吡嗪酰腙席夫堿單核Co(Ⅱ)配合物的合成與磁性
陳 瑜 王召東
(重慶文理學院化學與環境工程學院,環境材料與修復技術重慶市重點實驗室,重慶 402160)
0 Introduction
Spin-crossover(SCO)complexes have attracted much attention due to their promising applications in molecule-based memory devices,sensors and switches[1-2].Most of the work has focused on Fe(Ⅱ)[3-4]and Fe(Ⅲ)[5-6]complexes,there are still some mononuclear Co(Ⅱ)complexes with abrupt or gradual SCO properties based on derivatives of terpyridine(terpy)[7]and Schiff base ligands[8].The central Co(Ⅱ)ion is coordinated by N6[9-10]and N4O2donor sets[11].To the best of our knowledge,there are no reported Co(Ⅱ)SCO complexes with N2O4donor sets.The hydrazone Schiff base ligands have been well studied due to their ease of synthesis,modularity and various coordination modes with ketoenol tautomerism[12].Pyrazine-based hydrazone ligand H2L as shown in Scheme 1 has been used to construct single-molecule magnets(SMMs)with rare earth metals[13-17].As a complementary work to this ligand system,herein,we synthesized a mononuclear Co(Ⅱ)complex(NHEt3)[Co(HL)2]·3H2O(1)and characterized it with IR,thermogravimetric analysis(TGA),X-ray single diffraction and magnetic susceptibility.
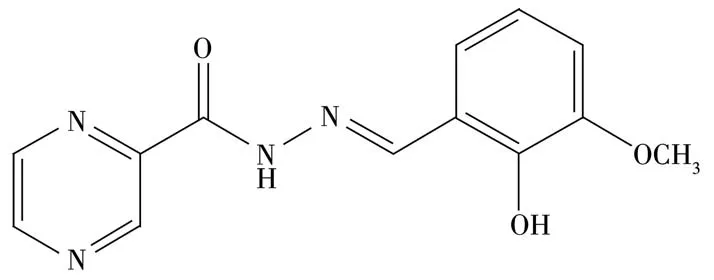
Scheme 1 Structure of H2L
1 Experimental
All chemical reagents were commercially available and used without further purification.H2L was synthesized according to the reported method[13].FT-IR spectra were recorded in a range of 4 000~400 cm-1on a PerkinElmer FT-IR spectrometer Frontier.Powder X-ray diffraction(PXRD)patterns were recorded on a Rigaku Smartlab X-Ray diffractometer with CuKαradiation(λ=0.154 178 nm,U=40 kV,I=26 mA)in a range of 5°~50°(2θ).Elemental analyses for C,H and N were measured on an Elementar Vario MICRO analyzer.TGA curves were measured in a Al2O3crucible using a PerkinElmer TGA instrument in a temperature range of 25~800℃ under a N2flow at a heating rate of 20℃·min-1.Magnetic susceptibility measurements for the samples were performed on the Quantum Design MPMS-XL instrument operating under a field of 1 000 Oe in a temperature range of 2~300 K.
1.1 Synthesis of(NHEt3)[Co(HL)2]·3H2O(1)
The methanol solution of CoC2O4·2H2O(0.035 5 g,0.20 mmol)was added dropwise to the mixed solution of H2L(0.054 5 g,0.20 mmol)in CH3OH and CH2Cl2with the addition of NEt3(5 drops)as a deprotonation reagent.The color of the mixture changed from yellow to brown slurring.After stirring for 6 h and the filtration,reddish brown filtrate was obtained.After slow evaporation of the filtrate for two weeks,needle like brown crystals were obtained for X-ray diffraction analysis.Yield:52%(based on CoC2O4·2H2O).Anal.Calcd.for C32H43CoN9O9(%):C,50.79;H,5.73;N,16.66.Found(%):C,51.36;H,5.28;N,17.27.IR(KBr,cm-1):3 431(s),2 934(w),2 831(w),2 681(w),1 623(s),1 600(s),1 534(s),1 475(s),1 437(s),1 345(m),1 243(w),1 219(s),1153(s),1 083(m),1 019(m),976(m),934(m),858(w),744(m),643(w),581(w),453(w).
1.2 Structure determination
Single-crystal diffraction data were recorded on a Bruker SMART APEXⅡCCD diffractometer with MoKα(λ=0.071 073 nm)radiation at 298 K.The crystal structure was solved by direct methods and refined by full-matrix least-squares method onF2using the SHELXTL 2014/7 program and OLEX2[18-19].All nonhydrogen atoms were refined anisotropically.Hydrogen atoms on water molecules were generated by the Q peaks.A water solvent molecule was severely disordered without adding hydrogen atoms and was modeled using the squeeze program.The details of singlecrystal diffraction data and selected bond lengths and bond angles are listed in Table 1 and 2,respectively.
