A polypropylene melt-blown strategy for the facile and efficient membrane separation of oil–water mixtures
Zhenqiang Zhang,Danfeng Yu,,Xiubin Xu,Huayi Li,Taoyan Mao,Cheng Zheng,Jianjia Huang,Hui Yang,Zihan Niu,Xu Wu,
1 School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006,China
2 CAS Key Lab of Engineering Plastics,Institute of Chemistry,Chinese Academy of Sciences,Beijing 100190,China
3 CAS Key Lab of Colloid,Interface and Chemical Thermodynamics,Institute of Chemistry,Chinese Academy of Sciences,Beijing 100190,China
Keywords:Separation Polypropylene membranes Surface Superhydrophobicity Superoleophilicity Environment
ABSTRACT Porous materials with selective wettability and permeability have significant importance in oil–water separation,but complex fabrication processes are typically required to obtain the desired structures with suitable surface chemistry.In this work,an industrial melt-blown strategy that utilized commercially available polypropylene(PP)was used for the large-scale fabrication of superhydrophobic/superoleophilic membranes with staggered fabric structures.These membranes could readily separate different oils including pump oil and crude oil from various aqueous solutions such as strongly acidic,alkaline,and saline media.In addition,the separation efficiencies of these membranes exceeded 99%,and they could remain functional even after exposure to corrosive media.We anticipate that this work will further the design of membranes and enhance their applicability in oil–water separation,and provide researchers and engineers with a more effective tool for performing challenging separations and mitigating pollution.
1.Introduction
Oil–water separation has become a focal point due to the frequent occurrence of oil-spills as well as the leakage of oily wastewater and hydrophobic organic solvents[1–3].All of the above issues cause financial losses and can lead to long-lasting damage to ecosystems[4–6].Traditionally,several types of techniques,such as gravity separation,flocculation separation,and electrolysis separation have been utilized to separate oil–water mixtures[7–10].However,those conventional methods are highly energy-intensive and inefficient,which have severely limited their development and applicability.Recently,membranes with special wettability have attracted considerable attention on account of their excellent separation selectivity and efficiencies[11,12].
Special wetting membranes are typically fabricated by designing suitable compositions and constructing appropriate surface structures,which can be roughly classified according to their contrasting wettabilities:superhydrophobic/superoleophilic and superhydrophilic/underwater superoleophobic [13].Superhydrophobic/superoleophilic membranes can selectively allow the permeation of oil,while blocking the passage of water[14,15].Chen et al.used an electrodeposition process to fabricate micro/nanoscale structures on a copper mesh and grafted polydopamine onto it to enable oil–water separation [16].In the case of the superhydrophilic/underwater superoleophobic membranes,Huang et al.prepared a TiO2composite membrane with oil–water selectivity[17].In addition,Li et al.combined a polyurethane sponge with a superhydrophobic fabric bag to enable the absorption of oil from water[18].Currently,the majority of these preparation methods are relatively complex and they often require toxic or environmentally harmful fluorine-containing reagents,which have drastically impeded their adoption for oil–water separation.
Polypropylene(PP)has been used as an oil absorbent material and in filtration membranes owing to its affordability,hydrophobicity,oleophilicity,good chemical resistance and high impact strength.Various approaches have been proposed for the fabrication of superhydrophobic PP fabric membranes for oil–water separation,such as electrospinning,spray deposition,dip-coating,phase inversion methods,in situ growth approaches,and so forth[19–23].For instance,Huang et al.immersed PP fibers in a suspension of SiO2/graphene oxide and then used a chemical vapor deposition strategy to fabricate hydrophobic silanes on PP fibers[24].Xu et al.fabricated superhydrophobic PP fabrics via the formation of a polydopamine membrane on fabrics to allow the deposition of Ag nanoparticles,which was followed by the fluorination with 1H,1H,2H,2H-perfluorodecanethiol[25].Kansara et al.grafted hydrophobic SiO2particles that were modified by alkyl siloxanes of different alkyl chain types on non-woven PP fabric films to achieve oil–water separation[26].The nanoparticles that were introduced onto the substrates in the aforementioned works were easily detached,thereby causing these materials to lose their superhydrophobicity[27].The superwettable fabrics warrants serious consideration,because it provides recyclability and durability,thus extending the lifetime and reducing the production costs,while there is still only a limited understanding of the oil–water separation mechanisms involving fabric membranes[28–30].
