葡萄鉀離子轉運基因VviHKT1;7在鹽脅迫下的功能鑒定
劉闖,高振,姚玉新,杜遠鵬
葡萄鉀離子轉運基因在鹽脅迫下的功能鑒定
劉闖,高振,姚玉新,杜遠鵬
山東農業大學園藝科學與工程學院/作物生物學國家重點實驗室,山東泰安 271018
【】探討在葡萄抗鹽機制中的作用,為后續培育抗鹽品種提供理論參考。利用DANMAN和MEGA軟件對葡萄HKT進行生物學信息分析。以抗鹽性較強的砧木SA15和SA17以及生產上常用砧木1103P組培苗為材料,用100 mmol·L-1NaCl分別處理0、3、6、12、24和48 h,以清水處理相應時間為對照,熒光定量PCR(qRT-PCR)檢測在葡萄根部的相對表達量;以SA17的cDNA為模板克隆基因,連接表達載體pRI101-AN-GFP,利用農桿菌侵染法侵染擬南芥花序,在抗性MS板上篩選直到獲得T3純合株系;將野生型與轉基因擬南芥種子播種于MS板和含有150 mmol·L-1NaCl的MS板上,觀察其發芽和生長情況并統計根長及鮮重;利用發根農桿菌技術獲得SA17轉基因葡萄根系,100 mmol·L-1NaCl處理24 h后,利用基于非損傷微測技術的NMT活體生理檢測儀檢測野生型和轉基因葡萄根系Na+的凈流量以及鹽脅迫下K+瞬時流量。多序列比對和系統進化樹分析表明,葡萄HKT之間同源性較高,其中開放閱讀框序列長度為1 380 bp,與的親緣關系最近。鹽脅迫顯著誘導了葡萄在3個品種中的表達,其中的相對表達量上調較高,長時間脅迫后表達量仍有上升趨勢,脅迫6或12 h時表達量達到峰值,且在耐鹽性強的SA17、SA15中表達量明顯高于1103P。擬南芥的發芽與生長結果表明,正常情況下野生型和轉基因擬南芥的發芽和生長情況無顯著差異,但鹽脅迫下轉基因擬南芥的發芽率、根長、鮮重明顯高于野生型。熒光檢測結果表明,轉基因葡萄根系在熒光下可以明顯看到綠色熒光,而野生型根系檢測不到熒光;進一步qRT-PCR檢測結果表明,轉基因葡萄根系中的表達量是野生型根系的20多倍。離子流速檢測結果表明,正常情況下野生型和轉基因根系Na+凈流量顯示出外排,各個時間段的波動幅度較小且無顯著差異,平均凈流量分別為208和205 pmol·cm-2·s-1;鹽脅迫后,兩者Na+凈流量明顯增大,各個時間段的波動幅度增大,平均凈流量分別為1 053和1 340 pmol·cm-2·s-1。正常情況下兩種根系K+吸收與外排處于動態平衡狀態,鹽脅迫顯著誘導K+外排,轉基因根系的外排量明顯小于野生型,分別為406和952 pmol·cm-2·s-1,表明轉基因植株根系的Na+外排、K+保持能力明顯大于野生型。在葡萄響應鹽脅迫中發揮著重要作用,過表達該基因可以提高擬南芥和葡萄根系在鹽脅迫下的適應能力。
葡萄;鹽脅迫;轉基因;功能鑒定
0 引言
【研究意義】土壤鹽堿化一直是世界各國面臨的嚴峻問題,制約著農業的可持續發展[1]。葡萄是一種世界分布較廣的植物,廣泛分布在五大洲,我國葡萄產量居世界第一位[2],土壤鹽漬化是制約我國西北干旱、半干旱產區葡萄產業發展的重要問題[3]。土壤鹽分對植物既有滲透脅迫又有離子毒害[4-5]。根區的高鹽會對葡萄產生滲透脅迫,從而降低植物的水分吸收和蒸騰作用[6]。鹽脅迫會使葡萄葉片柵欄組織的細胞由排列整齊的長柱狀變成無規則且有大量沉積物,海綿組織的細胞也減少,葉綠體變得腫脹,出現淀粉粒,液泡膜遭到破壞,從而使細胞出現大大小小的囊泡[7]。植物組織中過量的Na+和Cl-積累可引起光合作用降低、葉片壞死、果樹和漿果發育不均等離子毒性癥狀[8-9]。可見鹽脅迫直接破壞了葉片內在結構,使氣孔開度減小,從而損壞葉片光系統Ⅱ(PSII),降低葉片凈光合速率[10]。