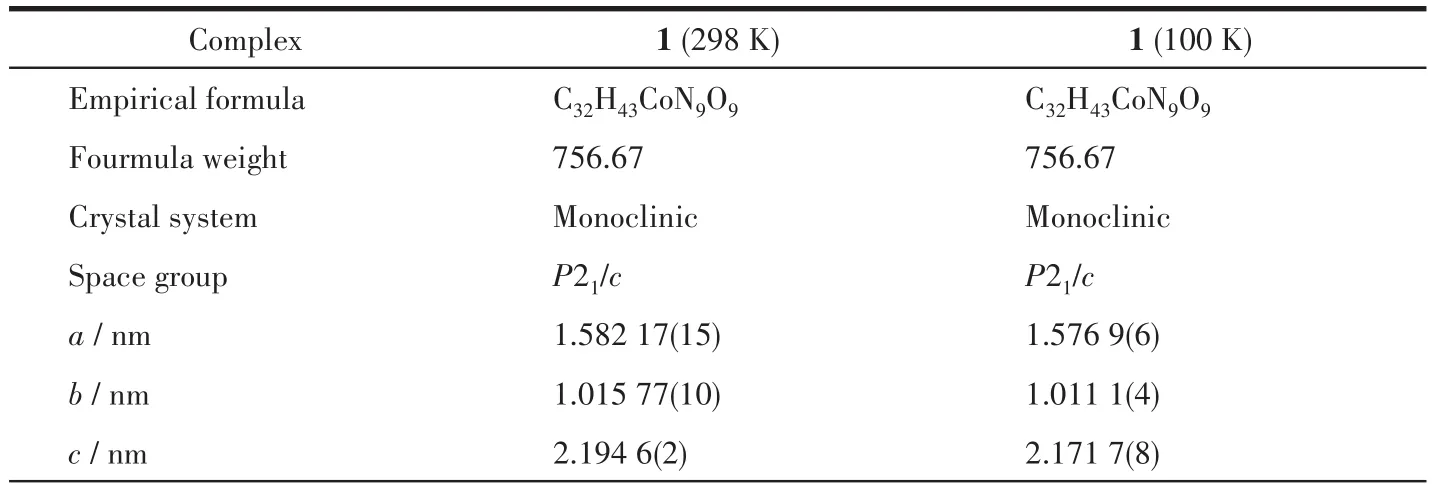
Table 1 Crystal data and structure refinement for complex 1

Continued Table 1
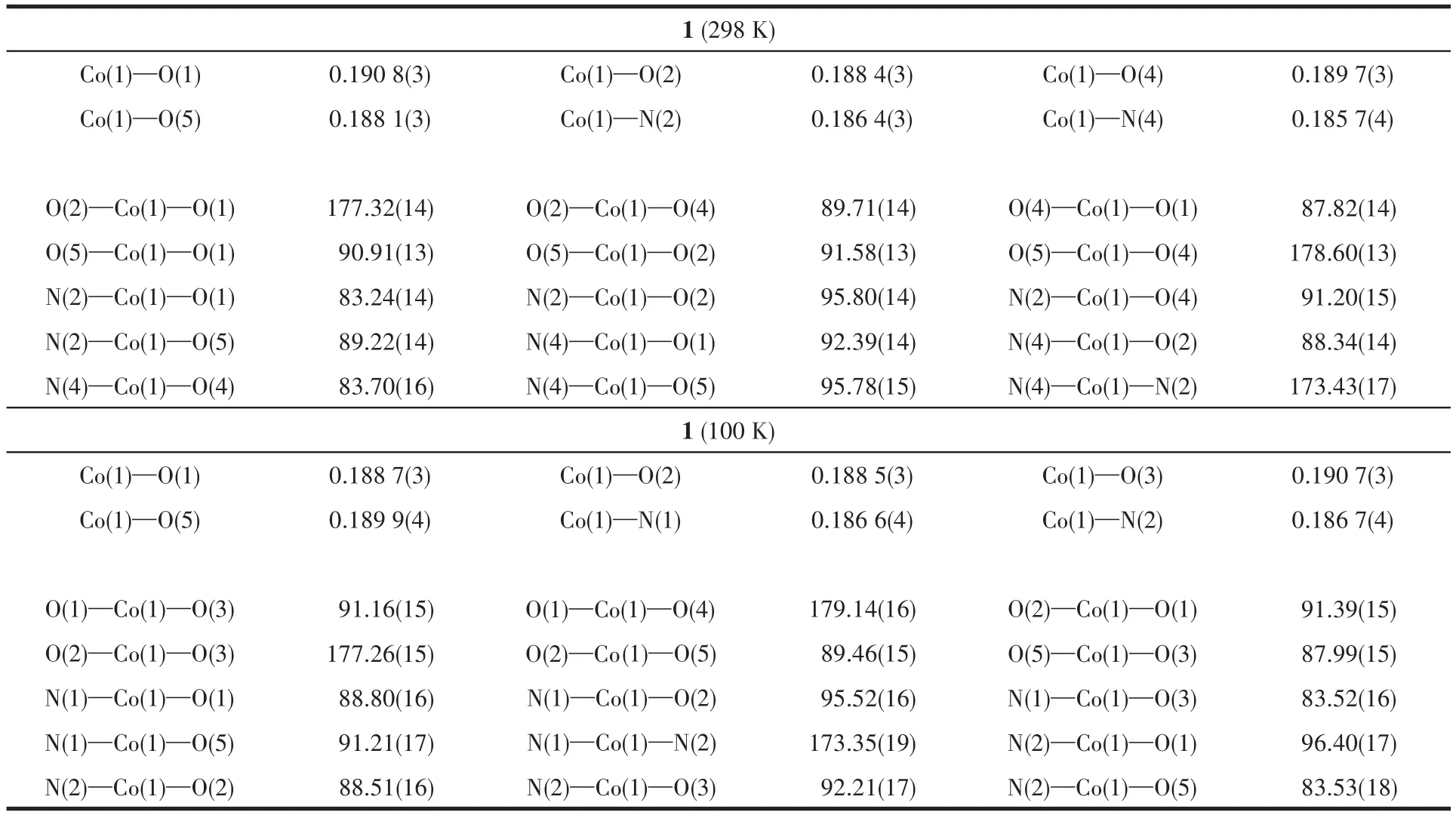
Table 2 Selected bond lengths(nm)and angles(°)for complex 1
CCDC:1973542,1(298 K);20274281,1(100 K).
2 Results and discussion
2.1 Crystal structure
Single-crystal X-ray diffraction analysis reveals that 1 crystallizes in monoclinic system,space groupP21/c.The coordination environment of Co(Ⅱ)in 1 is shown in Fig.1.It consists of a Co(Ⅱ)ion,two deprotonated HL-,one protonated NEt3and three free H2O molecules.The central Co(Ⅱ)is coordinated by two nitrogen atoms and four oxygen atoms from HL-.There could be amido-enol tautomerism in one HL-ligand and the enol was deprotonated in order to balance the charge of the complex.The Co(Ⅱ)ion has a distorted octahedral coordination geometry where four oxygen donors form the basal plane,and the two nitrogen donors occupy the apical position.The distortion parameter,∑,is defined as the sum of the deviation from the 12cis-O—Co—O,N—Co—O,N—Co—N angels[1].The smaller∑value of 35.63°for 1 shows a slightly distorted octahedral geometry.The Co—N bond lengths(Co1—N2 0.186 4(3)nm,Co1—N4 0.185 7(4)nm)are slightly