Protective effects of Fufang Ejiao Jiang against aplastic anemia assessed by network pharmacology and metabolomics strategy
HE Dan, ZHANG Haichao, YI Ziyang, ZHAO Di, ZHANG Shuihan
Hunan academy of Chinese Medicine, Hunan University of Chinese Medicine Changsha, Hunan 410013, China
Keywords Fufang Ejiao Jiang (復(fù)方阿膠漿,F(xiàn)FEJJ)Aplastic anemia Network pharmacology Metabolomics Lipid metabolomics Hematopoiesis microenvironment Acetylphenylhydrazine Cetylphenylhydrazine
ABSTRACT Objective To elucidate the mechanisms underlying the therapeutic effects of Fufang Ejiao Jiang (復(fù)方阿膠漿,F(xiàn)FEJJ) on aplastic anemia (AA) using integrated network pharmacology and serum metabolomics.Methods Traditional Chinese Medicine Systems Pharmacology(TCMSP), Pubmed, integrative pharmacology-based research platform of traditional Chinese medicine (TCMIP), and Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM) were used to identify the constituents and putative targets of FFEJJ.GeneCards and Dis-GeNET databases were used to identify AA-associated targets.We constructed a herb-component-target network and analyzed the protein-protein interaction (PPI) network.Potential mechanisms were determined using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses.In addition, an AA model was established using acetylphenylhydrazine (APH) and cetylphenylhydrazine (CTX).Ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS)-based serum metabolomics was applied to screen potential metabolites and the related pathways associated with AA and the potential anti-anemic effects of FFEJJ.Results A total of 30 active components of FFEJJ and 24 targets were related to AA.PPI network analysis showed that VEGFA,AKT1, IL-6, CASP3, and ICAM1 were key nodes overlapping with proteins known to be related to AA.KEGG pathway enrichment analysis revealed that the presumed targets of FFEJJ were mainly associated with pathways linked to the promotion of hematopoiesis and improvement of the hematopoietic microenvironment.A total of 423 metabolite biomarkers were identified between the control and AA models, which are involved in the development of AA.In contrast, FFEJJ reversed the 79 differential metabolites altered by AA.Pathway analysis suggested that the synergistic effects of FFEJJ were mainly enriched in 24 metabolic pathways.Among them, sphingolipid metabolism, glycerophospholipid metabolism, and arachidonic acid metabolism were related to promoting hematopoiesis and improving the hematopoietic microenvironment, which partially conforms with network pharmacology.The interaction network formed by three key differential metabolites, including hydroxy-eicosatetraenoic acid(HETE), sphingosine 1-phosphate (S1P), and lysophosphatidylcholine (lysoPC), and three predicted network targets (VEGFA,CASP3, and ICAM1) may be the potential mechanism underlying the anti-AA action of the multi-component of FFEJJ.Conclusion FFEJJ could be an alternative treatment option for AA.It acts by promoting hematopoiesis and improving the hematopoietic microenvironment.Network pharmacology-integrated metabolomics makes it possible to analyze TCMs from a systems perspective and at the molecular level.
1 Introduction
Aplastic anemia (AA) is a hematological disorder with subtle onset caused by various factors.Bone marrow hematopoietic failure is the main clinical manifestation of this condition[1].Moreover, it is responsible for high morbidity and mortality in the Asian population[2-4].Although frontline therapies such as immunosuppressive therapy (IST) and bone marrow transplantation (BMT) have been applied in the last decade, side effects and recurrence rates remain high[5].Thus, it is necessary to develop new and effective therapeutic strategies for AA.In addition, due to the multiple pathological mechanisms of AA, combination treatments instead of monotherapy should be considered.
Traditional Chinese medicine (TCM), particularly the TCM formula with multiple herbs, has become an important factor in the treatment and prevention of AA[6].According to TCM, AA is a physical condition that lacks Qi and blood.Fufang Ejiao Jiang(復(fù)方阿膠漿,F(xiàn)FEJJ)is characterized as having therapeutic efficacy against leukopenia and anemia,consisting of five herbs: Ejiao (Asini Corii Colla),Hongshen (Ginseng Radix et Rhizoma Rubra), Shudihuang (Rehmanniae Radix Preparata), Dangshen(Codonopsis Radix), and Shanzha (Crataegi Fructus).It is a commonly used prescription for “nourishing Qi and nourishing blood”.Previous studies have shown that FFEJJ alleviates myelosuppression by increasing the proliferation of hematopoietic stem cells (HSCs)and improving the bone marrow microenvironment[7,8].However, the therapeutic mechanism of FFEJJ in AA still needs further investigation to promote progress in the clinical application and development of FFEJJ.
Recently, network pharmacology combined with metabolomics has been widely employed to explore the molecular mechanisms of TCM at a holistic level.Several studies have indicated that network pharmacology enables the identification of drug-gene-disease co-module associations in a high-throughput manner[9-11].Furthermore, its "network target, multicomponents" is relatively consistent with the synergistic multi-compound characteristics of TCM.Metabonomics study plays an important role in comprehensively surveying the endogenous metabolites of the organism or organ and their relationship with internal or extrinsic factors.This combination is powerful for identifying the active components and mechanisms of action of TCMs.
In this study, network pharmacology was applied to identify the potential bioactive components in FFEJJ and elucidate its therapeutic mechanisms in AA.The metabolomics method was used to monitor alterations in endogenous substances after treatment with FFEJJ, aiming for an experimental gist associated with the metabolic mechanism of FFEJJ in AA.We performed network pharmacology and metabolomics analysis to elucidate the molecular mechanism of FFEJJ in AA.
2 Materials and methods
2.1 Database and software
(1) Herbs and components database: Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://lsp.nwu.edu.cn/tcmsp.php, ver.2.3), PubMed (https://www.ncbi.nlm.nih.gov/pubmed),Integrative Pharmacologybased Research Platform of Traditional Chinese Medi cine (TCMIP, v2.0, http://www.tcmip.cn), and Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM, http://bionet.ncpsb.org.cn/batman-tcm/).(2) Disease databases: GeneCards database (https://www.genecards.org/ ver.5.2) and the DisGeNET database (http://www.disgenet.org, last update: May 2020).(3) Protein database UniProt database (http://www.rcsb.org/).(4) Net work visualization database and software: STRING online database (https://string-db.org/), Cytoscape 3.7.2 software, and OmicShare cloud platform (http://www.omicshare.com).(5)Enrichment database:Database for Annotation, Visualization, and Integrated Discovery (DAVID) platform (https://david.ncifcrf.gov/) and MetaboAnalyst (https://www.metaboanalyst.ca/MetaboAnalyst/, ver.5.0).(6) Metabolite identification database: Human Metabolome Database (HMDB, http://www.hmdb.ca/), METLIN(http://metlin.scripps.edu).
2.2 Network pharmacology analysis
2.2.1 The prediction of potential targets for FFEJJ and AABased on the TCMSP database, the active components and targets of FFEJJ were identified based on oral bioavailability (OB ≥ 30%) and druglike (DL ≥ 0.18) characteristics.GeneCards and Dis-GeNet databases were employed to obtain the AAcorrelated therapeutic targets, and the keywords were set as “aplastic anemia” and “Homo sapiens”.
2.2.2 Enrichment analysisGene Ontology (GO)function and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed on the DAVID platform.The species was also set as“Homo sapiens”.The OmicShare cloud platform was used to visualize the histograms and bubble map charts.
2.2.3 Construction of networkThe component-targets-pathway network was established and visualized using Cytoscape 3.7.2 software.A protein-protein interaction (PPI) network was constructed using STRING.Topological parameters [including the degree and betweenness centrality (BC)] of the node were calculated to identify hub components, key targets, and pathways.
2.3 Metabolic analysis
2.3.1 Animal experimentsTwenty-one male specific pathogen-free (SPF) grade Sprague-Dawley (SD)rats aged eight weeks weighing 200 – 220 g were purchased from Hunan SJAC Laboratory Animal Co.,Ltd.(No.43004700058820).Before the experiment,animals were adaptively maintained and housed for one week in a SPF-grade facility at Hunan Academy of Chinese Medicine (License No.SYXK [Xiang] 2020-0008).All animal experiments were performed in accordance with the protocols and guidelines approved by the Animal Ethics Committee of the Hunan Academy of Chinese Medicine (Approval No.20190041).