高濃度鹽脅迫還會使葡萄果實汁液中含有較高濃度鹽分,并提高其pH[11-12]。【前人研究進展】為了應對鹽脅迫,植物需要進行滲透調節和離子分配,以盡量減少Na+對植物的危害。HKT轉運蛋白是一種高親和K+轉運蛋白,但同樣能轉運Na+,幫助植物更好地完成吸鉀排鈉,維持正常鈉鉀比[13]。HKT蛋白包含多個跨膜區和孔環(P-Loop),其中第一個P-Loop決定轉運底物類型,分為兩個亞家族,第一個亞家族只負責運輸Na+,因為第一個孔環中含有絲氨酸,絲氨酸是鈉離子特異性載體,主要存在于雙子葉植物[14],如擬南芥、桉樹、楊樹等。第二種存在于單子葉植物,是K+選擇性載體,第一個孔環含有甘氨酸,是Na+-K+的協同運輸體或Na+/K+的單一運載體[15-17]。在小麥中首次被發現并分離[18],在提高植物抗鹽方面發揮著重要作用[19]。例如,大麥有助于幼苗鈉離子的排出,對于維持鹽脅迫下大麥正常的Na+/K+至關重要[20]。水稻可以更好地阻止鈉離子在莖葉中的積累,可釋放木質部Na+,尤其在水稻生殖生長階段,可以提高其耐鹽性[21]。將擬南芥中在馬鈴薯中過表達,減少了馬鈴薯葉片中的Na+積累,并促進了K+/Na+穩態,從而最大程度地減少了滲透失衡,維持了光合作用和氣孔導度,并提高了植物的生產力[22]。【本研究切入點】關于家族基因在異源和同源轉基因材料中的功能鮮有報道;筆者課題組前期研究發現,NaCl脅迫明顯上調了葡萄根系家族基因相對表達量[23];本研究進一步對在鹽脅迫后的表達量及其功能展開研究。【擬解決的關鍵問題】通過獲得相關的轉基因材料,對其抗鹽性做進一步的驗證,為葡萄抗鹽基因篩選及分子育種提供理論基礎。
1 材料與方法
試驗于2018—2019年在山東農業大學園藝科學與工程學院葡萄抗逆與栽培實驗室進行。
1.1 植物材料及生長條件
葡萄材料選用‘左山一’(Rupr.)×SO4(×)雜種F1代中的耐鹽株系SA17、SA15組培苗以及生產中常用砧木1103P(×)的組培苗,所有植物均在MS固體培養基上進行體外培養,輔以30 g·L-1蔗糖、7.0—7.5 g·L-1瓊脂粉、0.2 mg·L-1的植物激素IBA及少量活性炭。使植物在25℃/20℃下以16 h光照/8 h黑暗的光周期生長,每月將至少有一個芽和葉的枝條用于繼代培養。擬南芥種子用75%乙醇表面消毒1 min,4%次氯酸鈉表面消毒10 min,再用無菌蒸餾水沖洗5—6次,將其放在含有MS培養基的平板上,4℃春化3 d后放入光照培養箱,待長出2—3片真葉時移入含有50%營養土和50%蛭石的混合基質中,覆膜兩周,在22℃、16 h光照/8 h黑暗的光照培養箱中生長。
1.2 基因擴增以及表達載體的構建
基因號為VIT_211s0103g00140,根據基因CDS區域設計特異性擴增引物,上、下游引物5′端分別添加合適的酶切位點,將擴增出的目的基因與克隆載體(pEASY-Simple-Blunt)連接并轉化大腸桿菌,菌落PCR篩選陽性克隆。將測序正確的目的基因酶切下來與表達載體pRI101-AN-GFP成功連接,雙酶切位點分別為I和I,轉化大腸桿菌并搖菌提取質粒。將構建好的表達載體采用凍融熱激法轉化農桿菌GV3101和K599。
1.3 葡萄轉基因根系和轉基因擬南芥的獲得