Herein,we present a facile one-step melt-blown route for the fabrication of a superhydrophobic and superoleophilic PP fabric membrane(MBPP membrane) that can enable the efficient separation of oil–water mixtures.Melt-blown is a solvent-free and high-throughput fabrication process typically used in producing nonwovens[31].During melt blowing,an appropriate viscosity polymer melt is extruded through an orifice die and drawing down the extrudate with hot highvelocity air jets to form ultrafine fibers,which are followed by cooling below the solidification temperature and collecting with a continuous fiber collector[32].By taking advantage of the melt-blown route,ultrafine fibers are staggered to form a desired structure with microscale roughness and superwetting properties [33].The MBPP membranes can be used directly without any other modifications,exhibiting outstanding separation efficiency,high flux,high breakthrough pressure,excellent stability and reusability during the separation procedure in various harsh environments.Moreover,the selectivity and wetting mechanisms were investigated,and the separation efficiency and flux were compared with other works to show the great potential of the MBPP membranes in oil-spill remediation,organic solvent separation,and oily wastewater treatment.
2.Experimental
2.1.Materials
Syndiotactic PP(MW=1500 g·mol?1,Beijing Junyi New Material Technology Co.,Ltd.),n-Hexane and toluene were all of A.R.grade,and purchased from Tianjin Damao Chemical Reagent Factory.n-Hexadecane,methyl blue and tony red were all of A.R.grade,and purchased from Macklin Chemical Reagent Factory.Pump oil was obtained from Huaxin Chemical Reagent Co.,Ltd.Crude oil was acquired from CNOOC Research Institute Co.,Ltd.Meanwhile,peanut oil and canola oil were purchased from a local shop (Guangzhou,China)and used as received.
2.2.Preparation of the MBPP membranes
Syndiotactic PP with a melt flow rate(MFR)of 1500 g/10 min was heated and melted at 230 °C and then fed into a melt blowing die with 350 μm diameter holes.Subsequently,the molten polymer was drawn to diameters of 1000 to 10,000 nm by hot air jets (230 °C,0.12 MPa)and subsequently cooled via exposure to the ambient air.Melt-blown fiber mats were collected with a continuous fiber collector located at a distance of 40 cm away from the melt blowing die tip.The fibers were efficiently collected by a weak suction force that was generated by an induction fan behind the continuous fiber collector(the details regarding this procedure are shown in Fig.1).The extracted membranes with an average thickness of approximately 0.5 mm were dried in the air for 24 h prior to use.
2.3.Instruments and characterization
The morphological structures of the MBPP membrane were observed by a field emission scanning electron microscope (FE-SEM,JSM-7001F,JEOL).Water contact angles(WCAs)and oil contact angles(OCAs)were respectively measured using a contact angle measuring device(JC2000A,Zhongchen Powereach,Shanghai,China)with water and oil droplets(2.0 μl in volume)at ambient temperature.Each reported CA represents the average CA value of measurements performed at five different positions on the membrane.
The oil–water separation experiment was examined by initially fixing the MBPP membrane between two glass-vessels with diameters of 25 mm,and clamping it with a clip.Subsequently,the oil–water mixtures(v/v:20 ml/40 ml)were carefully poured into the right glass vessel,and the separation was achieved after the oil had permeated through the membrane to the left vessel.
The chemical resistance and stability of the membrane were evaluated by exploring the influence on the wettability and the separation efficiency of membranes after immersion in three types of corrosive media including acidic(HCl,pH~0),alkaline(NaOH,pH~14)and saturated saline(NaCl,1.0 mol·L-1)solutions for several hours.The thermal stability of the membrane was investigated by measuring the wettability of the membrane after it was placed at a certain temperature for 2 h.The recycling tests were conducted by measuring the changes in the flux and efficiency after multiple separations had been performed with the same membrane.With regard to the abrasion stability tests,200.0–1000.0 g of commercial silicas(425–800 μm in diameter)were employed to impose impact abrasion to the membrane samples from a height of 15 cm.After the abrasion tests,WCAs and oil–water separation efficiencies of the membranes were tested.
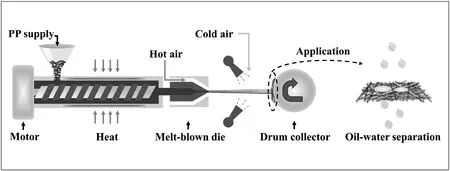
Fig.1.Schematic illustration of the protocol employed to fabricate the MBPP membrane via the melt-blown process.