After adaptation, the SD rats were randomized into three groups: control group (n=7), model group(n=7), and FFEJJ treatment group at a dose of 5.4 mL/kg (n=7)[12].On the 8th day of drug administration, the model and FFEJJ groups received an intraperitoneal injection of 30 mg/kg, 1-acetyl-2-phenylhydrazine (APH).On the 12th day, 100 mg/kg cyclophosphamide (CTX) +APH was injected[13].The control group was injected equal amounts of normal saline and distilled water.All the rats were euthanized after drug treatment.In a previous study, histological examination and peripheral blood(PB)routine tests were applied to evaluate the quantity of blood cells and the AA model[12](Figure 1).
2.3.2 Sample collection and preparationThe following procedures were used to determine serum metabolism.First, 100 μL serum samples were thawed at 4 °C for 30 min.300 μL pre-cooling mixture of methanol and acetonitrile (2/1, v/v) was added to the sample to completely remove the protein.In addition, 10 μL internal standard (0.3 mg/mL 2-chloro-l-phenylalanine (HPLC ≥ 98%, Adamasbeta) was also dispersed in this mixture.Thereafter,they were vortexed for 30 s.Subsequently, the above mixture was continued at 13 000 rpm and 4 °C for 15 min.The supernatant was filtered through a microfilter (0.22 μm).Finally, 10 μL supernatant was detected by ultra-performance liquid chromatographyquadrupole time-of-flight mass spectrometry (UPLCQTOF/MS).All samples were maintained at 4 °C prior to analysis.
Before sample preparation, quality control (QC)was prepared using an equal volume (100 μL) of samples from each group to evaluate the repeatability of the experimental methods and ensure the reliability of the metabolomic data.In our study, one QC sample was inserted into every six samples.Moreover, some ions were removed when their relative abundance was less than 30% of the QC.
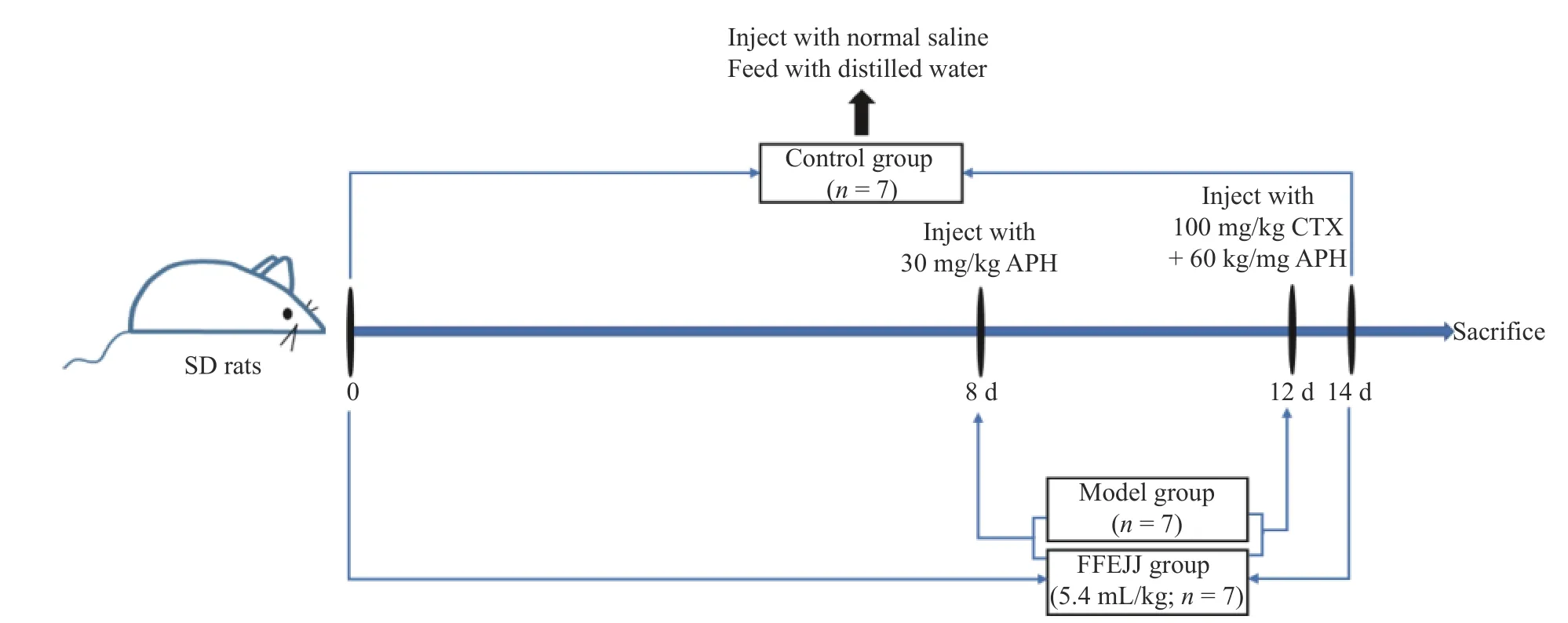
Figure 1 Animal experiments flow chart
2.3.3 UHPLC-MS analysisSerum metabolic profiles were obtained using a UPLC-QTOF/MS system in positive and negative ESI ion modes.An ACQUITY UPLC BEH C18 column (100 mm ×2.1 mm, 1.7 μm,Waters, USA) was used to optimize the gradient elution.In addition, 0.1 % formic acid water (A) and methanol (B) were used as the mobile phases.The working process is as follows: 0.01 – 1.5 min, 5% B; 1.5 –3 min, 5% – 30% B; 3 – 7 min, 30% – 60% B; 7 – 9 min,60% – 90% B; 9 – 11 min, 90% – 100% B; 11 – 12 min,100% B; 12 – 15 min, 100% – 5% B.The injection volume,flow rate column, and temperature were 10 μL,0.35 mL/min, and 45 °C, respectively.The following are the mass spectrum parameters for this experiment: the ion source temperature was set at 320 °C( + ) and 320 °C (?); ion spray voltage was 3 500 V ( + )and 3 100 V (?); curtain gas, 30 PSI; mass scan, 60 –900 ( + ) and 60 – 900 (?); and collision energy was 30 eV.
2.3.4 Identification and quantitative analysisPrognosis QI software (Waters Corporation, Milford,USA) was used to calibrate the peak, including alignment, peak picking, and deconvolution[14].Based on the human metabolome database (HMDB) and a metabolite mass spectral database (METLIN), metabolites were identified according to the characteristic rule of retention time (RT), m/z, and the ionic fragments of MS/MS results[15,16].The relative content of each metabolite was calculated by comparing the relative peak areas of the internal standard samples.The identification of biomarkers was enriched on the MetaboAnalyst platform.
2.4 Multivariate statistical analysis
Attest was used to identify significantly changed metabolites.Hierarchical cluster analysis (HCA) and orthogonal partial least squares discriminant analysis(OPLS-DA) were employed to distinguish different groups (model group, control group, and FFEJJ group)[17,18].Potential biomarkers were required to meet the variable importance projection (VIP) >1 andP< 0.05.