參考WANG等[24]的方法,準備好在無菌條件下進行侵染的葡萄組培苗的枝條,枝條最少有兩個芽和葉。取K599農桿菌菌液500 μL加入到20 mL含有卡那霉素(50 mg·L-1)和利福平(50 mg·L-1)的液體LB培養基中,并在28℃下搖動孵育過夜,二次活化菌液至金黃色,離心收集菌體,并使用含有100 μmol·L-1乙酰丁香酮的無菌1/2 MS液體培養基重懸至600=1.0。在250 mL的錐形瓶中進行發根農桿菌的侵染,使剪下來的枝條浸沒在液面下,將錐形瓶在25℃下避光振搖15 min。用無菌濾紙將嫩枝插條吸干以除去多余的農桿菌,然后插入固體生根培養基(1/2 MS,20 g·L-1蔗糖,7.0 g·L-1瓊脂粉,200 mg·L-1頭孢霉素)。感染后4—7周,在芽插條的傷口周圍誘導了獨立的再生根。當根長約3 cm時,剪取須根在480 nm的熒光下進行檢測,根據綠色熒光識別轉基因和非轉基因的根,每條根系至少取3個須根進行觀測。
用已轉化的農桿菌GV3101采用花序侵染法侵染擬南芥,在含有卡那霉素(100 mg·L-1)的MS板上篩選擬南芥種子,直到獲得第三代純合體。
1.4 RNA的提取與實時熒光定量PCR(qRT-PCR)
RNA提取試劑盒、反轉錄試劑盒及定量SYBR染料均來自康為世紀生物科技有限公司。20 μL反應體系為:2×UltraSYBR Mixture 10 μL,上、下游引物(10 μmol?L-1)各1.0 μL(表1),cDNA 1.0 μL,RNase Free ddH2O 7.0 μL。每個樣本至少做3個重復。反應條件:95℃預變性10 min,95℃變性30 s,56℃退火30 s,65℃延伸10 s,40次循環,溶解溫度從65℃到95℃,每升高0.5℃保持5 s;停止反應。
1.5 葡萄根系離子流速測定
根系Na+和K+凈流量的檢測儀器為NMT活體生理檢測儀(Younger,美國)。參照高海波等[25]的方法制作電極,Na+和K+電極前端分別灌充45和180 μm的液態交換劑液柱,電極尖端垂直于根系,顯微鏡調節電極尖端與根尖的距離,在600 μm左右最佳,測定過程中電極尖端盡可能靠近,但不接觸材料表面。
Na+電極不適合在含高Na+濃度的溶液中進行測定[23],因此本研究比較鹽脅迫解除后野生型和轉基因根系根尖分生區Na+流速的變化。測定過程參考付晴晴[23]的方法,挑選生長狀態相對一致的對照或100 mmol·L-1NaCl脅迫24 h后的葡萄SA17組培苗根系,去離子水沖洗后剪取2 cm左右根尖并放入測試液中平衡30 min后進行測定,待離子流穩定后測定15 min,每個處理測定6條根尖。
測定瞬時K+流的動態變化時,先在緩沖液中測定8 min左右,再加入一定體積的NaCl母液(pH 6.0),使測定液中的NaCl濃度為100 mmol·L-1,定點測量20 min左右,每個處理測定6條根尖。
測試耗材和試劑均由北京旭月科技有限公司提供。通過Fick擴散定律公式:J=-D×(dc/dx),可獲得該離子的流動速率(pmol·cm-2·s-1),式中的J為離子流速,D是離子/分子特異的擴散常數(cm-2·s-1),dc/dx為離子濃度梯度。
1.6 數據分析
所有試驗都至少重復3次,利用Excel和spss24進行數據處理和差異顯著性分析。
2 結果
2.1 葡萄HKT生物信息學分析
用DANMAN軟件對葡萄HKT家族蛋白序列進行比對發現,6個葡萄HKT蛋白相似度為60.25%,利用Pfam軟件對6個蛋白序列進行預測,發現都含有HKT蛋白家族特有的結構功能域TrkH(圖1)。
2.2 葡萄HKT系統進化樹分析
利用MEGA軟件將葡萄HKT蛋白序列與其他物種蛋白序列構建系統進化樹,發現VviHKT1;7、VviHKT1;6和VviHKT1;8的親緣關系較近;VviHKT1;1和VviHKT1;3親緣關系最近;而VviHKT1;2則與大豆中的GmHKT1親緣關系最近(圖2)。
2.3 不同葡萄品種中HKT家族成員的鹽脅迫響應分析