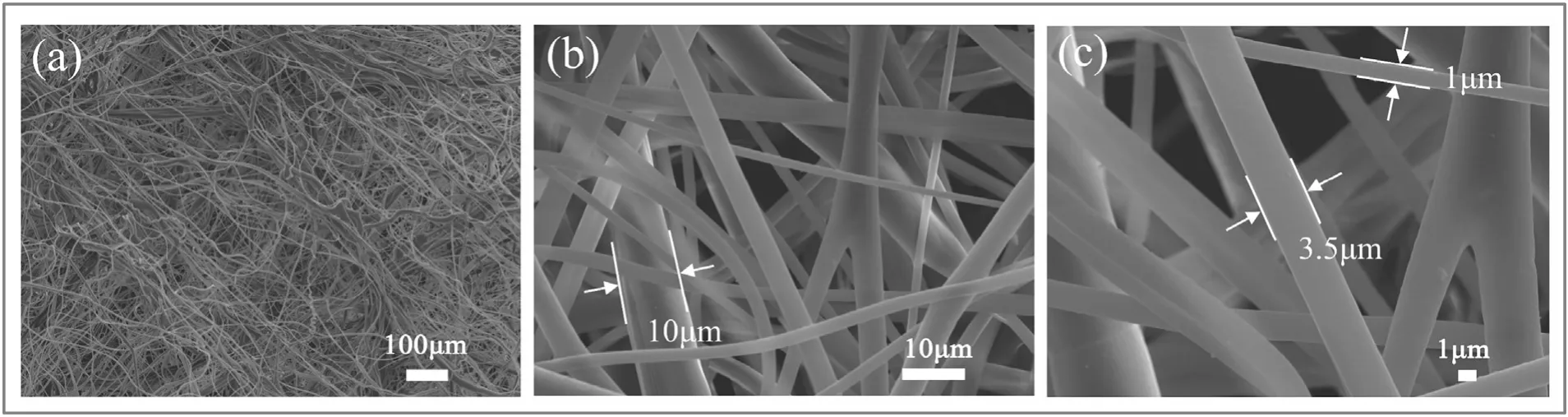
Fig.2.The 100×(a),1500×(b)and 3000×(c)FE-SEM images of the MBPP membrane.
3.Results and Discussion
3.1.Surface morphologies and wetting behavior of the MBPP membrane
The melt-blown route results in a large overall roughness through the arrangement of staggered fibers with different diameters.The morphologies of the MBPP membrane are shown in Fig.2(a)–(c),where it is readily apparent that the diameters of the melt-blown PP fibers were in the range of 1.0–10.0 μm.The roughness of fabric structures and dense microchannel structures can enhance the hydrobicity and oleophilicity of PP to the superhydrophobic and superoleophilic wettability[34].
The water was blocked outside the microchannels and the oil could spontaneously wet and pass through the membrane via the capillary effect[12].As shown in Fig.3(a),water droplets residing on the membrane exhibited a quasi-spherical shape,while n-hexane,pump oil,and peanut oil droplets rapidly diffused and permeated through the membrane.In addition,water droplets could readily roll off the surface of the membrane when it was tilted at a modest angle(Fig.3(b)and Movie S1).
The wettability of the MBPP membrane was further demonstrated in Fig.4(a)by the WCA and OCA of n-hexane,with the former exceeding 150° and the latter at nearly 0°,thus demonstrating both the superhydrophobicity and the superoleophilicity of the membrane.To investigate the superwetting mechanism of the melt-blown PP membrane,a wetting model was proposed as illustrated in Fig.4(b).PP fibers with different diameters exhibit a staggered arrangement to form a special microscale overall roughness,and the air becomes trapped in the gaps between these fibers.Once the water comes into contact with the surface,the hydrophobic PP fibers and the air play important roles in providing superwettability and preventing water penetration.Furthermore,the Cassie-Baxter model[16]was applied to demonstrate that fibers with different diameters interweave to produce a structure with special wettability behavior,as expressed by Eq.(1):

where θ and θ*denote the contact angle on the smooth surface and on the rough surface,respectively.Meanwhile,f indicates the state of the liquid that is in contact with air.According to Eq.(1),the f value of the MBPP membrane was 87.51%,which indicated that a significant amount of air was trapped in the fibers,increasing the water-air contact areas.The air content requirement and the roughness for changing a hydrophobic/oleophilic surface to a superhydrophobic/superoleophilic surface can be satisfied via a facile melt-blown route.
3.2.Oil–Water separation by the MBPP membrane
A series of experiments were conducted to demonstrate the oil–water separation performance of the MBPP membrane.As shown in Fig.5(a) and Movie S2,after an n-hexane-water mixture (v:v=20 ml:40 ml)was poured into the right glass vessel,the oil immediately permeated through the membrane to the left glass vessel while in contrast the passage of water was blocked.