3 Results
3.1 Network pharmacology analysis
3.1.1 The identification of chemical compounds and potential targets for FFEJJ on AAA total of 301 chemical compounds in FFEJJ were identified from the TCMSP, TCMIP, and BATMAN-TCM databases.Among them, 74 chemical compounds were present in Hongshen (Ginseng Radix et Rhizoma Rubra), 76 in Shudihuang (Rehmanniae Radix Preparata), 134 in Dangshen (Codonopsis Radix), and 32 in Shanzha(Crataegi Fructus).Of the 301 chemical compounds,we further screened 22 active components that conformed to OB ≥ 30% and DL ≥ 0.18 (Table 1).In addition, 17 amino acids in Ejiao (Asini Corii Colla)were identified using PubMed.Four chemical compounds, including arginine, histidine, lysine, and threonine, were taken into account in the network pharmacology analysis[19-22].According to previous study, four compounds (including panaxynol, ginsenoside rf, ginsenoside rh2, and panaxydol) in Hongshen (Ginseng Radix et Rhizoma Rubra) were also selected as presumptive active components[23].For the 30 active components in FFEJJ, the TCMSP and UniProt databases were used to identify 138 protein targets.Among these targets, 21 were related to Hongshen (Ginseng Radix et Rhizoma Rubra), 39 to Ejiao (Asini Corii Colla), 26 to Shudihuang (Rehmanniae Radix Preparata), 91 to Dangshen (Cadonopsis Radix), and 21 to Shanzha (Crataegi Fructus).The details are listed in Table 2.A total of 238 targets and 651 targets for AA were identified using the DisGe-NET and GeneCards databases, respectively.There were 133 overlapping targets in the above two databases.Consequently, after eliminating redundancy,24 targets were found to be related to the 30 active components in FFEJJ and were associated with AA.
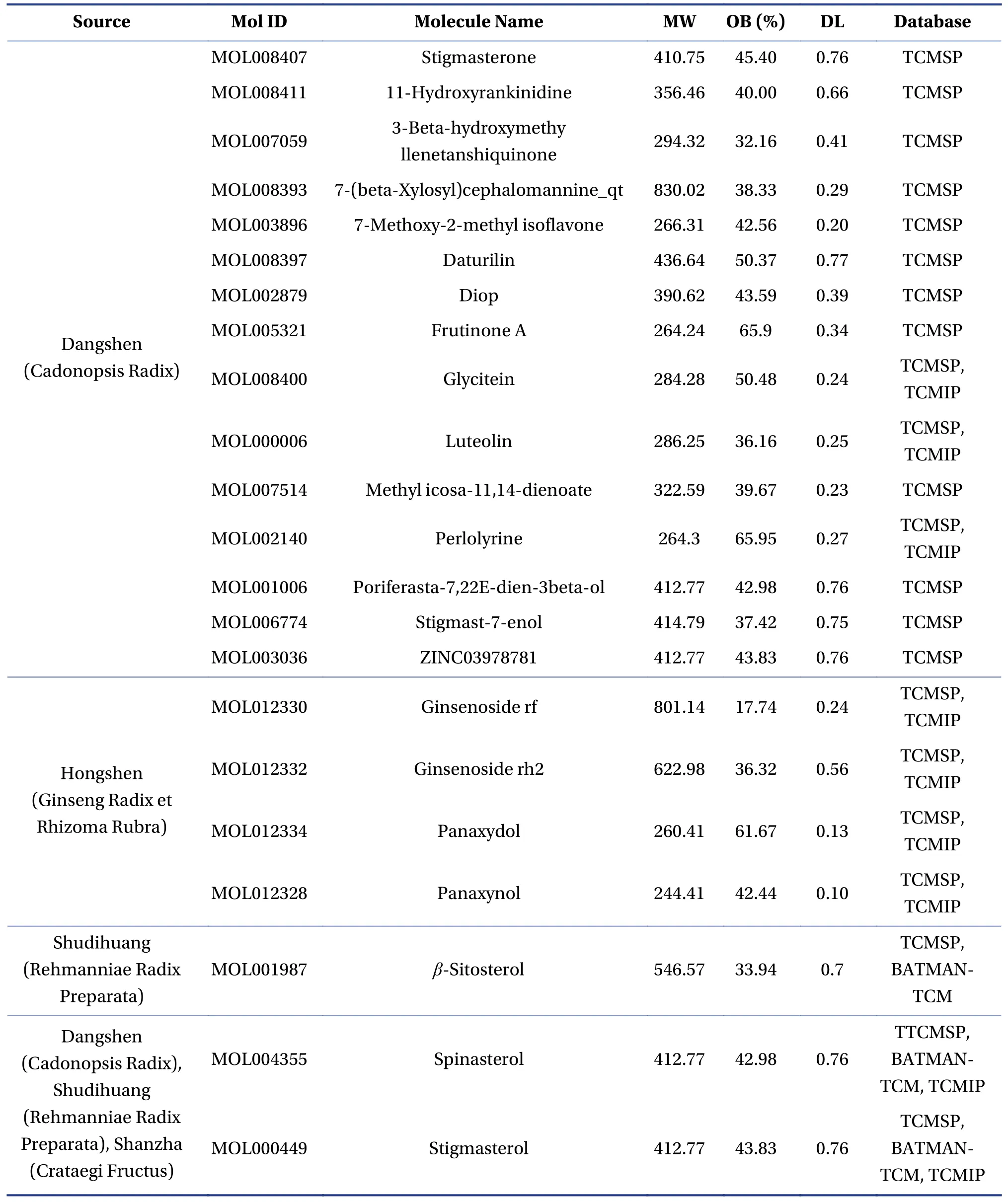
Table 1 Compounds information of FFEJJ
3.1.2 Herbs-components-targets network analysisOur study established an herbs-components-targets(containing 168 nodes and 273 edges) network (Figure 2A) for FFEJJ via Cytoscape.The topological analysis showed that 10 components with degree >5 were involved in the regulation of multiple targets,including three for flavonoids (luteolin, 7-Methoxy-2-methyl isoflavone, and glycitein), 3 for sterols (stigmasterol, ginsenoside rh2, and ginsenoside rf), and 4 for others (3-beta-hydroxymethyllenetanshiquinone,frutinone A, panaxynol, and panaxydol).These findings suggest that FFEJJ works through multiple components and targets.
3.1.3 PPI networkRepetitive targets between the GeneCards and the DisGeNET databases were excluded, and 756 AA-correlated targets were collected.To identify the targets with essential contributions,we imported the 24 co-targets of FFEJJ-AA and constructed FFEJJ-AA PPI networks via STRING (Figure 2B and 2C).Our PPI network showed that the top five proteins most closely associated with the anti-AA effects of FFEJJ were VEGFA, AKT1, IL-6, CASP3, and ICAM1, which conformed to the results of previous animal experiments (Figure 2D).