如圖3所示,在100 mmol·L-1NaCl脅迫下,所有在SA17、SA15、1103P根系中的表達量都有升高的趨勢,各個基因在1103P中的表達量普遍低于SA17、SA15。脅迫12 h后,、、、、的表達量普遍呈下降趨勢,而的表達量呈現下降后又上升的趨勢,且在3個品種中的表達量也普遍較高,在脅迫6或12 h時達到峰值,分別上升了14.73、16.8、10.32倍。
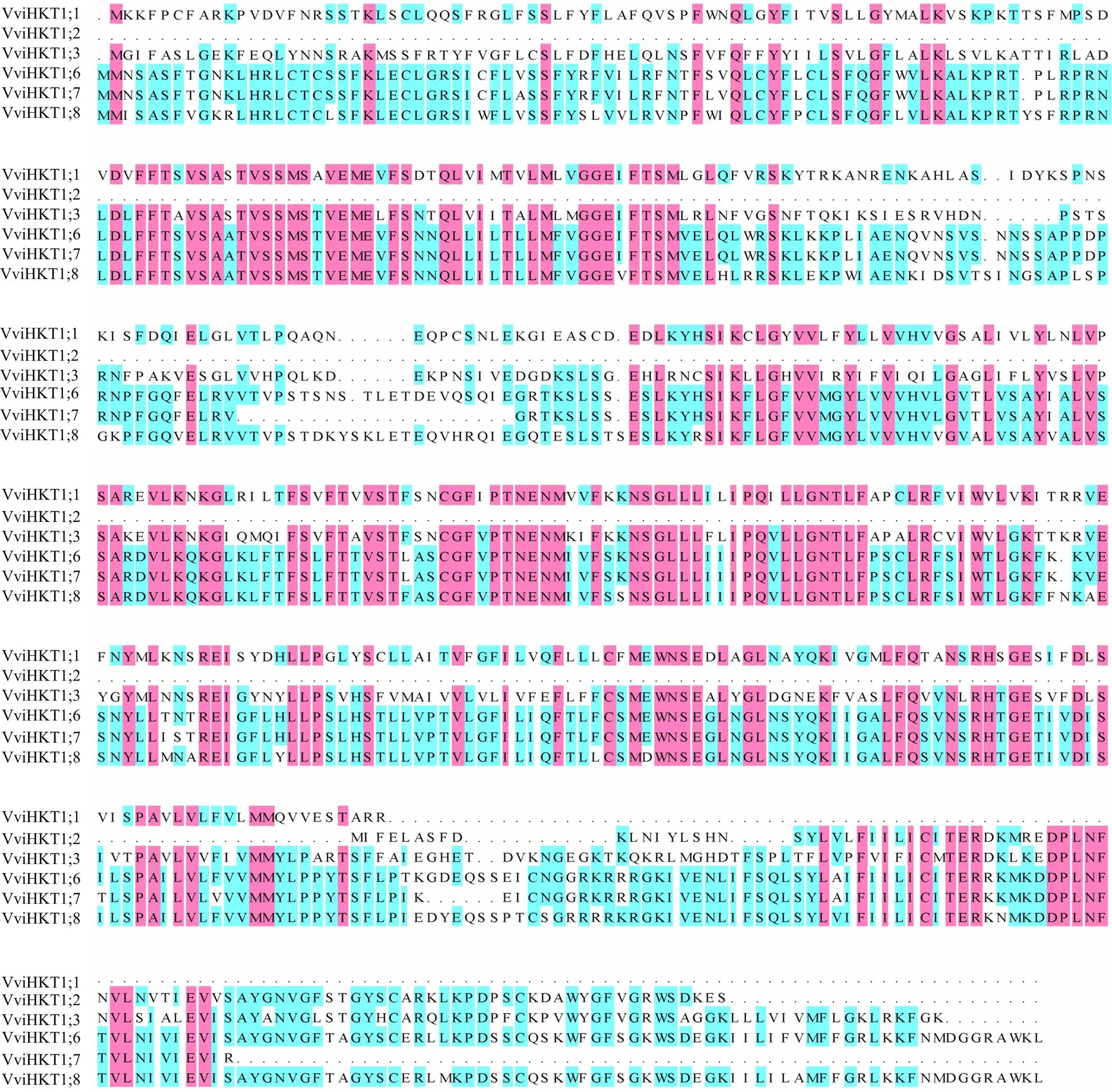
圖1 葡萄HKT蛋白序列比對
2.4 VviHKT1;7的克隆
以SA17的cDNA為模板克隆基因,經PCR擴增獲得了一條約1 400 bp的條帶(圖4),測序后發現與葡萄基因組數據庫中的序列一致。
2.5 鹽脅迫對野生型和轉基因擬南芥種子的發芽情況影響
如圖5-A、B所示,在正常生長條件下,野生型和轉基因種子發芽率無明顯差異,鹽脅迫抑制了種子的發芽,且對野生型種子抑制更明顯。野生型與3個轉基因株系種子的發芽率分別為46%、85%、90.3%、95%(圖5-C),可見鹽脅迫下轉基因種子的發芽情況明顯好于野生型。
2.6 鹽脅迫對野生型和轉基因擬南芥的生長影響
將野生型和轉基因擬南芥種子在MS板上生長一周后,挑選根長相對一致的擬南芥轉移到MS板和添加150 mmol·L-1NaCl的MS板上,觀察8 d后的生長情況(圖6-A、B)。MS板上的擬南芥各株系間根長、鮮重都無顯著差異,鹽脅迫下野生型及轉基因株系主根平均長度分別為19.3、30.2、30.6和31.4 mm(圖6-C),平均鮮重分別為0.027、0.057、0.057、0.053 g(圖6-D),可見轉基因擬南芥在鹽脅迫下的生長狀況明顯好于野生型。

圖2 葡萄HKT與其他物種HKT系統進化樹分析

圖3 鹽脅迫后不同葡萄株系根部HKT的表達分析
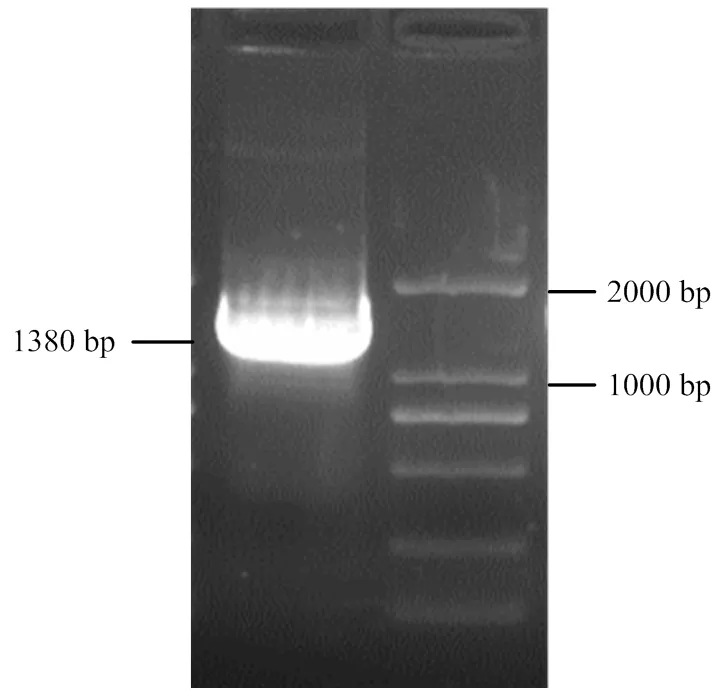
圖4 VviHKT1;7編碼區全長的PCR擴增
2.7 轉基因葡萄根系的獲得
如圖7-A所示,剪取須根進行檢測,因為基因表達載體帶有GFP標簽,所以在熒光下檢測時轉基因根系會觀察到綠色熒光,而非轉基因根系無綠色熒光。剪取少量側根而不損壞主根,以便后續進行離子流速試驗,利用qRT-PCR對篩選出的根系進一步進行鑒定,結果顯示轉基因根系目的基因的表達量顯著高于野生型(圖7-B)。
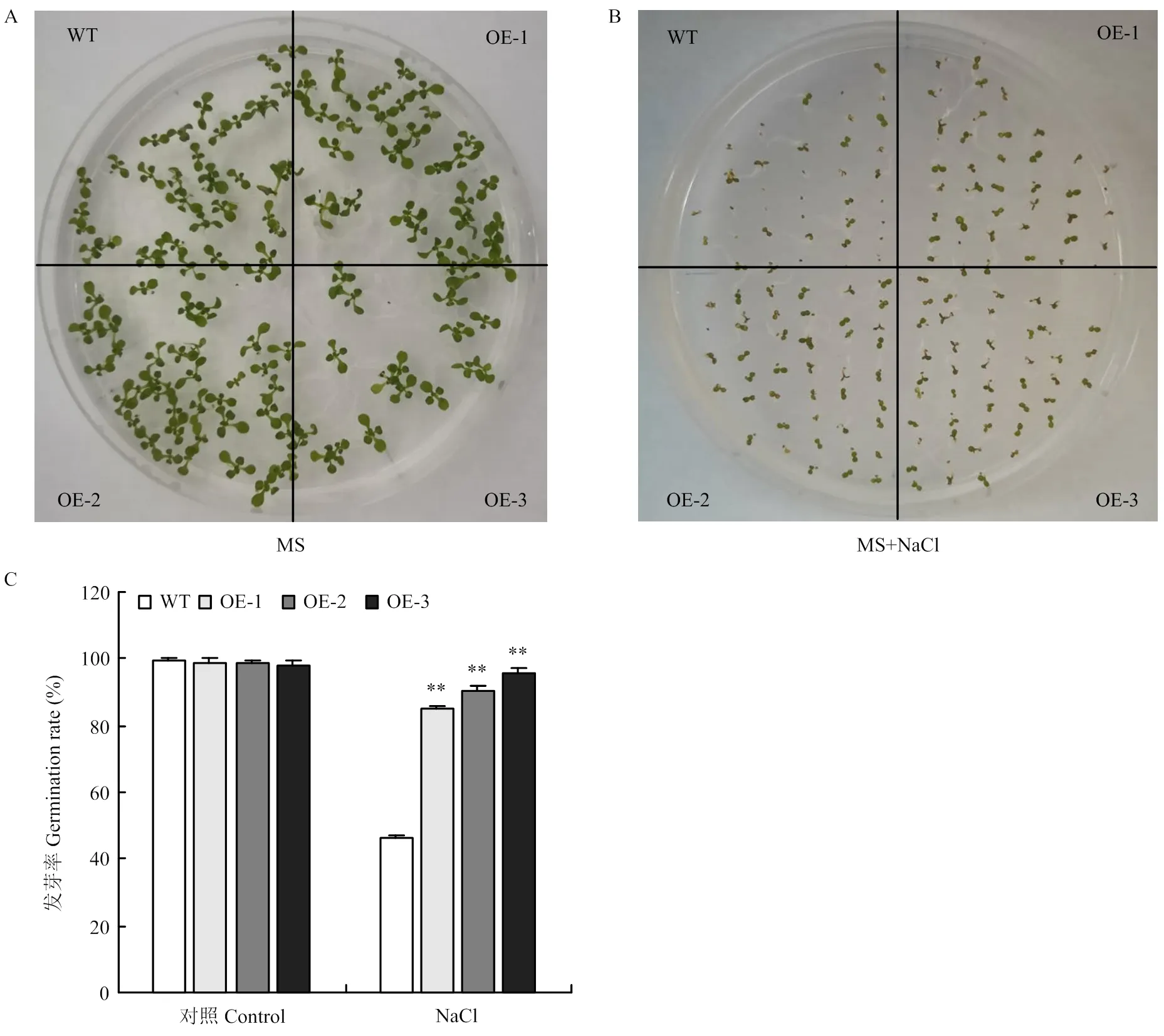
A:擬南芥種子在MS板上一周后的發芽狀況;B:擬南芥種子在添加150 mmol·L-1 NaCl的MS板上一周后的發芽情況;C:NaCl脅迫后發芽率統計。**表示差異極顯著(P<0.01);WT代表野生型,OE-1、OE-2、OE-3代表不同的轉基因株系。下同
2.8 野生型和轉基因葡萄根系Na+凈流量的檢測
正常情況下,野生型和轉基因葡萄根系Na+外排較低,約200 pmol·cm-2·s-1;100 mmol·L-1NaCl處理24 h后,Na+外流的凈流量明顯增大,轉基因根系外排能力明顯大于野生型(圖8-B)。如圖8-C所示,在15 min測量過程中,正常條件下根系Na+平均凈流量無明顯差異,鹽脅迫后轉基因根系平均凈流量明顯高于野生型,進一步說明轉基因根系可以更有效地調控Na+外排。

*表示差異顯著(P<0.05)* indicates significant difference (P<0.05)

A:轉基因根系的熒光檢測;B:轉基因根系的定量檢測

A:根系離子流測試位點;B:野生型和轉基因葡萄根系的Na+凈流量的檢測;C:Na+平均凈流量