The intrusion pressure,another factor that is used to evaluate the separation performance,was calculated via Eq.(2)[35]:

where ρwateris the density of water(kg·m?3),g denotes the gravitational acceleration(N·kg?1),and hmaxrepresents the maximum height of water that the membrane can support without penetration(m).As illustrated in Fig.5(b),hmaxwas~0.45 m,which corresponded to an intrusion pressure of 4.41 kPa.
The corresponding separation efficiencies for the various oil–water mixtures are presented in Fig.6(a).In particular,these values refer to the purity of the collected oil and are calculated from Eq.(3):

Fig.3.Photograph of water and various oil droplets on the surface of the MBPP membrane(a).Photograph taken as water droplets roll off the surface of the membrane(b).
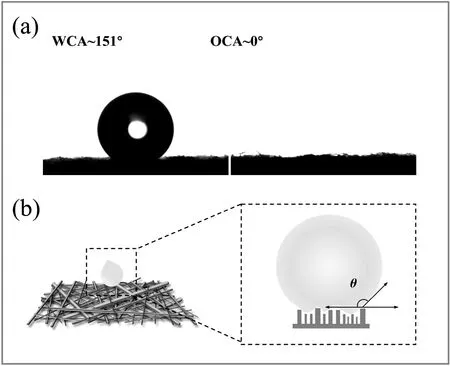
Fig.4.The contact angles of water and n-hexane on the MBPP membrane(a).The wetting model of the membrane with fabric and microchannel structures(b).

where w1and w2respectively denote the water and oil content in the filtrates after the oil–water separation(mg).