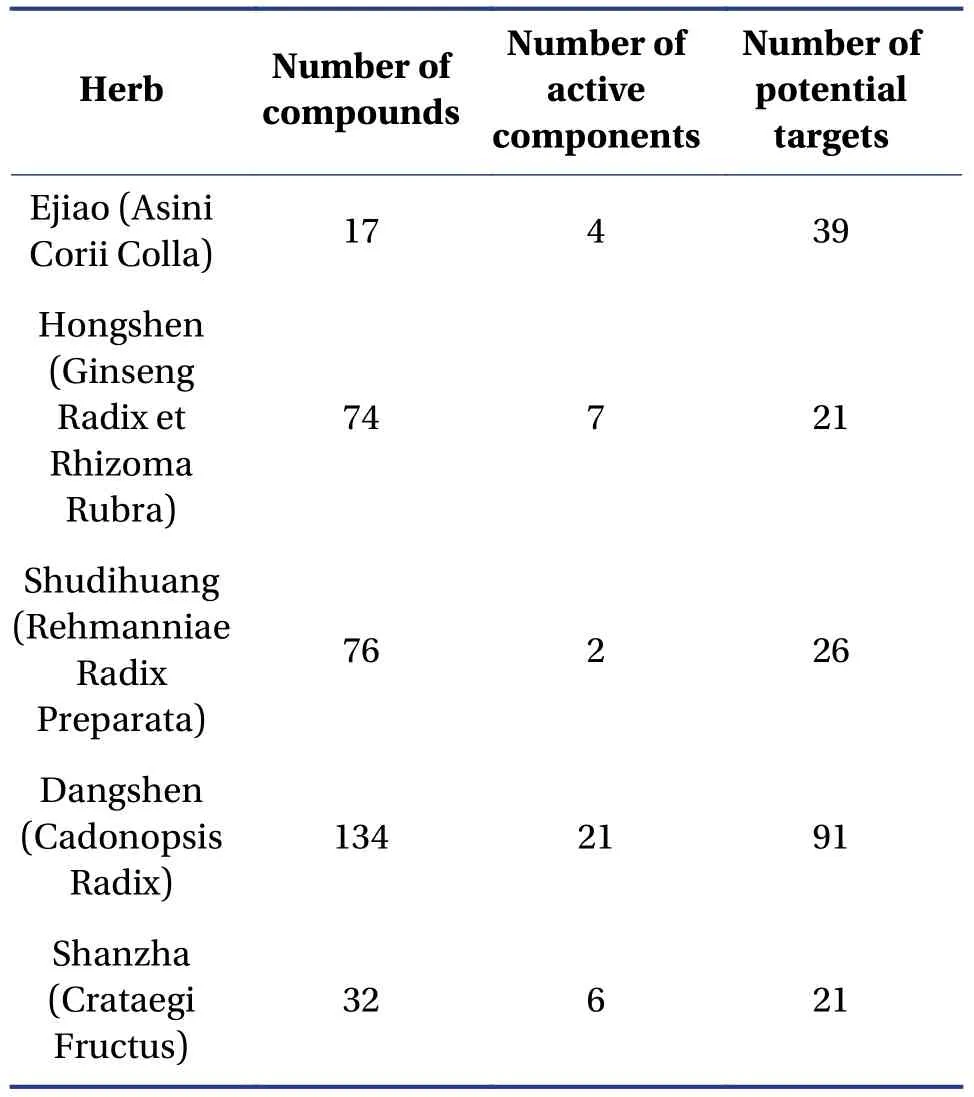
Table 2 Herb-compound-active components-potential targets for FFEJJ
3.1.4 GO analysis and KEGG pathway enrichment analysisThe DAVID platform was used to investigate the biological processes and functions of the candidate targets of FFEJJ with respect to AA.In this study, 24 co-targets were annotated by GO function analysis and KEGG pathway analysis.A total of 30 GO terms are displayed in Figure 3, including biological processes (BP), cellular components (CC), and molecular function (MF).The top five enriched GO terms for FFEJJ (Figure 3A) indicate that FFEJJ elicited its anti-AA effects through the following pathways: negative regulation of the apoptotic process,response to drug, positive regulation of chemokine biosynthetic process, positive regulation of nitric oxide biosynthetic process, and positive regulation of sequence-specific DNA binding transcription factor activity.
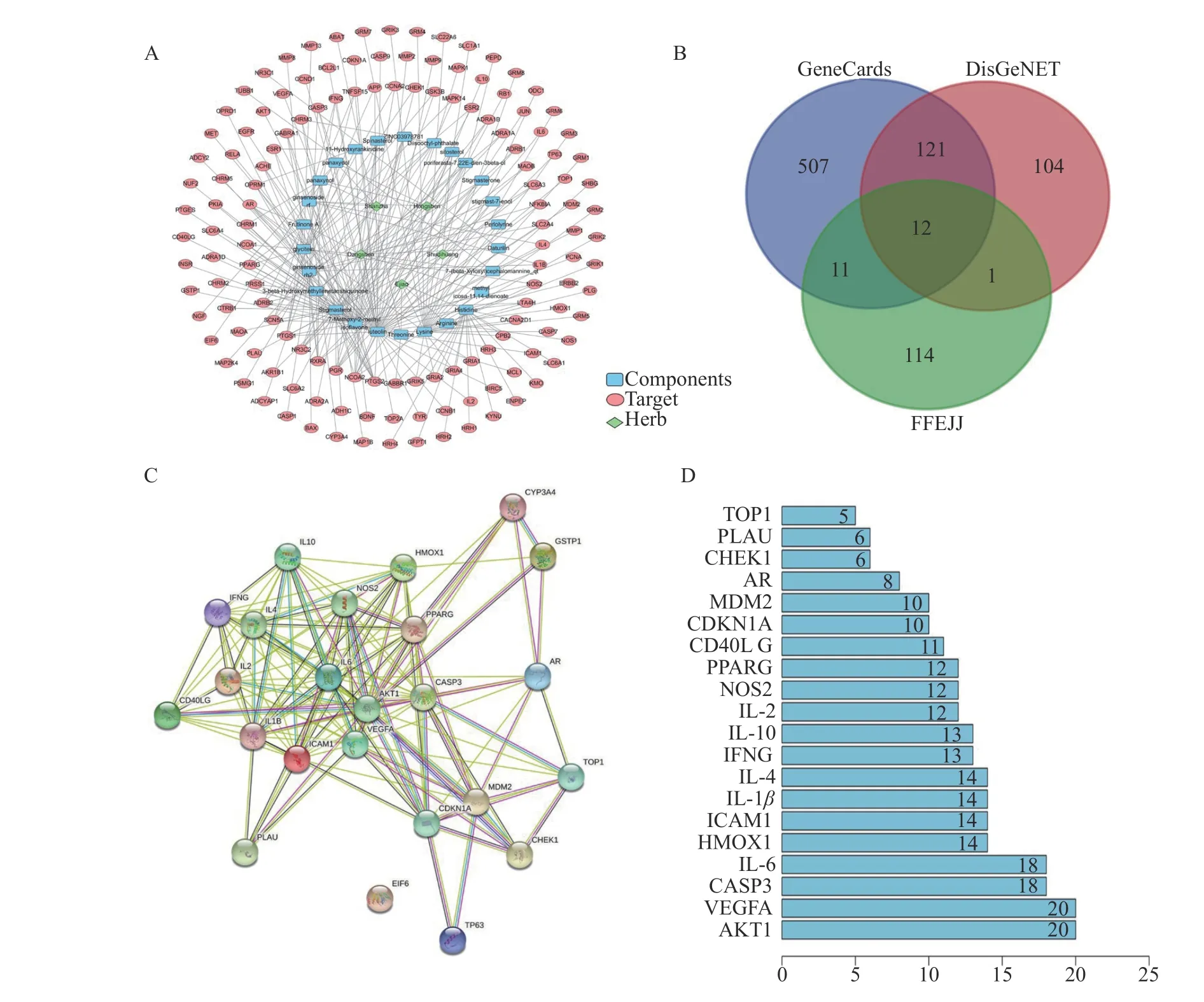
Figure 2 Network pharmacology analysis for FFEJJ
Pathway enrichment was conducted to clarify the activities of FFEJJ.The top 13 KEGG pathways (Figure 3B and 3C) withP< 0.05 were identified for FFEJJ, including immune regulation-related pathways (T cell receptor signaling pathway, intestinal immune network for IgA production, Jak-STAT signaling pathway, and Toll-like receptor signaling pathway), inflammatory regulatory corelated pathways (TNF signaling pathway, PI3K-Akt signaling pathway, NFkappa B signaling pathway), hematopoiesis-related pathways (osteoclast differentiation, hematopoietic cell lineage), oxidative stress-related pathways (HIF-1 signaling pathway, FoxO signaling pathway), and other pathways (cytokine-cytokine receptor interaction, p53 signaling pathway).In summary, these results suggest that FFEJJ exerts an anti-AA effect by combining effective targets with various bioactivities pathways.Pathway information is presented in Table 3.
3.2 Serum metabolomic analysis
3.2.1 Metabolic profilesSerum metabolomics analysis was conducted to further characterize the metabolite changes in AA rats, and the functional activities of FFEJJ were verified via metabolic profiles.According to the HMDB and Mrtlin platforms, 895 and 610 endogenous metabolites were identified in the positive and negative ion modes, respectively.