2.9 野生型和轉基因葡萄根系K+凈流量的檢測
如圖9所示,在加入NaCl前,野生型和轉基因根系K+流呈現吸收與外排的動態平衡中,且流速相對比較平穩。當施加100 mmol·L-1NaCl后,迅速增加了葡萄根系K+的外流,但轉基因根系的增加幅度明顯小于野生型。
3 討論
在鹽脅迫條件下,離子穩態受到嚴格的調控,使必需離子積累而毒性離子保持在較低水平[26-27]。HKT作為K+和Na+轉運蛋白,在維持植物細胞離子穩態方面扮演著不可或缺的角色。

圖9 野生型和轉基因葡萄根系的K+凈流量的檢測
葡萄含有6個高親和鉀離子轉運蛋白基因(),包括、、、和,其中被認為是轉運鈉離子的主要候選基因,在根中表達量較高,而3是非功能性的[28]。的終止密碼子出現在第一個成孔結構域的上游,因此其編碼功能性蛋白的可能性較小[29]。、和在葡萄根尖、花、種子中的表達水平較低,在根中表達更低[30],但這并不能否定它們的功能。SA15、SA17的耐鹽性較強,而1103P的耐鹽性相對較弱[23]。本研究中,在不同的鹽處理時間下,在SA15、SA17中的表達量都顯著高于1103P,初步表明鹽脅迫可以誘導的表達,表達量高低與葡萄的抗鹽能力有關。所以在特定的生長階段,相關刺激物誘導,以及植物不同的耐鹽能力都可能改變基因表達量。
在胡楊中,過表達可以通過提高抗氧化系統的效率來增強耐鹽性[31];在大麥中,過表達通過增強Na+和K+的轉運能力提高其耐鹽性[32],在棉花中過表達可以通過增加K+吸收、K+/Na+動態平衡和清除活性氧的能力來提高其耐鹽性[33]。在番茄中,沉默增加了鹽脅迫下花中Na+的積累,從而降低了果實產量[34]。WU等[30]用轉化酵母,在含有50 mmol·L-1NaCl的培養基上培養,半乳糖誘導轉基因表達并觀察其生長狀況,結果初步表明在酵母系統中起著Na+轉運體的作用。用雙電極電壓鉗(TEVC)電生理試驗檢測Na+和K+電導性,結果表明、和是強Na+轉運體,亞細胞定位表明和定位于質膜,定位于細胞內細胞器。本試驗中利用花序侵染法獲得轉基因擬南芥并進行抗性試驗,發現轉基因擬南芥在鹽脅迫下發芽與根的生長能力明顯高于野生型,表明過表達可以提高擬南芥的耐鹽性,與其他植物中的功能相似[31-34]。利用發根農桿菌技術獲得轉基因葡萄根系,并且對其鹽脅迫后Na+和脅迫下K+凈流量進行檢測,發現鹽脅迫后轉基因葡萄根系的Na+排出能力明顯強于野生型,鹽脅迫下轉基因葡萄根系能更好地防止K+流失,進一步驗證了的Na+轉運能力,與WU等[30]研究結果一致,也說明了過表達可以更好地幫助葡萄維持細胞離子滲透勢的穩態,從而減輕鹽脅迫對植物的離子毒害。作為細胞器定位基因,可能在調節細胞內Na+含量方面發揮一定作用。有關葡萄家族的相關信號通路及分子機制依然值得進一步研究。
4 結論
鹽脅迫顯著誘導葡萄家族成員表達量上調,其中上調最顯著;異源過表達可以提高擬南芥在鹽脅迫下的適應能力,同源過表達該基因可以提高葡萄根系在鹽脅迫下的Na+排出和K+保持能力。
[1] JAMES R A, BLAKE C, BYRT C S, MUNNS R. Major genes for Na+exclusion, Nax1 and Nax2 (wheatand), decrease Na+accumulation in bread wheat leaves under saline and waterlogged conditions. Journal of Experimental Botany, 2011, 62(8): 2939-2947.
[2] 劉俊, 晁無疾, 亓桂梅, 劉寅喆, 漢瑞峰. 蓬勃發展的中國葡萄產業. 中外葡萄與葡萄酒, 2020(1): 1-8.