As shown in Fig.6a,the MBPP membrane exhibited excellent separation capabilities for various oil–water mixtures with high efficiencies(>99.3%),including n-hexane,toluene,n-hexadecane,peanut oil,canola oil,pump oil and crude oil.It is noteworthy that the viscosity of the respective oil has little effect on the separation efficiency of the MBPP membrane.Remarkably,the membrane provides very efficient separation of highly viscous oil–water mixtures(>99.5%)without requiring any external energy inputs,which has not been reported with previously developed devices.
Aside from the separation efficiency and intrusion pressure,the oil flux was also measured to further evaluate the separation performance of the membrane by recording the time that was required for the passage of a certain volume of oil.This flux was calculated via Eq.(4):

where F is the liquid flux(L·m?2·h?1),V denotes the liquid volume(L) permeating through the effective contact area of the membrane(4.91×10?4m2)expressed as S,and t represents the time required for the oil to pass through the membrane(h).
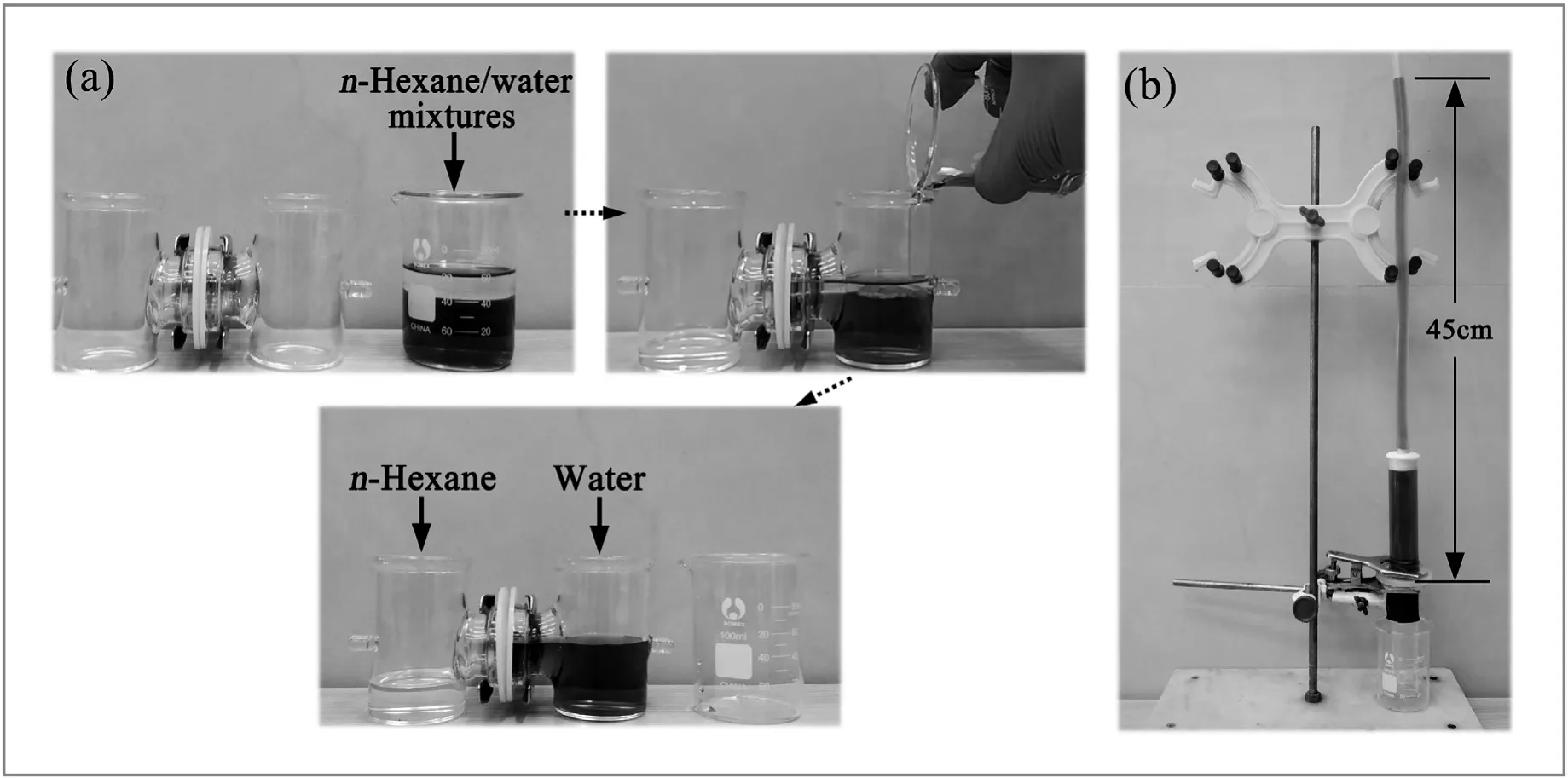
Fig.5.Oil–water separation experiments on the MBPP membrane(the water was dyed with methylene blue)(a).Intrusion pressure tests on the membrane(b).
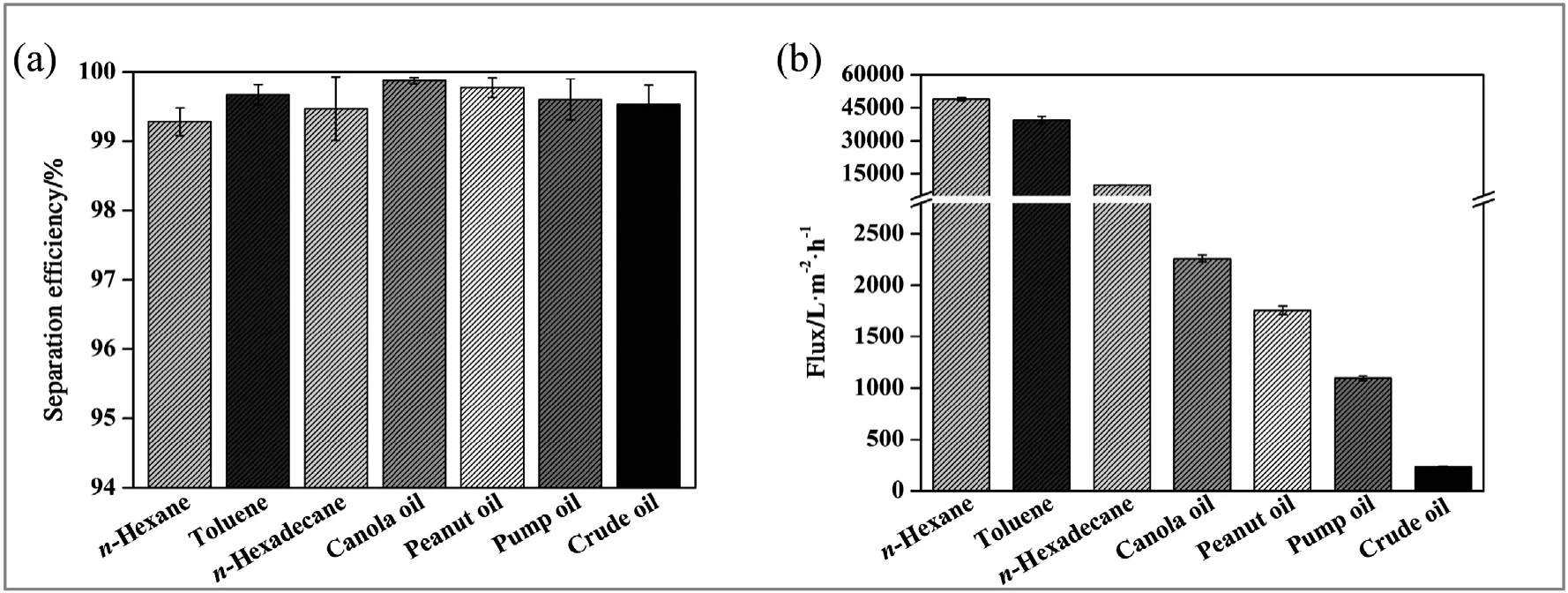
Fig.6.The oil–water separation efficiency(a)and oil flux(b)experiments on the MBPP membrane.