Figure 3 Enrichment analysis for FFEJJ on AA
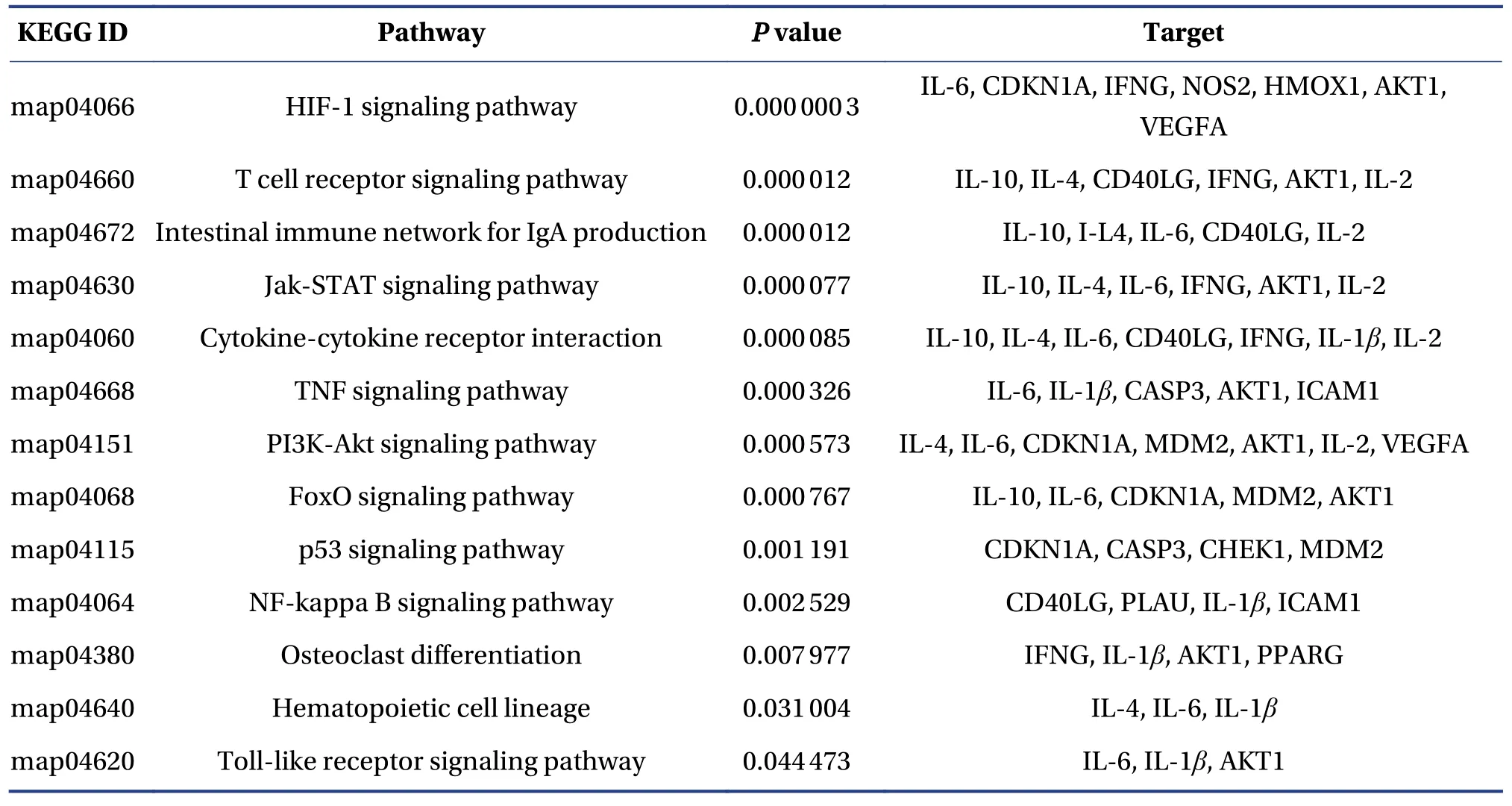
Table 3 The top 13 KEGG pathways associated with FFEJJ in relation to AA
HCA is described as a probe tool that distinguishes natural groups and associates similar classes in a batch of data.As shown in Figure 4, the AA model group were briefly separated from the control group, indicating that APH combined with CTX caused a serious metabolic disorder in rats.Compared with the model group, animals treated with FFEJJ showed a trend towards the control group, suggesting that FFEJJ can regulate the metabolic disorder caused by AA (Figure 4A).In addition, OPLSDA, a supervised analysis strategy, was used to screen and identify the differential metabolites responsible for class separation.OPLS-DA scoring showed good division between groups (Figure 4B).Endogenous metabolites with fold change (FC) > 2 or FC < 0.5,andP< 0.05, were regarded as potential biomarkers.Based on the positive and negative ion modes, 423 biomarkers (58 upregulated and 365 downregulated)were identified between the control and model groups.Similarly, 79 biomarkers (15 upregulated and 64 downregulated) were identified as differential metabolites between the FFEJJ and model groups(Figure 5A and 5B).
Furthermore, to develop our understanding of the regulation rules for FFEJJ, 35 overlapping altered metabolites were identified, and the metabolic profiles are shown in the heat map (Figure 5C and 5D).Notably, FFEJJ reversed 26 metabolites compared to rats without treatment (Table 4).
3.2.2 Metabolic pathway analysisEnrichment analysis for biomarkers was performed to reveal the correlative metabolic pathways and function and illustrate the potential mechanisms underlying the therapeutic effects of FFEJJ at the metabolic level.Overall,423 biomarkers were altered in the control and AA models enriched in 24 metabolic pathways.Similarly,79 metabolites with significant changes between FFEJJ therapy and the AA model were involved in 11 metabolic pathways.Moreover, 10 overlapping metabolic pathways were observed between the control vs.model and FFEJJ vs.model groups.Among them,five metabolic pathways with impact > 0.1 were considered key metabolic pathways for FFEJJ in the treatment of AA,including retinol metabolism,sphingolipid metabolism, pentose and glucoronate interconversions, glycerophospholipid metabolism,and arachidonic acid metabolism, which may also be responsible for the metabolic changes in AA rats (Figure 6).
Sphingolipids(C00319)and sphinganine(C00836) are affected by sphingolipid metabolism.PC [15∶0/20∶3 (8Z, 11Z, 14Z)] (C00157) and Lyso-PC [22∶1 (13Z)] (C04230) were responsible for metabolite nodes in glycerophospholipid metabolism.Arachidonic acid metabolism was regulated by 15(S)-HPETE (C05966) and PC [15∶0/20∶3 (8Z, 11Z,14Z)] (C00157).In addition, pentose and glucuronate interconversions and retinol metabolism were significantly affected by retinal (C00376) and 6-Hydroxy-5-methoxyindole glucuronide (C03033), respectively.The regulatory effects of FFEJJ are mainly involved in lipid metabolism.In this study, compared to the model group, FFEJJ decreased the serum levels of LysoPC [P-18∶1 (9Z)] (P< 0.001), Lyso-PC[20∶1(11Z)],LysoPC[22∶1(13Z)],and LysoPC(P-18∶0) (P< 0.05), while it increased PC[16∶0/16∶1 (9Z)] (P< 0.01).In addition, FFEJJ decreased the sphinganine (P< 0.001) and cer(d18∶ 1/2∶0) (P< 0.05), while it upregulated the level of S1P (P< 0.05).Our results suggest that FFEJJ is involved in the regulation of sphingolipid metabolism via multiple metabolites.