LIU J, CHAO T J, Qi G M, QI G M, LIU Y Z, HAN Y F. Booming development of Chinese grape industry. Sino-Overseas Grapevine & Wine, 2020(1): 1-8. (in Chinese)
[3] MAAS E V, HOFFMAN G J. Crop salt tolerance-current assessment. Journal of the Irrigation and Drainage Division, 1977, 103(2): 115-134.
[4] CHINNUSAMY V, ZHU J H, ZHU J K. Salt stress signaling and mechanisms of plant salt tolerance. Genetic Engineering, 2006, 27: 141-177.
[5] DEINLEIN U, STEPHAN A B, HORIE T, LUO W, XU G H, SCHROEDER J I. Plant salt-tolerance mechanisms. Trends in Plant Science, 2014, 19(6): 371-379.
[6] NEUMANN P M. Chapter 2-recent advances in understanding the regulation of whole-plant growth inhibition by salinity, drought and colloid stress. Advances in Botanical Research, 2011, 57: 33-48.
[7] 秦玲, 康文懷, 齊艷玲, 蔡愛軍. 鹽脅迫對釀酒葡萄葉片細胞結構及光合特性的影響. 中國農業科學, 2012, 45(20): 4233-4241.
QIN L, KANG W H, QI Y L, CAI A J. Effects of salt stress on mesophyll cell structures and photosynthetic characteristics in leaves of wine grape (spp.). Scientia Agricultura Sinica, 2012, 45(20): 4233-4241. (in Chinese)
[8] BABY T, COLLINS C, TYERMAN S D, GILLIHAM M. Salinity negatively affects pollen tube growth and fruit set in grapevines and cannot be ameliorated by silicon. American Journal of Enology & Viticulture, 2016, 67(2): 218-228.
[9] WALKER R R, CLINGELEFFER P R. Rootstock attributes and selection for Australian conditions. Australian Viticulture, 2009, 13(4): 70-76.
[10] 李晨, 李秀杰, 韓真, 劉莉萍, 李勃. 非生物脅迫對葡萄光合作用的影響研究進展. 山東農業科學, 2017, 49(12): 144-148.