shorter than the reported Co complex with N2O4donor sets.While the Co—O bond lengths(Co1—O1 0.190 8(3)nm,Co1—O2 0.188 4(3)nm,Co1—O4 0.189 7(4)nm,Co1—O5 0.188 1(3)nm)are comparable to the reported Co complex[20].We also determined the crystal structure of 1 at 100 K as shown in Fig.1,it reveals the same structure composition and coordination environment of the central Co ion.The bond lengths of Co—O and Co—N are slightly different with 1 at 298 K.The∑value of 37.29°also shows a slightly distorted octahedral geometry.The free water molecules form O—H…O hydrogen bonds with O from phenol and methoxy group as shown in Fig.2.There are also O—H…N hydrogen bonds with N from pyrazine N and N atom from the protonated triethylamine.

Fig.1 Coordination environment of Co(Ⅱ)ion in complex 1(left:298 K,right:100 K)with 50% probability thermal ellipsoids
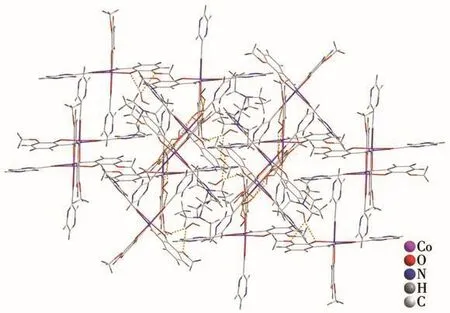
Fig.2 Packing structure of complex 1(298 K)with hydrogen bonding
2.2 PXRD patterns and TGA analysis
PXRD pattern of the complex was determined at room temperature to check the phase purity as shown in Fig.3.The peak positions of the experimental PXRD match well with the simulated one,indicating the purity of the complex.