As shown in Fig.6(b),the flux of n-hexane(~0.3 MPa·s)and toluene(~0.5 MPa·s) both exceeded 27,000 L·m?2·h?1.Meanwhile,in the cases with n-hexadecane(~3.3 MPa·s),canola oil(~60 MPa·s),peanut oil(~80 MPa·s),pump oil(~200 MPa·s),and crude oil(~500 MPa·s),it was evident that the flux values decreased gradually with an increasing viscosity.The flux still exceeded 200 L·m?2·h?1even for crude oil,demonstrating the great potential applicability of the MBPP membrane for the separation of various viscous oils from water.
The Hagen–Poiseuille model is commonly used to further describe the transport mechanism across a membrane[36].Therefore,the volume flux of n-hexane and toluene through the MBPP membrane was described by the Hagen-Poiseuille equation.Assuming that all of the pores in the MBPP membrane have the same radii,this relationship can be described by Eq.(5)[37]:


where J is the theoretical liquid flux(L·m?2·h?1),ε denotes the porosity of the membrane,r represents the pore radius(m),ΔP is the applied pressure(Pa),μ denotes the viscosity of the oil(Pa·s),L is the thickness of the membrane(m),and τ represents the tortuosity factor.The porosity of the membrane ε could be described by Eq.(6)[38]:where Wddenotes the mass of a dry membrane(kg),Wwis the mass of a membrane after it has been wet by pure liquid(kg),ρwrepresents the density of the pure liquid (kg·m?3) at room temperature,and Vwdenotes the volume of a wet membrane(m3).The predicted porosity of the membrane is 0.81.The theoretical Jn-hexaneand Jtolueneas calculated with the Hagen–Poiseuille equation are 75,650 and 57,130 L·m?2·h?1,respectively.In comparison with the experimentally measured n-hexane flux of 48,900 L·m?2·h?1and toluene flux of 39,250 L·m?2·h?1,the theoretical flux values are higher because the oil droplets actually come into contact with the pores of the MBPP membrane gradually as time increases rather than coming into immediate contact with all of the pores.
With regard to the flux and separation efficiency,the toluene's experimental flux value of 39,250 L·m?2·h?1and separation efficiency of 99.5%are better than those reported in earlier literature,which are shown in Table 1.In contrast with some reports,the MBPP membrane can also separate oil from various harsh environments.It is noteworthy that with either low or high viscosity oil,the separation efficiency of the MBPP membrane exceeds 99.3%.The key factors providing this high flux were the special wettability in combination with abundance of crosslinked fibers on the MBPP membrane.Due to the presence of interconnected pores with tortuous paths in the membranes and the superhydrophobicity/superoleophilicity of these membranes[12,19],oil permeated rapidly through the MBPP membrane under gravity while the passage of the water phase was blocked.Furthermore,because of the presence of interconnected microscale pores in the membrane,the pores were filled with oil during the separation process,which would enhance the lipophilicity of oil and resistance of water[3].Therefore,the MBPP membrane enabled the rapid separation of oil and water mixtures while maintaining a high separation efficiency.