LI C, LI X J, HAN Z, LIU L P, LI B. Research advances on effects of abiotic stress on photosynthesis of grape. Shandong Agricultural Sciences, 2017, 49(12): 144-148. (in Chinese)
[11] WALKER R R, BLACKMORE D H, CLINGELEFFER P R, CORRELL R L. Rootstock effects on salt tolerance of irrigated field-grown grapevines (L. cv. Sultana) 2. Ion concentrations in leaves and juice. Australian Journal of Grape and Wine Research, 2004, 10(2): 90-99.
[12] STEVENS R M, HARVEY G, PARTINGTON D L. Irrigation of grapevines with saline water at different growth stages: Effects on leaf, wood and juice composition. Australian Journal of Grape & Wine Research, 2011, 17(2): 239-248.
[13] Francisco R, Walter G, Julian I S. Sodium-driven potassium uptake by the plant potassium transporterand mutations conferring salt tolerance. Science, 1995, 270(5242): 1660-1663.
[14] Uozumi N, Kim E J, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker E P, Nakamura T, Schroeder J I. Thegene homolog mediates inward Na+currents in Xenopus laevis oocytes and Na+uptake in Saccharomyces cerevisiae. Plant Physiology, 2000, 122(4): 1249-1259.
[15] HORIE T, YOSHIDA K, NAKAYAMA H, YAMADA K, OIKI S, SHINMYO A. Two types oftransporters with different properties of Na+and K+transport in. The Plant Journal, 2001, 27(2): 129-138.
[16] GARCIADEBLáS B, SENN M E, BA?UELOS M A, RODR?GUEZ- NAVARRO A. Sodium transport andtransporters: The rice model. Plant Journal, 2003, 34(6): 788-801.
[17] MASER P, ECKELMAN B, VAIDYANATHAN R, HORIE T, FAIRBAURN D J, KUBO M, YAMAGAMI M, YAMAGUCHI K, NISHIMURA M, UOZUMI N, ROBERYSON W, SUSSMAN M R, SCHROEDER J I. Altered shoot/root Na+distribution and bifurcating salt sensitivity inby genetic disruption of the Na+transporter. FEBS Letters, 2002, 531(2): 157-161.
[18] SCHACHTMAN D P, SCHROEDER J I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature, 1994, 370(6491): 655-658.
[19] WATERS S, GILLIHAM M, HRMOVA M. Plant high-affinity potassium () transporters involved in salinity tolerance: structural insights to probe differences in ion selectivity. International Journal of Molecular Sciences, 2013, 14(4): 7660-7680.
[20] BEZOUW R F H M V, JANSSEN E M, ASHRAFUZZAMAN M, GHAHRAMANZADEH R, KILIAN B, GRANER A, VISSER R G F, VAN DER LINDEN C G. Shoot sodium exclusion in salt stressed barley (L.) is determined by allele specific increased expression of. Journal of Plant Physiology, 2019, 241: 153029.
[21] SUZUKI K, YAMAJI N, COSTA A, OKUMA E, KOBAYASHI N I, KASHIWAGI T, KATSUHARA M, WANG C, TANOI K, MURATA Y, SCHROEDER J I, MA J F, HORIE T.mediated Na+transport in stems contributes to Na+exclusion from leaf blades of rice at the reproductive growth stage upon salt stress. BMC Plant Biology, 2016, 16(1): 22.
[22] WANG L, LIU Y H, LI D, FENG S J, YANG J W, ZHANG J J, ZHANG J L, WANG D, GAN Y T. Improving salt tolerance in potato through overexpression ofgene. BMC Plant Biology, 2019, 19(1): 357.
[23] 付晴晴. ‘左山一’雜交砧木株系耐鹽評價及鈉離子吸收分配特征研究[D]. 泰安: 山東農業大學, 2018.
Fu Q Q. Salt tolerance identification and mechanism of hybrid rootstocks from ‘Zuo Shan 1’ [D]. Tai’an: Shandong Agricultural University, 2018. (in Chinese)
[24] WANG F P, ZHAO P P, ZHANG L, ZHAI H, DU Y P. Functional characterization of). Horticulture Research, 2019, 6(1): 803-814.
[25] 高海波, 張淑靜, 沈應柏. 灰斑古毒蛾口腔反吐物誘導沙冬青細胞Ca2+內流及H2O2積累. 生態學報, 2012, 32(20): 6520-6526.
GAO H B, ZHANG S J, SHEN Y B. Regurgitant fromGermar induces calcium influx and accumulation of hydrogen peroxide in Ammopiptanthus mongolicus () Cheng f. cells. Acta Ecologica Sinica, 2012, 32(20): 6520-6526. (in Chinese)
[26] ZHU J K. Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology, 2003, 6(5): 441-445.
[27] HAMAMOTO S, HORIE T, HAUSER F, DEINLEIN U, SCHROEDER J, UOZUMI N.transporters mediate salt stress resistance in plants: from structure and function to the field. Current Opinion in Biotechnology, 2015, 32: 113-120.
[28] HENDERSON S W, DUNLEVY J D, WU Y, BLACKMORE D H, WALKER R R, EDWARDS E J, GILLIHAM M, WALKER A R. Functional differences in transport properties of naturalvariants influence shoot Na+exclusion in grapevine rootstocks. The New Phytologist, 2018, 217(3): 1113-1127.
[29] HAUSER F, HORIE T. A conserved primary salt tolerance mechanism mediated bytransporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ratio in leaves during salinity stress. Plant, Cell & Environment, 2010, 33(4): 552-565.