Fig.3 PXRD patterns for 1
TGA of 1 was carried out in a range of 25~800 ℃under nitrogen atmosphere.As shown in Fig.4,the first weight loss of 6.9% from 95 to 185℃is attributed to the loss of free H2O molecules(Calcd.6.89%).The second weight loss of 13.1% from 195 to 260℃is attributed to the loss of one protonated NEt3(Calcd.13.7%).After that,the complex began to decompose.

Fig.4 TGA curve of 1
2.3 Magnetic analysis of complex 1 and dehydrated 1
The magnetic susceptibilitiesχmof 1 and dehydrated 1 were measured in the 2~300 K temperature range.TheχmTversusTplots for both in cooling and heating mode are shown in Fig.5.TheχmTvalue of 1.40 cm3·mol-1·K was lower than the theoretical value of 1.88 cm3·mol-1·K of the high-spin mononuclear Co(Ⅱ),indicating a mixture of high-spin and low-spin state.TheχmTdecreased gradually as the temperature lowered.It reached 0.848 cm3·mol-1·K at about 168 K,then it began to increase slowly to 0.935 cm3·mol-1·K at 147 K,indicating a slightly reverse spin transition due to possible phase changes during this period of temperature range[21-23].This was confirmed by the slightly different bond lengths and the∑value for complex 1 at 298 K and 100 K.TheχmTwas 0.44 cm3·mol-1·K at 59 K corresponding to the mononuclear Co(Ⅱ)low-spin state,and it decreased with decreasing temperature until it reached about 0.02 cm3·mol-1·K at 2 K,there was a small hysteresis loop in the cooling and heating mode as shown in Fig.5.In order to check the solvent effects on the magnetic properties,the dehydrated complex 1 was obtained by annealing 1 at 115 ℃ for 6 hin vacuo.TheχmTvalue of 0.89 cm3·mol-1·K for dehydrated 1 was significantly lower than the theoretical value of 1.88 cm3·mol-1·K of the highspin mononuclear Co(Ⅱ),indicating a majority of lowspin state.TheχmTdecreased gradually as the temperature lowered.It reached 0.43 cm3·mol-1·K at about 78 K corresponding to the mononuclear Co(Ⅱ)low-spin state.It also kept decreasing toca.0.02 cm3·mol-1·K at 2 K,and no hysteresis loop was observed in the cooling and heating mode.The difference of 1 and the dehydrated 1 can be assigned as the hydrogen bonding effects on the magnetic properties[24].
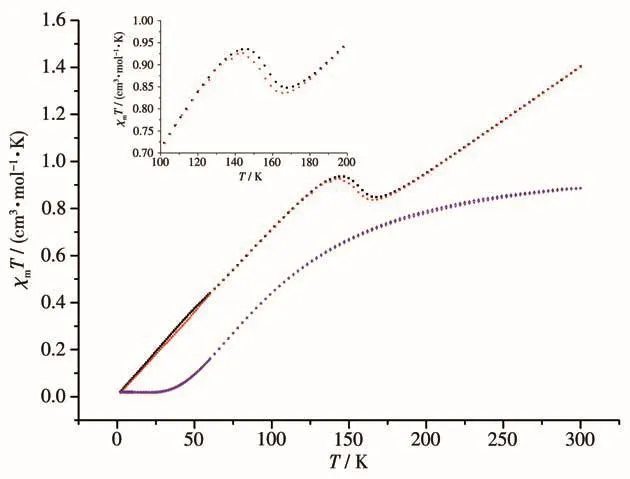
Fig.5 χmT vs T plots for complex 1(cooling:black,heating:red)and dehydrated 1(cooling:blue,heating:pink)in cooling and heating mode
3 Conclusions
In summary,we have successfully synthesized and characterized a new mononuclear Co(Ⅱ)complex based on a pyrazine-containing hydrazone Schiff base ligand with rare N2O4donor sets.The complex 1 exhibited a gradual spin transition with an unusual reverse spin state transition due to possible phase changes.The dehydrated 1 also showed a gradual spin transition,and the differences can be assigned as the hydrogen bonding effects on the magnetic properties.