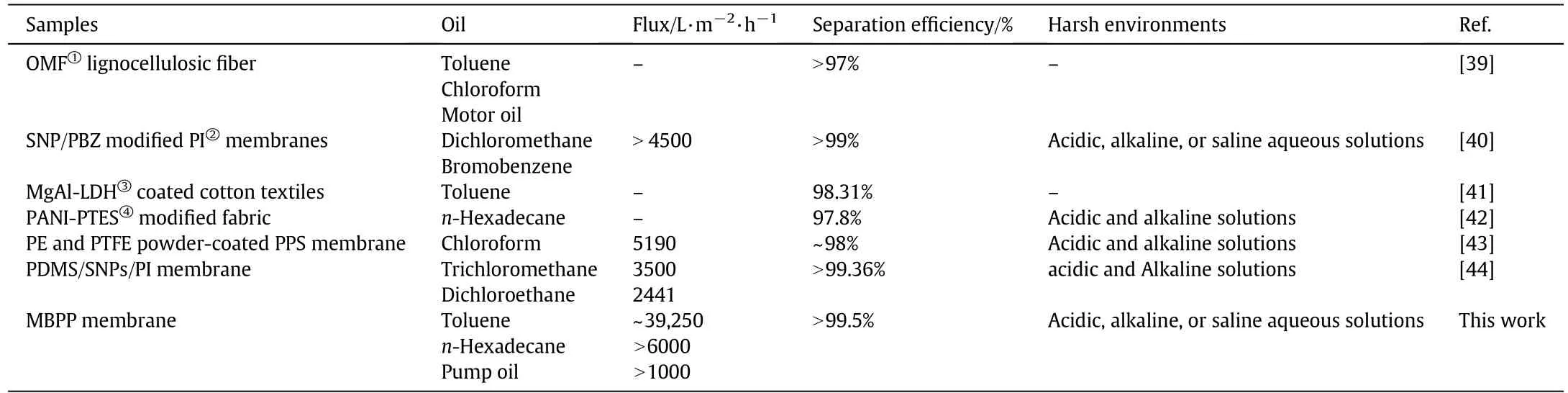
Table 1 Comparison of the flux and separation efficiency values with those found in other works
3.3.The chemical stability of the MBPP membrane
In the case of practical oil–water separation,it is crucial that filtration materials are resistant to extreme environments.Strikingly,our prepared membranes exhibited stable superhydrophobicity and high oil–water separation efficiency even up to exposure to various harsh environments,such as highly acidic,alkaline,and salt-containing liquids.
As shown in Fig.7(a),after the MBPP membrane had been immersed in different solutions for various durations(ranging from 0 to 48 h),the superhydrophobicity of the membrane did not change significantly,with the WCA remaining at~150°.These membranes were subsequently used to separate the corresponding mixtures of n-hexane-H2SO4(pH 0),n-hexane-NaOH(pH 14),and n-hexane-saturated NaCl,respectively.Remarkably,the corresponding separation efficiency of the membranes remained above 99.0%(Fig.7(b)),indicating that the membrane had stable superhydrophobicity in the corrosive solutions.The excellent corrosion resistance of the MBPP membranes was attributed to chemical inertness of polypropylene itself[45]and low corrosive liquid contact areas of the membranes.Moreover,the WCAs of the MBPP membrane did not change significantly after exposure to hightemperature environments or immersion in various oils(Fig.7(c)and(d)),further demonstrating the broad range of applications for the membrane.
3.4.Recyclability and durability of the MBPP membrane
To test the recyclability and durability of the MBPP membrane,recycling tests and silica abrasion tests were also performed.As illustrated in Fig.8(a) and (b),n-hexane was chosen as the sample to study the influence of the number of separation cycles on the flux and efficiency.As the number of separation cycles increased,the flux gradually diminished but remained above 37,000 L·m?2·h?1after 27 separation cycles.It is noteworthy that the flux was almost returned to its original state after it had received simple washing and drying treatment.Furthermore,the membrane can be reused for at least 20 cycles while retaining high efficiency (>99.0%),thus demonstrating the excellent recyclability of the as-prepared membrane.Meanwhile,the silica abrasion tests (Fig.8(c) and (d)) revealed that not only were the WCAs of the membrane not significantly influenced by abrasion,but it was also observed that the membrane maintained its high separation efficiency (>98.5%) after these tests.Moreover,Fig.8(e) showed that the MBPP membrane could support a 500 g mass without forming cracks,further demonstrating the mechanical durability of the membrane.Fig.8(f) presented a new application that the MBPP membrane could be used to recover oil floating on water at a rapid rate of 1.86 L·h?1due to its flexibility(the n-hexane was dyed with tony red).It also could be observed from Movie S3 that the oil permeated rapidly through the membrane while the water was blocked even under high water pressure.