[30] WU Y, HENDERSON S W, WEGE S, ZHENG F, WALKER A R, WALKER R R, GILLIHAM M. The grapevine NaE sodium exclusion locus encodes sodium transporters with diverse transport properties and localisation. Journal of Plant Physiology, 2020, 246/247: 153113.
[31] XU M, CHEN C H, CAI H, WU L. Overexpression ofimproves salt tolerance in. Genes, 2018, 9(10): 475.
[32] MIAN A, OOMEN R J, LSAYENKOV S, SENTENAC H, MAATHUIS F J, VéRY A A. Over-expression of an Na+and K+permeabletransporter in barley improves salt tolerance. Plant Journal, 2011, 68(3): 468-479.
[33] GUO Q, MENG S, TAO S C, FENG J, FAN X Q, XU P, XU Z Z, SHEN X L. Overexpression of a samphire high-affinity potassium transporter geneenhances salt tolerance in transgenic cotton. Acta Physiologiae Plantarum, 2020, 42(3): 36.
[34] ROMERO-ARANDA M R, GONZáLEZ-FERNáNDEZ P, PéREZ- TIENDA J R, LóPEZ-DIAZ M R, ESPINOSA J, GRANUM E, TRAVERSO J á, PINEDA B, GARCIA-SOGO B, MORENO V, ASINS M J, BELVER A. Na+transporterreduces flower Na+content and considerably mitigates the decline in tomato fruit yields under saline conditions. Plant Physiology and Biochemistry, 2020, 154: 341-352.
Functional Identification of Grape Potassium Ion TransporterUnder Salt Stress
LIU Chuang, GAO Zhen, YAO YuXin, DU YuanPeng
College of Horticultural Science and Engineering, Shandong Agricultural University/State Key Laboratory of Crop Biology, Tai’an 271018, Shandong
【】The aim of this study was to explore the role ofin the salt tolerance mechanism of grapes, so as to provide a theoretical reference for the subsequent cultivation of new salt-tolerant varieties. 【】DANMAN and MEGA software were used to analyze the biological information of VviHKT. The strongly salt resistant rootstocks SA15, SA17 and the commonly used rootstock 1103P tissue cultured seedlings were used as materials. Seedlings were treated under 100 mmol·L-1NaCl for 0, 3, 6, 12, 24, 48 h, and the corresponding time of water treatment were taken as control. Real-time quantitative PCR (qRT-PCR) was used to detect the relative expression ofin the roots of grapes.was cloned from SA17 cDNA and then linked with pRI101-AN-GFP, and the inflorescence ofwas infected by. Subsequently, T3homozygous lines were screened out from resistant MS plates. Wild-type and transgenicseeds were sowed on MS plates and MS plates (150 mmol·L-1NaCl added), their germination and growth were observed, and the root length and fresh weight were counted. The SA17 transgenic grape roots were obtained byrhizogenes technology. After being treated with 100 mmol·L-1NaCl for 24 hours, the NMT in vivo physiological detector based on non-damaging micro-measurement technology was used to detect the net flow of Na+and K+instantaneous flow under salt stress in the roots of wild-type and transgenic grapes. 【】Multiple sequence alignment and phylogenetic tree analysis showed that VviHKT had high homology, among which theopen reading frame sequence length was 1 380 bp and it was the closest to VviHKT1;6. Salt stress significantly induced the expression ofgene in three varieties of grapes. Among them, the relative expression ofwas up-regulated, which was still increased after long-term stress. The relative expression ofreached the peak at 6 or 12 h under salt stress, and its relative expression in SA17 and SA15 was significantly higher than 1103P. Results of germination and growth experiments inshowed that there was no significant difference between wild-type and transgenicunder normal conditions, but the germination rate, root length and fresh weight of transgenicwere significantly higher than those of wild type under salt stress. Fluorescence detection experiments showed that green fluorescence could be seen in the transgenic grape roots under fluorescence, rather than in the wild-type roots. Further, qRT-PCR results also showed that the relative expression ofin the transgenic grape roots was 20-folds higher than that in the wild-type roots. The results of ion flow rate detection showed that the net flow of Na+both in wild-type and transgenic roots showed efflux under normal conditions. Besides, no significant difference was found between wild-type and transgenic roots (208 and 205 pmol·cm-2·s-1) and the fluctuation range of ion flow rate in each time period was small. After salt stress, the Na+net fluxes of them increased significantly, and the fluctuations in each time period also increased; the average net fluxes of wild-type and transgenic roots were 1 053 and 1 340 pmol·cm-2·s-1, respectively. Under normal conditions, the K+absorption and efflux of the two roots were in a dynamic equilibrium. Salt stress significantly induced K+efflux, and the efflux of K+in transgenic roots was significantly smaller than that in the wild type, which were 406 and 952 pmol·cm-2·s-1, respectively. The results indicated that the ability of removing Na+and keeping K+of transgenic roots was significantly greater than that of wild type. 【】played an important role in the response of grapes to salt stress, and the overexpression of this gene could improve the adaptability ofand grape roots under salt stress.
grape;; salt stress; transgene; functional identification

10.3864/j.issn.0578-1752.2021.09.012
2020-07-30;
2020-10-14
國家重點研發計劃(2019JZZY010727)、國家現代農業產業技術體系建設專項(CARS-29-zp-2)、山東省重大科技創新工程(2018CXG0306)
劉闖,E-mail:18364030521@163.com。通信作者杜遠鵬,E-mail:duyuanpeng001@163.com
(責任編輯 趙伶俐)

