as direct substrate take part in photosynthetic O2evolution.Photosystem II core-complex should be definited inorganic carbon oxidase/hydrolase opposite to Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco).The dehydration of extrinsic CA provides protons,and the hydration of intrinsic CA provides bicarbonate for photosynthetic oxygen evolution.PSII core-complex combined with extrinsic CA provides two basic substrates,proton and bicarbonate,for photosynthetic oxygen evolution.The equation of photosynthetic oxygen evolution in Photosystem II was written as following:
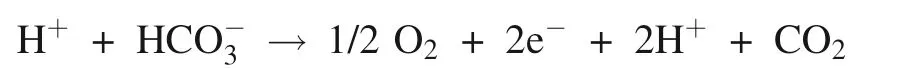
Additionally,the bicarbonate-bind as ligand to the nonheme iron on the acceptor side of Photosystem II depended on the pH,which was influenced by the coupling of Photosystem I.When the protons produced by photosynthetic O2evolution were moved through Photosystem I,the pH increased,and the ligand is present.Otherwise,the ligand is absent (Bowden et al.1991).The consumption of protons by Photosystem I regulates the electron flow and photophosphorylation in Photosystem II (Carr and Axelsson 2008;Fedorchuk et al.2018),and eventually,adjusts photosynthetic O2evolution.That is to say,another function of bicarbonate is to regulate photosynthetic O2evolution.
Evolution of Earth’s environment versus photosynthetic O2 evolution using bicarbonate as a direct substrate
Obviously,if plants would not consume bicarbonate as a direct substrate during photosynthesis,it is difficult to explain the evolution of Earth’s environment.The concentration of CO2in the Archean atmosphere before 2500 million years was 0.9~900 kPa,and that in Contemporary atmosphere only 0.03 kPa.Correspondingly,the concentration of bicarbonate in the Archean seawater was 15~15,000 mM,and that in Contemporary seawater 2 mM (Dismukes et al.2001).If the photosynthetic organisms assimilated only CO2in the Archean atmosphere,bicarbonate in the Archean seawater decreased depending on dissociation equilibrium.The hydroxide ion in the Archean seawater would not increase.As the system in the Archean seawater with high concentration of carbonic acid and bicarbonate was buffer,the pH value would not change.In fact,it is estimated that seawater pH gradually increased from~6.5 and 7.0 of Archean to~7.5 and 9.0 of Phanerozoic (Halevy &Bachan 2017).It partly accounts for the increase of pH in seawater which marine organisms utilized bicarbonate as the direct substrate during photosynthesis.In the Archean seawater,organisms used bicarbonate as a direct substrate,and reduced the concentration of bicarbonate during photosynthesis.Meanwhile,hydrogen ions in environment simultaneously entered the organism to achieve an electrochemical balance,resulting in the rise of pH in the Archean seawater.
Thermodynamic convenience of bicarbonate photolysis
Thermodynamically,the standard free energy differences in the chemical equilibria,H2O →1/2 O2+2e-+2H+,was 37.3 kcal/mol,and that in the chemical equilibria,24.8 kcal/mol (Dismukes et al.2001).The free energy required for O2evolution using bicarbonate as the substrate is significantly lower than that using water as the substrate during photosynthesis of organisms.Therefore,the photosynthetic organisms may be more likely to photolysis bicarbonate to release O2and CO2,then CO2incorporate into the Calvin cycle for carbon fixation.
Reexamining the isotopic evidence
We reexamine the isotopic evidence that bicarbonate is not the direct substrate of photosynthetic O2release according to our previous work (Wu et al.2015).From the experiment of Metzner et al.(1979),it can be found that at least 39% of oxygen release from bicarbonate during light reaction in photosynthesis ofAnkistrodesmus brauniiby using isotope binary mixture model when the algae were absent in CA activity.The proportion of photosynthetic O2from bicarbonate will be larger whenAnkistrodesmus brauniiwere present in CA activity.

Conclusions
It is generally accepted that O2released by plants come from water in photosynthesis,which should be debated.In fact,bicarbonate is directly used as substrate to participate in photosynthetic O2evolution,resulting in the chemical equilibria,+CO2,which provides with electrons,and accumulates CO2into Calvin cycle for photosynthetic carbon assimilation.
AcknowledgementsWe thank the foundations of the National Key Research and Development Program of China [2016YFC0502602],the National Natural Science Foundation of China[No.U1612441-2],and Support Plan Projects of Science and Technology Department of Guizhou Province [No.(2021)YB453].
Declarations
Conflicts of interestThe author declares no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http://creativecommons.org/licenses/by/4.0/.
- Acta Geochimica的其它文章
- Coupling effects of hydrological characteristics and nutrient load in sediments on the trophic state of reservoirs
- Geochemical constraints and uranium potential of the younger granitic rocks in El Maghrbia area,Central Eastern Desert,Egypt
- Carboniferous tectono-magmatic evolution of the northern Luliang arc:evidence from geochemistry and petrography of Carboniferous volcanic rocks in the northern Luliang Uplift,NW China
- Geochemistry and spectrometric prospection of younger granites and granitic pegmatites bearing uranium mineralization at G.Kab El Rakeb area,Central Eastern Desert,Egypt
- Zinc,copper,and strontium isotopic variability in the Baiyangping Cu–Pb–Zn–Ag polymetallic ore field,Lanping Basin,Southwest China
- Olivine and Cr-spinel as indicators of the petrogenesis and partial melting conditions of the high-MgO ultramafic volcanic rocks from NW Ad Dhala Province—Yemen