4.Conclusions
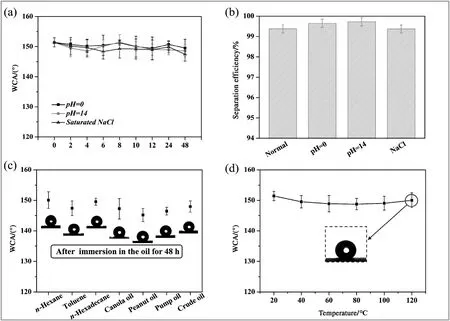
Fig.7.Changes in the WCAs of the MBPP membrane under various conditions(a).The n-hexane-water separation efficiency of the membranes after exposure to various harsh environments for 48 h,respectively.(b).WCAs of the membrane after immersion in various oils(c)or exposure to high temperature environments(d).
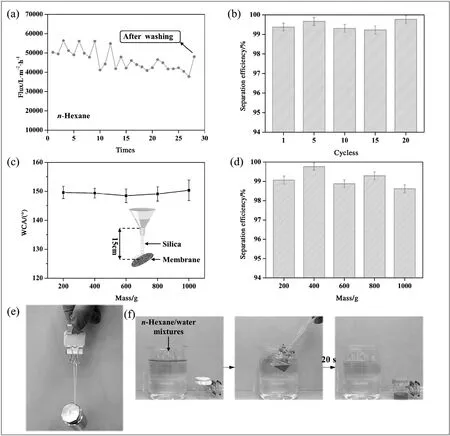
Fig.8.Changes in the flux(a)and separation efficiency(b)of the MBPP membrane after various separation cycles.The influence of different mass of silica abrasion on n-hexane-water separation efficiencies(c)and WCAs(d)of the membrane.The inset diagram illustrates the method employed for the silica abrasion test.Photograph of the membrane supporting a 500 g suspended mass(e).Photographs taken during the operation of using the membrane to recover oil from the water surface(f).
A facile,cost-effective,and environmentally-friendly melt-blown route has been developed to fabricate a superhydrophobic and superoleophilic PP membrane with outstanding oil–water separation performance,including excellent separation efficiency,high flux,and high intrusion pressure.The as-prepared membrane also showed excellent durability even after exposure to various harsh environments (highly acidic,alkaline,and saline media) and good recyclability that allowed the membrane to be reused for at least 20 cycles while still retaining high separation efficiency (>99.0%).The hydrophobicity of PP combined with the rough and porous structure that is formed by the interweaving of these low-surfaceenergy fibers of various diameters is the key to produce a membrane with selective permeation of oil rather than water.Utilizing the melt-blown strategy proposed in this work,commercially available PP can be used to fabricated superhydrophobic and superoleophilic membranes on a large scale,which can be directly applied to wastewater treatment,organic solvent separation,and oil-spill remediation.
Nomenclature
F Liquid flux,F=V/(S×t),L·m?2·h?1
f Air fractions of water/solid contact interface,%
g Gravitational acceleration,N·kg?1
hmaxMaximum height of water,m.
J Theoretical liquid flux,J=
L Thickness of the membrane,m
P Intrusion liquid pressure,P=ρwaterg hmax,kPa
R Purity of the collected oil,R=
r Pore radius,m
S Contact area of the membrane,m2
t Time required for the oil to pass through the membrane,h
V Liquid volume,L
VwVolume of a wet membrane,m3
WdMass of a dry membrane,kg
WwMass of a membrane after wetting by pure liquid,kg
w1Water content in the filtrates after the oil–water separation,mg
w2Oil content in the filtrates after the oil–water separation,mg
ΔP Applied pressure,Pa
ε Porosity of the membrane,ε=
θ Contact angle on the smooth surface,(°)
θ*Contact angle on the rough surface,cos θ?=?f+(1 ?f)cos θ,(°)
μ Viscosity of the oil,Pa·s
ρwaterDensity of water,kg·m?3
ρwDensity of the pure liquid at room temperature,kg·m?3
τ Tortuosity factor
Acknowledgements
We wish to thank the National Natural Science Foundations of China(Nos.21878059,21878058,21808044),the Science and Technology Project of Guangdong Province (2017A050501040),the Science and Technology Project of the Guangzhou Education Bureau(201831830,201831825)for sponsoring this research.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2020.03.033.
 Chinese Journal of Chemical Engineering2021年1期
Chinese Journal of Chemical Engineering2021年1期
- Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Preparation and properties of a low-cost porous ceramic support from low-grade palygorskite clay and silicon-carbide with vanadium pentoxide additives
- An efficient preparation of porous polymeric microspheres by solvent evaporation in foam phase
- Engineering practice and economic analysis of ozone oxidation wet denitrification technology
- Application of an immobilized microbial consortium for the treatment of pharmaceutical wastewater:Batch-wise and continuous studies
- Pyrolysis of single large biomass particle:Simulation and experiments