4 Discussion
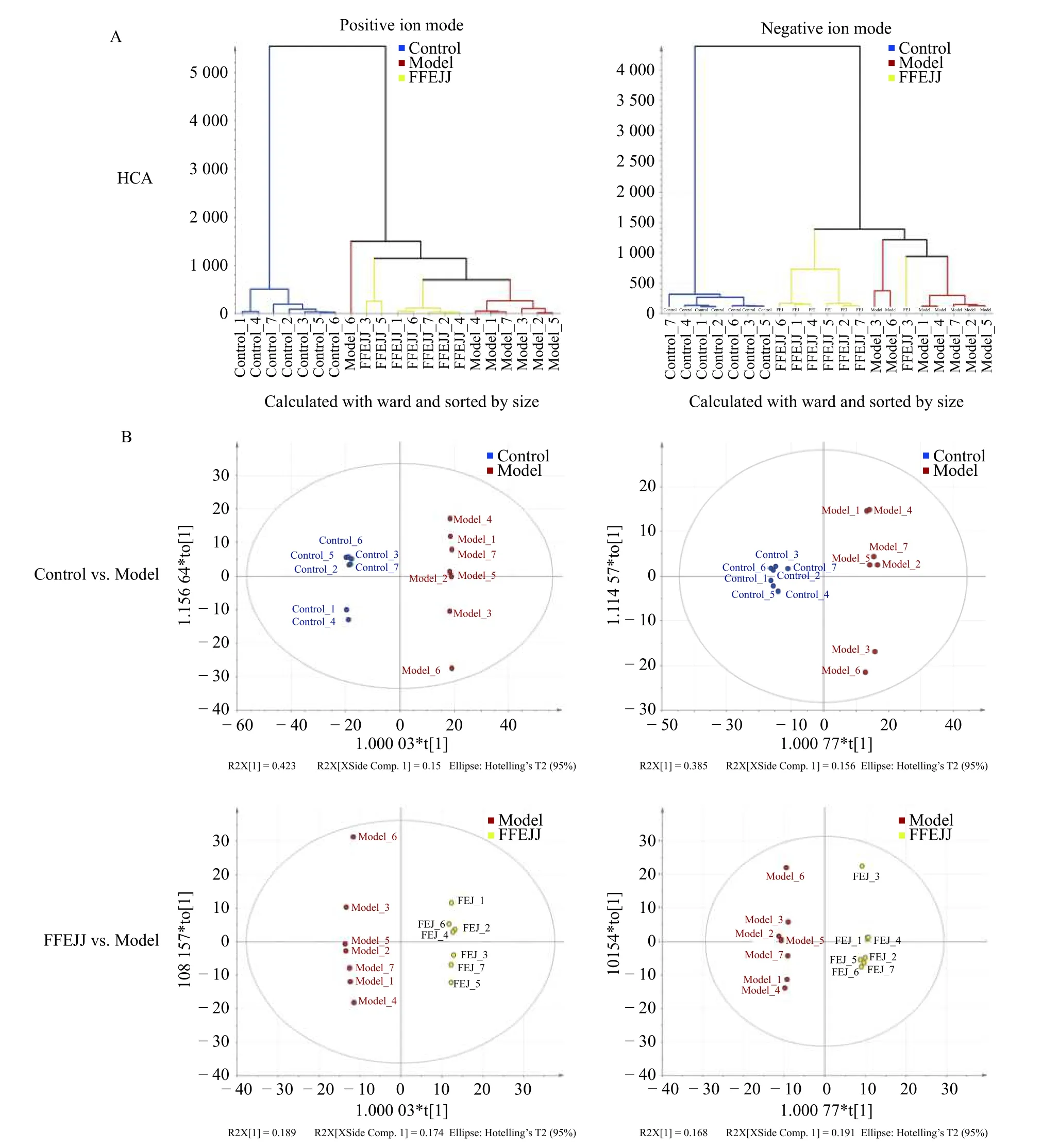
Figure 4 Multivariate statistical analysis of control vs.model and FFEJJ vs.model
AA is considered a fatal blood disease that can occur at any age and is more prevalent in Asia[24].TCMs comprise a combination of multi-flavored herbs and are considered to be effective for bone mar row failure-related diseases, and has the characteristics of high safety, low toxicity, and fewer side effects.Network pharmacology and associated databases were used to forecast the dominant targets of FFEJJ on AA.The development of network pharmacology, containing herbs, ingredients, and disease platforms, has accelerated the advancement of approaches employed to reveal the bioactive constituents and underlying mechanisms of TCMs[25].
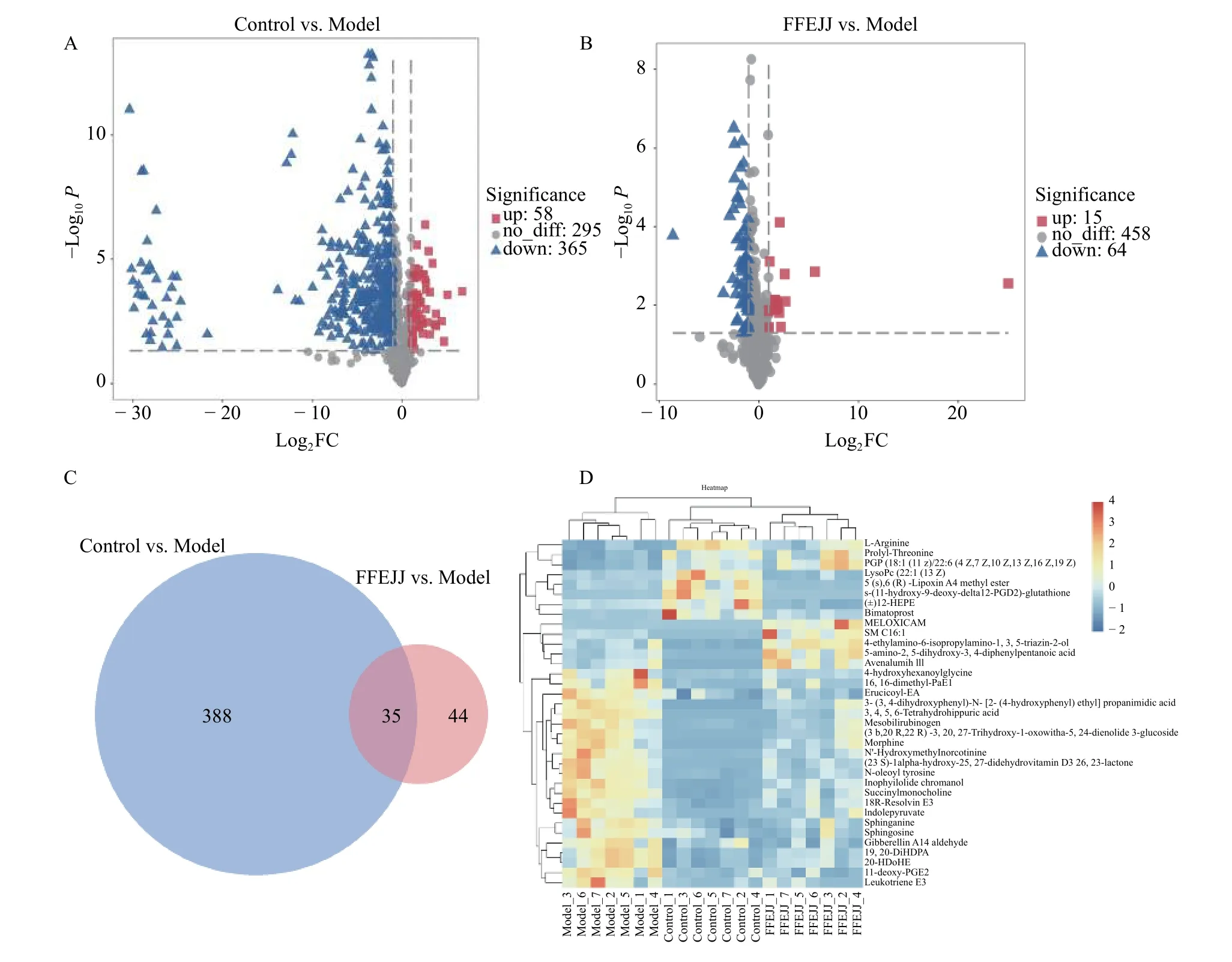
Figure 5 Differential metabolites analysis of control vs.model and FFEJJ vs model
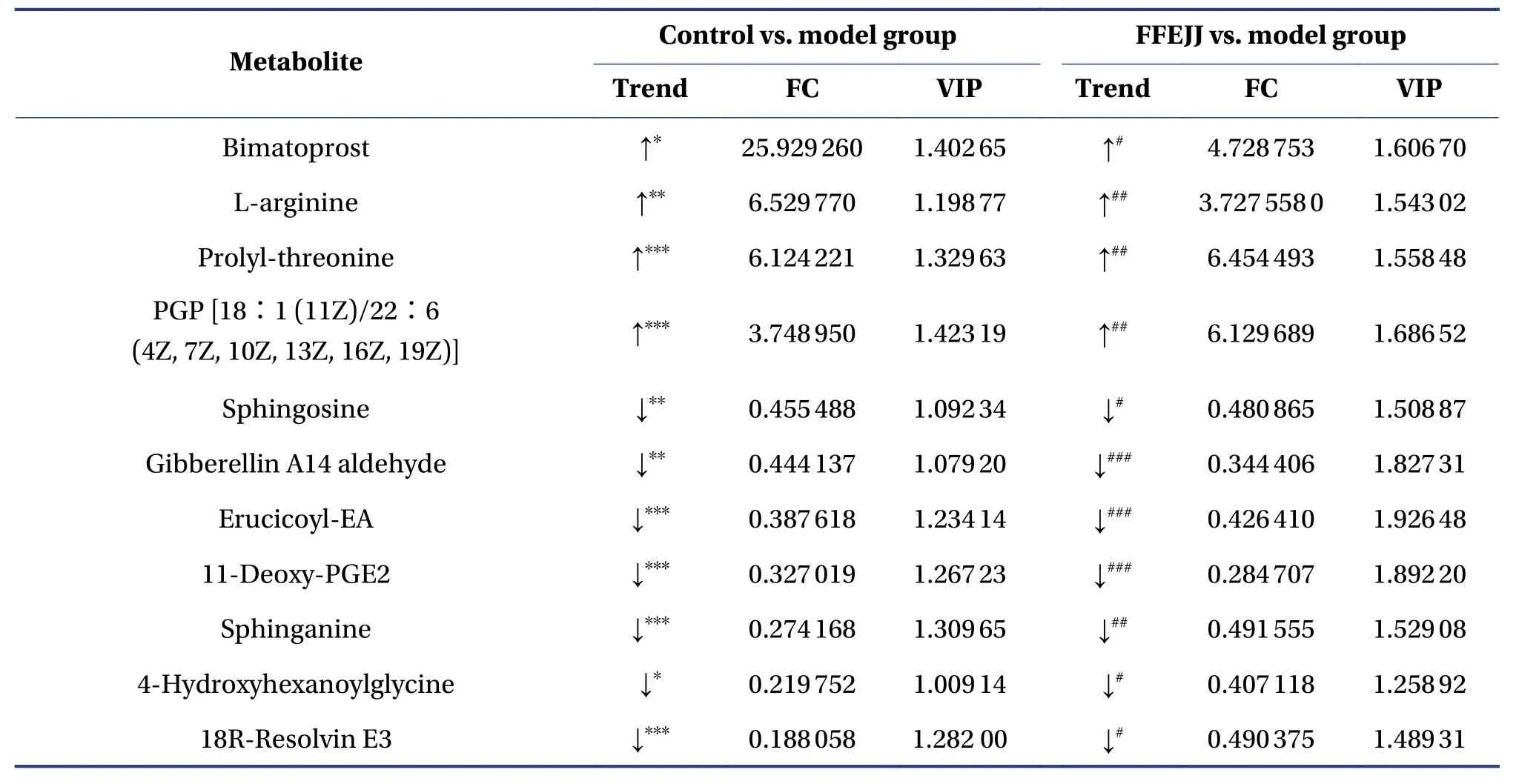
Table 4 Differentially expressed endogenous metabolites of 35 overlapping biomarkers between the control vs.model and FFEJJ vs.model groups

Table 4 Continued
Luteolin and ginsenosides were identified in this study.Previous studies showed that luteolin alleviates HgCl2-induced AA by regulating the Sirt1/Nrf2/TNF-αsignaling pathway[26,27].Accumulating evidence indicates that ginsenosides might be an alternative strategy for the treatment of myelosuppression induced by chemotherapy or radiotherapy[23].In addition, ginsenosides possess dual activities in AA,restoring hematopoiesis and regulating immunity[28].These studies indicate that FFEJJ has the advantages of multiple components and multiple targets in the treatment of AA.Network pharmacology analysis plays an important role in clarifying this complex relationship by linking the chemical space of components with biomolecules.The upregulation of VEGFA accelerates the process of anemia, whereas the upregulation of adhesive molecules in bone marrow mesenchymal stem cells (BMMSCs), such as VLA-4,VCAM-1, and ICAM-1, participates in AA protection[29,30].CASP3 overexpression occurs during erythropoietic stress, especially under conditions of tissue hypoxia during the period of anemia[31].Research demonstrates that irradiation and chemotherapeutic drugs increase IL-6 expression in AA,which may disturb the balance of the bone marrow hematopoietic microenvironment[32].Our study found that luteolin in Dangshen (Codonopsis Radix)is capable of concurrently regulating VEGFA, AKT1,CASP3, and ICAM1.Panaxytol, ginsenoside rh2, and luteolin synergistically regulated CASP3.Network pharmacology suggests that it is possible to analyze complex systems and establish relationships between multiple targets in the chemical compounds of TCM and specific diseases.According to our results, FFEJJ may be involved in regulating bone marrow hematopoietic microenvironment-related targets to achieve the therapeutic effect of AA.
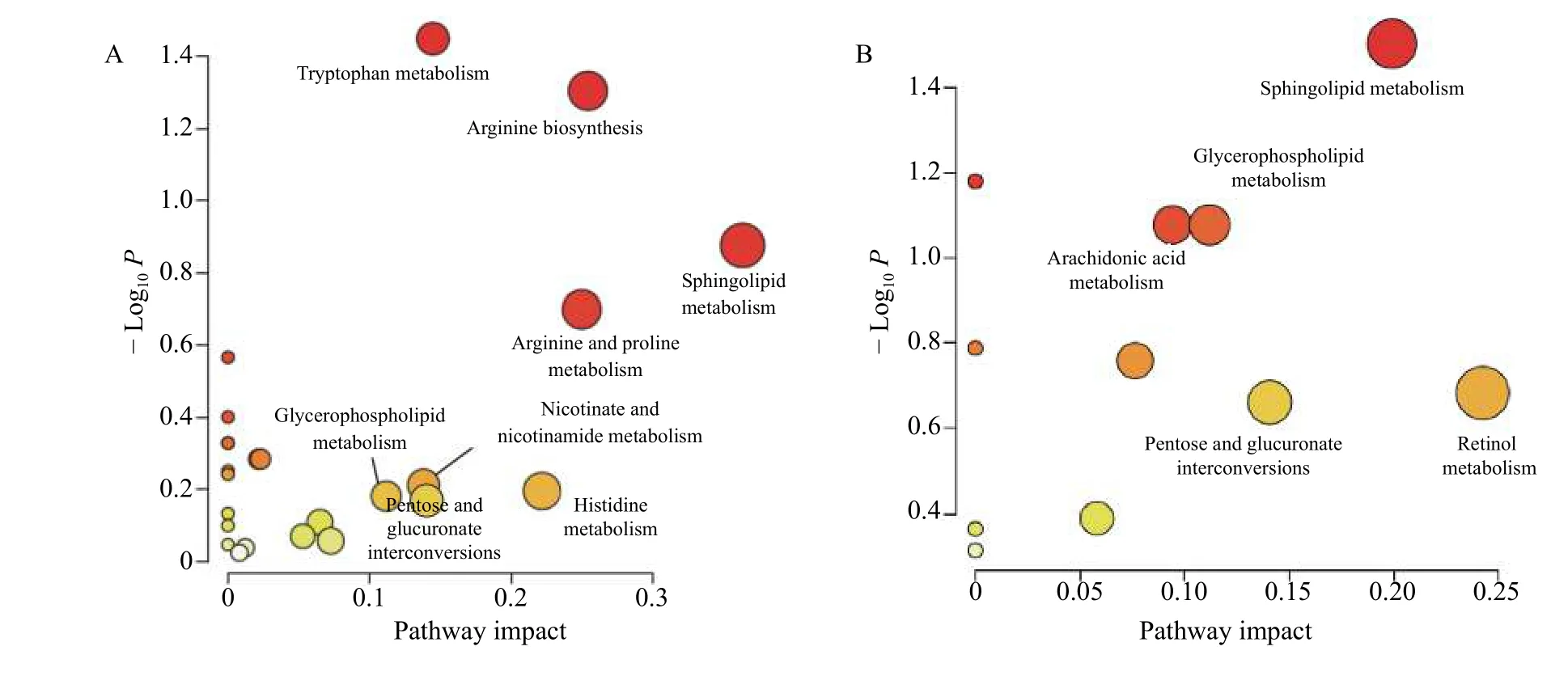
Figure 6 Metabolic pathway analysis
It has been demonstrated that lipid metabolism is a vital aspect of hematopoiesis in the cell-autonomous and bone marrow (BM) microenvironment[33].A previous study showed that the levels of lysophosphatidylcholines(lysoPCs)containing lysoPC(18∶0), lysoPC (20∶4), lysoPC (16∶0), lysoPC(18∶2),sphinganine,thiamine pyrophosphate,phytosphingosine, and glycerophosphocholine increased significantly in hemolytic and AA rats[34].PCs are the precursors of lysoPC and a primary constituent of membrane lipids.During terminal erythropoiesis, the levels of PC and phosphocholine are decreased and metabolized to choline[35].In addition, PC also serves as a substrate reservoir for polyunsaturated fatty acids, such as eicosanoids and hydroxyeicosatetraenoic acid (HETE).HETE participates in bone marrow repopulation and limits the selfrenewal of long-term hematopoietic stem cell (LTHSC)[33].15-HETE is produced in erythroid progenitor cells when treated with erythropoietin[36].The 15(S)-HPETE inhibits the TNF-α-triggered expression of adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which partly conforms to the results of the network pharmacology analysis[37].Thus, FFEJJ may alleviate AA by maintaining the BM microenvironment.
Sphingolipids play important roles in membrane integrity and cellular signaling.Ceramide and sphingosine-1-phosphate (S1P) are two types of central bioactive lipids associated with sphingolipids.S1P has been shown to trigger CXCL12 secretion from mesenchymal stem cells, thereby increasing the excretion of progenitor cells via a reactive oxygen species(ROS)-dependent pathway and modulating hematopoiesis[38,39].Our results suggest that FFEJJ is involved in the regulation of sphingolipid metabolism via multiple metabolites.
Changes in amino acids are also one of the causes for hematopoietic dysfunction[40].Previous studies have shown that Ejiao (Asini Corii Colla) can regulate the metabolism of arginine and threonine and participate in the treatment of diseases[41,42].Our results showed that FFEJJ can increase serum levels of L-arginine and prolyl-threonine in anemia and improve the symptoms of aplastic anemia.It is suggested that Ejiao (Asini Corii Colla) , as a blood-enriching component of FFEJJ, may play an important role in regulating the metabolism of organisms.
This study focused on the relationship between metabolites and targets regulated by active components.Network pharmacology combined with metabolomic analyses was used to investigate this correlation.In addition, two crucial pathways, hematopoiesis-related pathways and hematopoietic microenvironment-related pathways, were enriched.Among them,luteolin, ginsenoside rh2, and panaxytol were the hub active components of FFEJJ.Ginsenoside Rh2 is involved in the regulation of VEGFA and CASP3[43,44].Luteolin can decrease the expression of ICAM1 and CASP3[45,46].Panaxytol normalizes CASP3 expression[47].This is consistent with our prediction of the interaction network.In addition, these three targets are responsible for maintaining the stability of the BM microenvironment and regulating hematopoi esis.Lipid metabolism (including HETE, Lyso PCs,and S1P) is the main metabolic regulatory pathway altered by FFEJJ.Normalization of lipid metabolism is an important aspect in maintaining hematopoiesis.he above pathways partly account for the protective mechanism of FFEJJ against AA.Moreover, the results indicate that FFEJJ has an impact on network regulation through the synergistic action of multiple components on multiple targets, thus playing an important role in the treatment of AA (Figure 7).
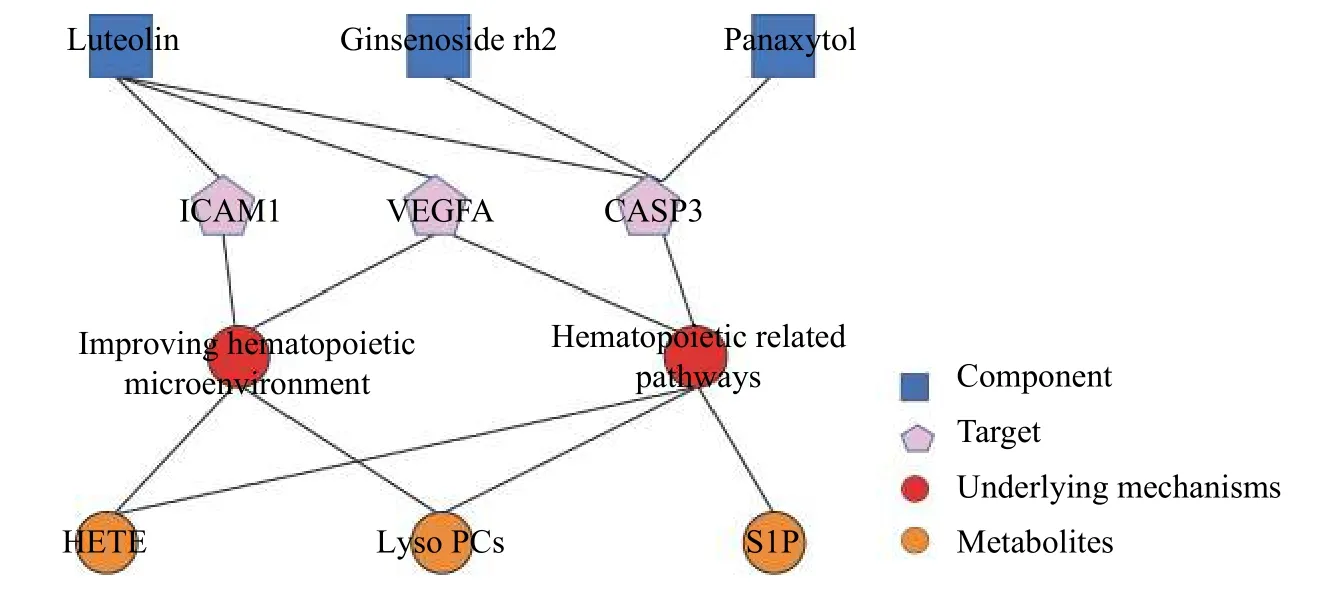
Figure 7 Interaction network of components, metabolites, targets, and the mechanisms regulated by FFEJJ in treating AA
5 Conclusion
In summary, network pharmacology combined with serum metabolomics showed that FFEJJ provided protection against AA via a target network and multiple pathways.Three hub compounds (luteolin, ginsenoside rh2, and panaxytol) and three key targets(VEGFA, CASP3, and ICAM1) responsible for maintaining the stability of hematopoietic cells and for supporting bone marrow hematopoiesis were identified.The integrated network also revealed that metabolic regulation by FFEJJ, particularly lipid metabolism, is an important part of its anti-AA activity.These results suggest that FFEJJ may be a new treatment option for AA.Moreover, the emergence of network pharmacology integrated metabolomics makes it possible to analyze TCM from a systems perspective and at the molecular level.
Acknowledgements
We thank for the funding support from the Natural Science Foundation of China (No.81673585, No.81874493, No.81573956); Program of Survey of Chinese Medicines of China (No.[2017]66), Science Foundation of Hunan Province (No.2019JJ50345, No.2020JJ5325, No.2021168), Key Research and Development Project of Changsha Science and Technology (No.kq1901067); Training Program for Excellent Young Innovators of Changsha (No.kq1802017); Research on the Comprehensive Development and Utilization of Characteristic Traditional Chinese Medicine Resources (No.2060302); and the Support of Hunan Province Traditional Chinese Medicine Preparation and Quality Traceability Engineering and Technology Center, and the 2011 Collaboration and Innovation Center for Digital Chinese Medicine in Hunan.
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年4期
Digital Chinese Medicine2021年4期
- Digital Chinese Medicine的其它文章
- Digital Chinese Medicine
- Effects of Fuke Qianjin Formula on hormones and their receptors and metabonomics study in uterine fibroids model rats
- Study on differential gene expression profile of serum exosomes in patients with acute cerebral infarction
- Rational drug design, synthesis, and biological evaluation of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides as potential antimalarial, antifungal, and antibacterial agents
- Traditional Chinese medicine compounds for the treatment of functional dyspepsia: an updated meta-analysis of randomized,double-blind, placebo-controlled trials
- Systematic review of robust experimental models of rheumatoid arthritis for basic research
